Abstract
Cisplatin is a chemotherapeutic agent, which is used in the treatment of various solid organ cancers, and its main dose limiting side effect of cisplatin is nephrotoxicity. The aim of this study is to investigate the role of pioglitazone and creatine on cisplatin nephrotoxicity in vitro. Real-time cell analyzer system (RTCA) was used for real-time and time-dependent analysis of the cellular response of HK-2 cells following incubation with cisplatin and combination with creatine or pioglitazone hydrochloride. First, half-maximal inhibitory concentrations (IC50) of cisplatin, creatine and pioglitazone were calculated by RTCA system. Afterwards creatine and pioglitazone was administered with serial dilutions under RTCA system. IC50 dose for cisplatin was 7.69 M × 10−5 at 24th hour and 3.93 M × 10−6 at 48th hour. IC50 dose for pioglitazone was 1.61 M × 10−3 at 24th hour and 2.85 M × 10−4 at 48th hour. Although cells were treated the dose of 40,225 mM creatine, IC50 dose could not been reached. Neither pioglitazone nor creatine had additional protective effect in any dose. Consequently, beneficial effect of creatine and pioglitazone on cisplatin-induced cell death could not be found. Further studies and clinical trials are needed to evaluate the effect of different doses of these drugs in cisplatin-induced nephrotoxicity.
Introduction
Cisplatin is a chemotherapeutic agent which is used in the treatment of a variety of solid organ cancers such as lung, testis, neck, ovary and breast.Citation1 Nephrotoxicity is a serious dose limiting adverse effect of cisplatin treatment.Citation2,Citation3 Several possible mechanisms such as DNA damage, mitochondrial dysfunction, oxidative stress, activation of caspase formation and apoptosis have been proposed to explain mechanism of nephrotoxicity.Citation4–7 Nevertheless, cisplatin-induced nephrotoxicity has not been clearly illuminated and prevention strategies remain unclear. Therefore, more effective preventive ways are still being investigated.
Creatine is a popular supplement to enhance physical performance. The dietary intake and endogenous production of creatine matches the spontaneous degradation of phosphocreatine and creatine to creatinine.Citation8 Recently, several articles have indicated the various effects of creatine including cytoprotective features through antioxidant-like effect, mitochondrial genome stabilization and decrease in oxygen consumption besides its ergogenic role.Citation9
Pioglitazone is an anti-diabetic drug that belongs to thiazolidinediones and acts as an agonist of peroxisome proliferator activated receptor gamma (PPARg). Along with its anti-inflammatory and antioxidant effects, pioglitazone has been reported to have protective effects on cerebral, myocardial and renal ischemia.Citation10–15
Real-time cell analyzer (RTCA) system is a label-free and non-invasive method which works with the basis of measurement of the electrical impedance on integrated microelectrodes in microwell plates. After the interaction of cells with electrodes, ionic environments alter and modifying impedance is determined in each well by RTCA system. Hence, cell number monitoring can be done and viability can be measured in real time. Results are displayed as cell index value. RTCA is a useful method since the system has several advantages like less interfering with normal cell metabolism and dynamic real-time cell viability measurement.Citation16,Citation17
The aim of this study is to investigate the role of pioglitazone and creatine on cisplatin nephrotoxicity in vitro. This is the first study to search for the possible effects of pioglitazone and creatine in the HK-2 cells exposed to cisplatin.
Material and methods
Cell culture
Experimental studies were carried out using HK-2 cells (CRL-2190-American Type Culture Collection), which are human proximal tubular cells immortalized by transduction with human papilloma virus 16 E6/E7 genes. Cells were cultured in keratinocyte serum-free (KSF) medium (Gibco/BRL 17005-042) supplemented with 5 ng/mL recombinant epidermal growth factor and 0.05 mg/mL bovine pituitary extract at 37 °C in 5% CO2 in a humidified incubator.
Cell treatment and viability
Real-time cell viability analysis The xCELLigence RTCA system (Roche Applied Science, Mannheim, Germany) was used for real-time and time-dependent analysis of the cellular response of HK-2 cells following incubation with cisplatin (ORNA, Istanbul, Turkey) and combination of cisplatin with creatine (General Nutrition Corporation, Pittsburgh, PA) or pioglitazone hydrochloride (CHEMOS GmbH, Regenstauf, Germany).
Cells were seeded at a density of 5 × 103 cells/well in a 96-well plate and allowed to attach for 24 hour. The medium was then replaced with the medium containing cisplatin and creatine or pioglitazone was combined with serial dilutions. The medium and drug combinations renewed at 48th hour. First, half-maximal inhibitory concentrations (IC50) of cisplatin and pioglitazone were calculated by RTCA system to determine the drug potency and the concentration of the drug that is required for 50% inhibition of cell viability. Although we treated creatine with the dose of 40,225 mM, IC50 dose could not been reached.
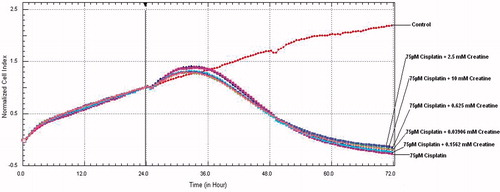
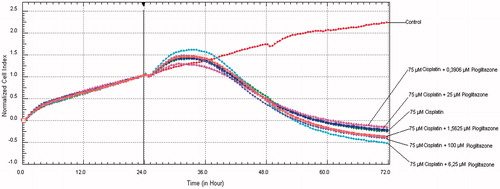
Creatine and cisplatin were dissolved in medium, while PI was dissolved in DMSO. Cisplatin was used as IC50 drug concentration of 75 µM. Creatine concentrations were 10 mM, 2.5 mM, 0.625 mM, 0.1562 mM and pioglitazone concentrations were 100 µM, 25 µM, 6.25 µM, 1.5625 µM, 0.3906 µM. After treatment, the cells were allowed to grow for 72 hours with monitoring every 30 minutes. Control experiments were carried out using the only growth culture medium without any additive. Cell proliferation was then monitored by RTCA system (xCELLigence and RTCASoftwareV1.2.1). Each experiment was carried out in triplicate. Obtained cell index measurements were normalized according to time of drug administration and referred as normalized cell index in figures.
Results
IC50 dose for cisplatin was 7.69 M × 10−5 (R2: 0.97) at 24th hour and 3.93 M × 10−6 (R2: 0.95) at 48th hour. IC50 dose for pioglitazone was 1.61 M × 10−3 (R2: 0.96) at 24th hour and 2.85 M × 10−4 (R2: 0.91) at 48th hour. After addition of creatine to cisplatin treated cells with serial dilutions at 24th hour, all cell series proliferated at 33rd hour and from this point cell viability progressively decreased to end of 72nd hours (). In cell line treated with cisplatin and 6.25 µM pioglitazone cell proliferation retained until 34th hour and cell viability began to decrease at the end of 72nd hours. In cell line treated with cisplatin and 100 µM pioglitazone cell proliferation retained until 31st hour, beginning from 34th hour cell viability decreased to end of 72nd hours. Other pioglitazone dose combinations were parallel to free cisplatin administration (). These results indicate that neither pioglitazone nor creatine addition had protective effect in any dose.
Discussion
Cisplatin is accumulated in both proximal and distal nephrons. The S3 segment of the proximal tubules has the highest concentration of cisplatin, followed by the distal collecting tubule and the S1 segment in the proximal tubule.Citation18,Citation19 Cisplatin-induced cytotoxicity results from its interaction with DNA and resultant DNA damage.Citation20 Cisplatin especially tends to accumulate in mitochondria and cells which have densely mitochondria are much more sensitive to toxicity. Since renal proximal tubule cells have highest densities of mitochondria in the kidney, this segment is more susceptible to cisplatin-induced cell damage.Citation21 Therefore, we used renal proximal tubular cell line in this toxicity model. In addition to DNA damage, cisplatin inhibits fatty acid oxidation that is the main source of energy for the proximal tubule through reduction in PPAR-α mediated expression of genes involved in cellular fatty acid utilization.Citation21–23 Cisplatin also inhibits the mitochondrial complexes I to IV of the respiratory chain and induces decrease in intracellular ATP levels.Citation24
Creatine can prevent cell damage with two possible mechanisms: stabilization of cellular membranes and maintenance of ATP.Citation18 In addition to these Nomura et al. Citation25 reported that creatine has an anti-inflammatory effect on endothelial cells. After ischemic insults, ATP production through oxidative pathways is reduced resulting in cell damage. Sharov et al.Citation26 showed that exogenous phosphocreatine administration attenuated ischemic damage and reduced necrotic zone with maintenance of membrane integrity. Berneburg et al.Citation27 reported that there was a link between mitochondrial DNA mutations and cellular energy metabolism. In addition, authors showed that mitochondrial mutagenesis could be normalized by creatine supplementation. On the contrary of this in a limited number of patients (n = 15), Kornblum et al.Citation28 reported that high dose creatine treatment has no beneficial effect on skeletal muscle energy metabolism in patients with genetically proven single mitochondrial DNA deletion.
Besides, activation of intrinsic and extrinsic apoptotic cascades, production of reactive oxygen species are other known pathways of cisplatin-induced renal cell death.Citation21 In this sense, several substances were used to ameliorate cisplatin-induced cell damage such as free radical scavengers, iron chelators and other antioxidants.Citation29–33 Oxidative damage to mtDNA may lead to loss of membrane potential, reduced ATP synthesis and cell death.Citation34 Several reports have been published arguing that creatine supplementation enhances oxidative phosphorylation.Citation35–37 Guidi et al.Citation38 reported that creatine has a protective effect on oxidatively-injured mitochondrial and nuclear DNAs. Sestili et al.Citation39 reported that there is a cytoprotective effect of creatine on oxidatively injured cultured mammalian cells through direct antioxidant activity via a scavenging mechanism. Due to all these mechanism and cytoprotective effects of creatine, we used creatine to prevent cisplatin-induced cell death but we have not seen any significant effect on cell viability in RTCA system. Since there was no cytotoxic effect of creatine, further studies with higher doses of creatine might be carried out in vitro or in vivo.
Apoptosis is induced by several pathways in cisplatin-induced cell damage.Citation7 Wei et al.Citation40 reported that cisplatin-induced apoptosis was attenuated in the Bax protein-deficient mice and authors showed Bax protein and cisplatin-induced apoptosis association. In a renal ischemia-reperfusion injury animal model, Hu et al.Citation15 reported that pioglitazone pretreatment provide up-regulation of expression of Bcl-2 proteins and down-regulation of expression of Bax protein in kidney. Through this way mitochondrial activity and cell integrity are maintained. Pioglitazone has been shown to have beneficial effect on septic shock via suppression of inflammatory responses.Citation12 Cisplatin treatment activates mitogen-activated protein kinase (MAPK) pathways and inhibition of these ways reduces apoptosis, caspase activation, inflammation and renal injury.Citation7 Pioglitazone has ability of suppression of MAPK, nuclear factor-kappa B signaling pathways and inhibition of tumor necrosis factor alpha.Citation10,Citation41 In addition to this, pioglitazone can suppress oxidative stress and damage through improving antioxidant capacity.Citation42,Citation43 Recent studies showed that there is a relationship between cisplatin and sirtuin genes.Citation44 One of the sirtuin genes, SIRT1 is a part of cellular response to cisplatin and overexpression of SIRT1 ameliorated cisplatin-induced cytotoxicity.Citation45 Recent literature supported that pioglitazone treatment increased SIRT1 expression.Citation46,Citation47 For all these reason, in our study, we used pioglitazone as another possible protective drug for cisplatin-induced cell death. However, administration of pioglitazone does not seem to have a beneficial effect on cell viability compromised by cisplatin ().
In conclusion, although it is observed that cisplatin nephrotoxicity is attributed to several factors including accumulation, oxidative stress, apoptosis and inflammation, we could not find any beneficial effect of creatine and pioglitazone on cisplatin-induced cell death. Further studies and clinical trials are needed to evaluate the effect of different doses of these drugs and/or other possible drugs, which may affect the ways mentioned above in cisplatin-induced nephrotoxicity.
Declaration of interest
The authors report no declarations of interest. This study was supported by grants from the Ondokuz Mayis University Research Fund with the number PYO.TIP.1901.10.014
References
- Hartmann JT, Lipp H-P. Toxicity of platinum compounds. Expert Opin Pharmacother. 2003;4:889–901
- Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol. 2003;23:460–464
- Sastry J, Kellie SJ. Severe neurotoxicity, ototoxicity and nephrotoxicity following high-dose cisplatin and amifostine. Pediatr Hematol Oncol. 2005;22:441–445
- Kaushal GP, Kaushal V, Hong X, Shah SV. Role and regulation of activation of caspases in cisplatin induced injury to renal tubular epithelial cells. Kidney Int. 2001;60:1726–1736
- Leibbrandt ME, Wolfgang GH, Metz AL, Ozobia AA, Haskins JR. Critical subcellular targets of cisplatin and related platinum analogs in rat renal proximal tubule cells. Kidney Int. 1995;48:761–770
- Matsushima H, Yonemura K, Ohishi K, Hishida A. The role of oxygen free radicals in cisplatin induced acute renal failure in rats. J Lab Clin Med. 1998;131:518–526
- Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of Cisplatin nephrotoxicity. Toxins (Basel). 2010;2:2490–2518
- Walker JB. Creatine: biosynthesis, regulation, and function. Adv Enzymol Relat Areas Mol Biol. 1979;50:177–242
- Sestili P, Martinelli C, Colombo E, et al. Creatine as an antioxidant. Amino Acids. 2011;40:1385–1396
- Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435
- Shimazu T, Inoue I, Araki N, et al. A peroxisome proliferator activated receptor-gamma agonist reduces infarct size in transient but not in permanent ischemia. Stroke. 2005;36(2):353–359
- Haraguchi G, Kosuge H, Maejima Y, et al. Pioglitazone reduces systematic inflammation and improves mortality in apolipoprotein E knockout mice with sepsis. Intensive Care Med. 2008;34(7):1304–1312
- Ahmed LA, Salem HA, Attia AS, Agha AM. Pharmacological preconditioning with nicorandil and pioglitazone attenuates myocardial ischemia/reperfusion injury in rats. Eur J Pharmacol. 2011;663(1–3):51–58
- Medhi B, Aggarwal R, Chakrabarti A. Neuroprotective effect of pioglitazone on acute phase changes induced by partial global cerebral ischemia in mice. Indian J Exp Biol. 2010;48(8):793–799
- Hu H, Zou C, Xi X, Shi Z, Wang G, Huang X. Protective effects of pioglitazone on renal ischemia-reperfusion injury in mice. J Surg Res. 2012;178(1):460–465
- Solly K, Wang X, Xu X, Strulovici B, Zheng W. Application of real-time cell electronic sensing (RT-CES) technology to cell-based assays. Assay Drug Dev Technol. 2004;4:363–372
- Atienza JM, Yu N, Kirstein SL, et al. Dynamic and label-free cell-based assays using the real-time cell electronic sensing system. Assay Drug Dev Technol. 2006;4:597–607
- Persky AM, Brazeau GA. Clinical pharmacology of dietary supplement Cr monohydrate. Pharmacol Rev. 2001;53:161–176
- Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol. 2003;23:460–464
- Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins (Basel). 2010;2:2490–2518
- Qian W, Nishikawa M, Haque AM, et al. Mitochondrial density determines the cellular sensitivity to cisplatin-induced cell death. Am J Physiol Cell Physiol. 2005;289:C1466–C1475
- Li S, Wu P, Yarlagadda P, et al. PPAR alpha ligand protects during cisplatin-induced acute renal failure by preventing inhibition of renal FAO and PDC activity. Am J Physiol Renal Physiol. 2004;286:F572–F580
- Portilla D, Dai G, McClure T, et al. Alterations of PPARalpha and its coactivator PGC-1 in cisplatin-induced acute renal failure. Kidney Int. 2002;62:1208–1218
- Kruidering M, Van de Water B, de Heer E, Mulder GJ, Nagelkerke JF. Cisplatin-induced nephrotoxicity in porcine proximal tubular cells: mitochondrial dysfunction by inhibition of complexes I to IV of the respiratory chain. J Pharmacol Exp Ther. 1997;280:638–649
- Nomura A, Zhang M, Sakamoto T, et al. Anti-inflammatory activity of creatine supplementation in endothelial cells in vitro. Br J Pharmacol. 2003;139:715–720
- Sharov VG, Saks VA, Kupriyanov VV, et al. Protection of ischemic myocardium by exogenous phosphocreatine. I. Morphologic and phosphorus 31-nuclear magnetic resonance studies. J Thorac Cardiovasc Surg. 1987;94:749–761
- Berneburg M, Gremmel T, Kurten V, et al. Creatine supplementation normalizes mutagenesis of mitochondrial DNA as well as functional consequences. J Invest Dermatol. 2005;125:213–220
- Kornblum C, Schröder R, Müller K, et al. Creatine has no beneficial effect on skeletal muscle energy metabolism in patients with single mitochondrial DNA deletions: a placebo-controlled, double-blind 31P-MRS crossover study. Eur J Neurol. 2005;12:300–309
- Baliga R, Zhang Z, Baliga M, Ueda N, Shah SV. In vitro and in vivo evidence suggesting a role for iron in cisplatin-induced nephrotoxicity. Kidney Int. 1998;53:394–401
- Davis C, Nick H, Agarwal A. Manganese superoxide dismutase attenuates cisplatin-induced renal injury: importance of superoxide. J Am Soc Nephrol. 2001;12:2683–2690
- Dickey DT, Wu YJ, Muldoon LL, Neuwelt EA. Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. J Pharmacol Exp Ther. 2005;314:1052–1058
- Naziroglu M, Karaoglu A, Aksoy AO. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology. 2004;195:221–230
- Kroning R, Lichtenstein AK, Nagami GT. Sulfur-containing amino acids decrease cisplatin cytotoxicity and uptake in renal tubule epithelial cell lines. Cancer Chemother Pharmacol. 2000;45:43–49
- Van Houten VWB, Santos JH. Role of mitochondrial DNA in toxic response to oxidative stress. DNA Rep. 2006;5:145–152
- Rico-Sanz J. Creatine reduces human muscle PCr and pH decrements and P(i) accumulation during low-intensity exercise. J Appl Physiol. 2000;88:1181–1191
- Walsh B, Tonkonogi M, Soderlund K, Hultman E, Saks V, Sahlin K. The role of phosphorylcreatine and creatine in the regulation of mitochondrial respiration in human skeletal muscle. J Physiol. 2001;537:971–978
- Passaquin AC, Renard M, Kay L, et al. Creatine supplementation reduces skeletal muscle degeneration and enhances mitochondrial function in MDX mice. Neuromuscul Disord. 2002;12:174–182
- Guidi C, Potenza L, Sestili P, et al. Differential effect of creatine on oxidatively-injured mitochondrial and nuclear DNA. Biochim Biophys Acta. 2008;1780:16–26
- Sestili P, Martinelli C, Bravi G, et al. Creatine supplementation affords cytoprotection in oxidatively injured cultured mammalian cells via direct antioxidant activity. Free Radic Biol Med. 2006;40:837–849
- Wei Q, Dong G, Franklin J, Dong Z. The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int. 2007;72:53–62
- Wei-guo Z, Hui Y, Shan L, et al. PPAR-gamma agonist inhibits Ang II-induced activation of dendritic cells via the MAPK and NF-kappaB pathways. Immunol Cell Biol. 2010;88:305–312
- Inoue I, Goto S, Matsunaga T, et al. The ligands/activators for peroxisome proliferator-activated receptor alpha (PPARalpha) and PPARgamma increase Cu2+,Zn2+-superoxide dismutase and decrease p22phox message expressions in primary endothelial cells. Metabolism. 2001;50:3–11
- Hasegawa T, Okada K, Okita Y, Pinsky DJ. Antioxidant properties of pioglitazone limit nicotinamide adenine dinucleotide phosphate hydrogen oxidase and augment superoxide dismutase activity in cardiac allotransplantation. J Heart Lung Transplant. 2011;30:1186–1196
- Kelly GS. A review of the sirtuin system, its clinical implications, and the potential role of dietary activators like resveratrol: part 2. Altern Med Rev. 2010;15:313–328
- Jung YJ, Lee JE, Lee AS, et al. SIRT1 overexpression decreases cisplatin-induced acetylation of NF-κB p65 subunit and cytotoxicity in renal proximal tubule cells. Biochem Biophys Res Commun. 2012;419:206–210
- Makino N, Maeda T, Oyama J, Higuchi Y, Mimori K. Improving insulin sensitivity via activation of PPAR-gamma increases telomerase activity in the heart of OLETF rats. Am J Physiol Heart Circ Physiol. 2009;297:2188–2195
- Picard F, Kurtev M, Chung N, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776