Abstract
A role for transforming growth factor-β1gene has been suggested in the etiology of IgA nephropathy. However, results have been inconsistent. In this study, a meta-analysis was performed to further clarify the association between transforming growth factor-β1-509C/T gene polymorphism and the susceptibility of IgA nephropathy. PubMed, EMBASE, Web of Science, CNKI, WanFang, and VIP Data were searched for eligible studies. Pooled odds ratios (ORs) with 95% confidence intervals were calculated using a fixed-effects model or random-effects model. A total of eight publications involving 1355 IgA nephropathy patients and 1464 controls met the inclusion and were analyzed. The pooled ORs for the association between TGF-β1gene-509C/T polymorphism and IgA nephropathy risk were not statistically significant under all genetic models (for CT+TT vs. CC: OR = 1.09; 95% CI = 0.92–1.29, p = 0.490; for TT vs. CT+CC: OR = 1.14; 95% CI = 0.94–1.38, p = 0.081; for CC vs. TT: OR = 0.87; 95% CI = 0.69–1.08, p = 0.195; for C allele vs. T allele: OR = 0.92; 95% CI = 0.83–1.03, p = 0.149). In the stratified analysis by ethnicity, results also showed no significant association between TGF-β1 gene-509C/T polymorphism and IgA nephropathy risk in both European and Asian populations. This meta-analysis does not support the hypothesis that TGF-β1 gene-509C/T polymorphism is a risk factor for the development of IgA nephropathy.
Introduction
IgA nephropathy, also called Berger's disease, is the most common form of glomerulonephritis globally, accounting for 25–50% of primary glomerulonephritis patients.Citation1 Epidemiologic studies have indicated that up to 25–30% of IgA nephropathy progress to end-stage kidney disease within 20–25 years.Citation2,Citation3 The best treatment of IgA nephropathy is not well-defined and at present no causal therapy is available.Citation4 In the past 45 years, many hypotheses have been made since IgA nephropathy was first described. Of primary importance is the glycosylation pattern of IgA1. In IgA nephropathy, an increased fraction of circulatory IgA1 has a galactose deficiency in some carbohydrate side chains (O-glycan) that are attached to the hinge-region segment of the heavy chain.Citation5
In recent years, both experimental and clinical studies have shown that transforming growth factor-β1 (TGF-β1) plays an important role in tissue fibrosis and has been found to participate in the pathologic process of IgA nephropathy.Citation6–10 TGF-β1 is a major cause of tissue fibrosis. In human IgA nephropathy, on the one hand, TGF-β1 can promote the cells to synthesis and secrete extracellular matrix. The excessive accumulation of the extracellular matrix will cause glomerular sclerosis. On the other hand, TGF-β1 promotes the renal tubular epithelial cell transform to mesenchymal cell, which causes interstitial fibrosis of renal interstitium.Citation11,Citation12 Several common functional polymorphisms in the TGF-β1 gene have been reported. TGF-β1 is located in the 19q13 chromosome with seven exons and six introns and 509C/T polymorphism is located in the promoter region of TGF-β1 gene. The −509T allele was shown to be in association with the increased secretion of TGF-β1. Several previous studies have reported that TGF-β1 production was regulated at the transcriptional level,Citation13 and suggested that a mutation in the TGF-β1 gene region was accompanied by a change in TGF-β1 production.Citation14–17 In addition, a variety of epidemiologic studies have focused on the association between the polymorphisms of TGF-β1gene-509C/T and the susceptibility of IgA nephropathy. However, the association between the polymorphisms of TGF-β1gene-509C/T and the susceptibility of IgA nephropathy remains inconsistent.Citation17,Citation18 This condition can be explained by a number of factors: the limited sample size, the ethnicity, genotyping procedure, and geographical locations. Therefore, each single study is inadequate to achieve a comprehensive and reliable conclusion. So, we performed a meta-analysis from all eligible studies to derive a more precise estimation of the relationship between the polymorphisms of TGF-β1gene-509C/T and the susceptibility of IgA nephropathy.
Materials and methods
Literature search
Studies were identified by systematic searches of the PubMed, Web of Science, Embase, CNKI (China National Knowledge Infrastructure), WanFang, and VIP (Database of Chinese Scientific and Technical Periodicals) for relevant studies published in English or Chinese before June 2014 using the following terms: “Transforming growth factor-β” or “Transforming growth factor-beta” or “TGF-β”or “TGF-beta” and “IgA nephropathy” combined with “polymorphism” or “mutation” or “variant”. These computer searches did not include animal studies or non-English language articles.
Inclusion criteria
In the meta-analysis, the selected studies met the following criteria: (1) information on the evaluation of TGF-β1gene-509C/T polymorphism and the susceptibility of IgA nephropathy; (2) sufficient published data for estimating the odds ratio (OR) with 95% confidence interval (CI); (3) studies using a case-control design; (4) genotype distribution of control population should be consistent with Hardy–Weinberg equilibrium (HWE); (5) not republished data; (6) not animal studies.
Data extraction
Two investigators independently extracted data and reached a consensus on all items. For each of the included articles, information including the first author, year of publication, source of controls (population-based or hospital-based), study population (county, ethnicity), total numbers of patients and controls, and frequency of TGF-β1 gene-509C/T polymorphism in patients and controls was extracted.
Statistical analysis
The Hardy–Weinberg equilibrium for TGF-β1gene-509C/T genotype distribution in controls was tested using Pearson’s χ2 test.Citation19 The ORs with the corresponding 95% CIs were used to assess the strength of the association between TGF-β1gene-509C/T polymorphism and the risk of IgA nephropathy. The following contrasts for TGF-β1gene-509C/T polymorphism were evaluated: dominant model: TT+CT versus CC; recessive model: TT versus CC+CT; additive model: CC versus TT, CC versus CT; allelic model: C allele versus T allele. A subgroup analysis was carried out with respect to ethnicity. Heterogeneity among studies was assessed by the Q-test and the I2 of Higgins and Thompson.Citation20 If the result of the heterogeneity test was p > 0.10, ORs were pooled according to the fixed-effect model. Otherwise, the random effect model was used.Citation21 The sensitivity analysis was performed by omitting each individual study, and an individual study was suspected of excessive sensitivity if the point estimate of its omitted analysis was outside the 95% CI of the combined analysis. Funnel plot asymmetry was assessed by the method of Egger linear regression test (p < 0.05 was considered representative of statistically significant publication bias). The process of statistical analysis was performed with the Stata software (version 11.0; Stata Corporation, College Station, TX), all p values were two-tailed.
Results
Study characteristics
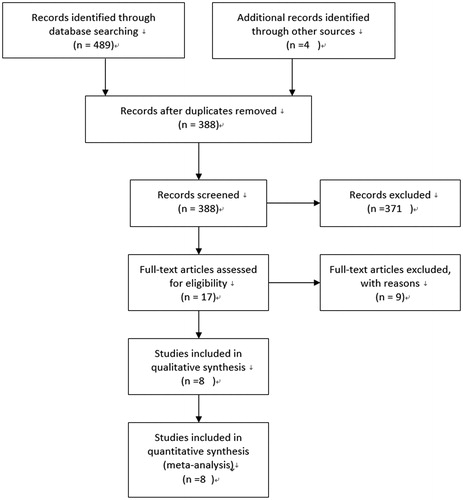
The flow diagram of the search process is exhibited in .
The initial search identified 493 potentially relevant results from databases, of which 476 articles were rejected due to their obvious irrelevance to purpose of our study by abstracts (including 105 repetitive publications and reviews 371 studies for not relevant TGF-β polymorphism or animal studies). In addition, nine papers with no usable data were excluded. Finally, a total of eight publicationsCitation22–28 involving 1355 IgA nephropathy patients and 1464 controls met the inclusion. The counties of these studies contained China, Korea, Sweden, Japan, and Italy. Five studies involving Asians included 1040 patients and 797 controls, two ones involving Europeans contained 315 cases and 667 controls. The total eight studies provided the number of allele C and allele T in both IgA nephropathy patients and controls. The Hardy–Weinberg equilibrium had been tested for all polymorphisms in the control groups, and all were found to be in the Hardy–Weinberg equilibrium. and present the main characteristics and data of these studies.
Table 1. Characteristics of studies included in this meta-analysis.
Table 2. Summary of different comparative results.
Quantitative synthesis
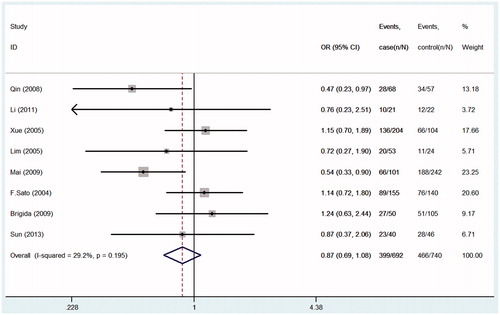
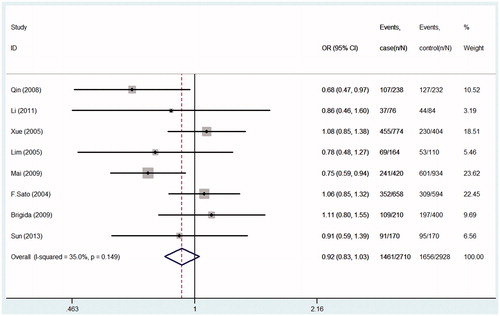
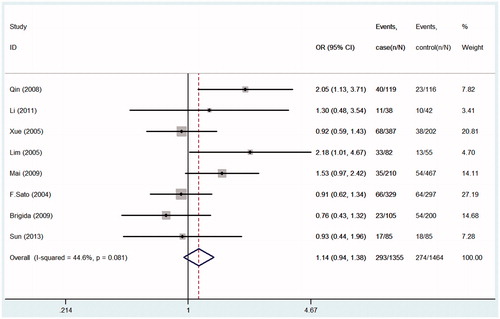
Seven studies were pooled into the meta-analysis, the summary results of this meta-analysis for the association between TGF-β1gene-509C/T polymorphism and the susceptibility of IgA nephropathy were presented in . In the overall analysis, no significant association was found when all studies were pooled into the meta-analysis (for CT+TT vs. CC: OR = 1.09; 95% CI = 0.92–1.29, p = 0.490, ; for TT vs. CT+CC: OR = 1.14; 95% CI = 0.94–1.38, p = 0.081, ; for CC vs. TT: OR = 0.87; 95% CI = 0.69–1.08, p = 0.195, ; for C allele vs. T allele: OR = 0.92; 95% CI = 0.83–1.03, p = 0.149; ). In order to analyze potential ethnic differences, we performed subgroup analysis. We analyzed Asians and Europeans, but Europeans have only two studies. In the stratified analysis by ethnicity, results also showed no significant association between TGF-β1gene-509C/T polymorphism and IgA nephropathy risk in both European and Asian populations ().
Figure 2. Forest plots of the susceptibility of IgA nephropathy associated with TGF-β1-509C/T (TT+CT vs. CC).
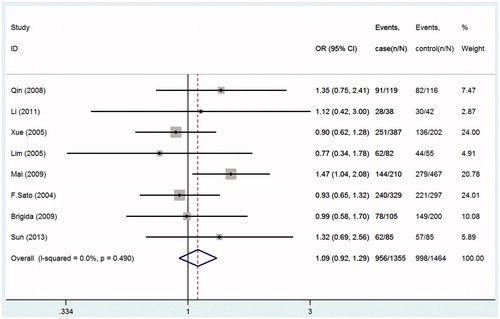
Figure 3. Forest plots of the susceptibility of IgA nephropathy associated with TGF-β1-509C/T (TT+CT vs. CC).
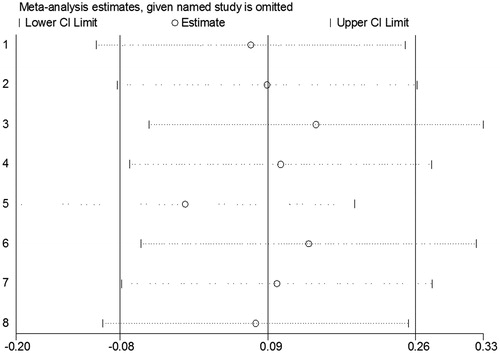
Sensitivity analysis
The sensitivity analysis, an individual study involved in the meta analysis was excluded each time to investigate the influence of the individual dataset on the pooled OR. The corresponding pooled OR and significant results did not change materially (), indicating that our results were statistically robust.
Publication bias
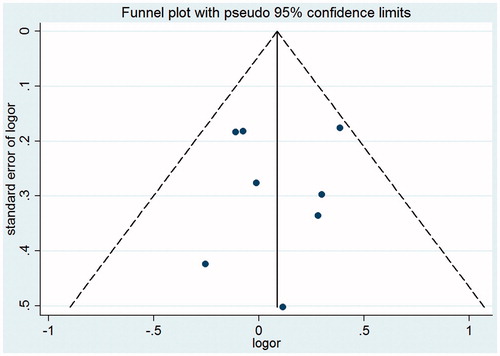
Begg’s funnel plot and Egger’s test were performed to assess the publication bias of literatures. As indicated in the , there existed no evidence of obvious asymmetry in the shape of funnel plots ( for the dominant model). The modified Egger linear regression test and Begg’s test indicated that no significant publication bias was observed (t = −0.27, p = 0.944; Z = 0.15, p = 0.707 for TT+CT vs. CC).
Discussion
In the present study, we failed to find any association between TGF-β1gene-509C/T polymorphism and risk of IgA nephropathy in overall analyses. After subgroup analyses according to ethnicity, we did not found any association between TGF-β1gene-509C/T polymorphism and IgA nephropathy in both Asians and Caucasians. These findings indicate that TGF-β1gene-509C/T polymorphism may not be a risk factor for IgA nephropathy.
IgA nephropathy, which is a complex disease with environment and genetic factors contributing to disease etiology, is the most common primary glomerulonephritis. Many studies have confirmed that genetic susceptibility is associated with IgA nephropathy. Associations between IgA nephropathy and polymorphisms in several other candidate genes, including TGF-β1, lymph toxin beta receptor,Citation29 ACE,Citation30 aldosterone,Citation31 toll-like receptors,Citation32 and retinoic acid receptor alphaCitation33 have been studied. TGF-β1 is considered as one of the primary candidate genes due to its crucial role in the development and progression of IgA nephropathy.
A number of case-control studies were performed to assess the effects of TGF-β1gene-509C/T polymorphism on IgA nephropathy, but the results have been inconsistent. Brezzi et al.Citation28 found that a significant association was observed in the haplotype analysis, but genotype frequencies were not significant. Lim et al.Citation17 have reported that there were significant differences in gene-509C/T genotype frequency between IgA nephropathy patients and normal controls (CC: CT: TT, 20:29:33 vs. 11:31:13, p = 0.043) in Korea. A study carried out by Qin et al.Citation23 indicated that the distribution of 509C/T SNP site allele is apparently different with normal control; the TT homozygote was significantly higher (33 vs. 22%, p = 0.02) and 509C/T SNP site genotype of TGF-β1 gene is associated with the susceptibility of IgA nephropathy. However, some studies did find no significant associations. Sato et al.Citation27 found that CC-509 genotypes, as well as C-C haplotype of TGF-β1 gene polymorphisms are specifically associated with marked proteinuria and increased mesangial cell proliferation in Japanese patients with IgA nephropathy, but they do not confer susceptibility to this disease. Two other studies were done in Chinese population with IgA nephropathy. Xue et al.Citation24 came to a conclusion that TGF-β1 gene-509C/T polymorphism may be associated with serious renal tissue lesion and albuminuria even glomerulosclerosis; IgA nephropathy patients with CC genotype or C allele were not benefited. However, this polymorphism was not associated with susceptibility to IgA nephropathy in Chinese Han nationality. Li et al.Citation18 verified that TGF-β1gene-509C/T polymorphism and allele might not relate to genetic susceptibility of Guangxi Zhuang minority group with IgA nephropathy, but it may be associated with quite serious injury of the kidney or renal deposition of the immune pathology.
Our study investigated the relationship between TGF-β1gene-509C/T polymorphism and the susceptibility of IgA nephropathy. The present meta-analysis with seven studies (include 1335 cases and 1464 controls) provides more comprehensive analysis on the relationship between TGF-β1gene-509C/T polymorphism and IgA nephropathy. Our study indicates that no significant association was found in any genetic model when all studies were pooled into the meta-analysis. However, mild heterogeneity within studies was detected in several models (). Considering the influence of genetic background on the result of this present study, we also performed subgroup analyses by ethnicity for these polymorphisms. Due to that study from Caucasian has only two, the subgroup analysis was carried out between Asians and Caucasians. The results show no significance was found in any genetic models (). However, only two published studies for Caucasians included in the meta-analysis make the stratified analysis not so reliable. The results should be interpreted with caution, and more additional studies with further large-scale sample are needed to validate the result. But maybe this provides some support for the difference between the races.
Although one studyCitation26 previously performed pooling analysis about this issue, several published studies were not included in this analysis and additional original studies with larger sample sizes have been published since then. Their meta-analysis included only three studies,Citation26–28 one of which was about Asians and two of which were about Caucasians. In addition, their meta-analysis did not cover Chinese databases, and did not perform subgroup analysis on different types of study population. Their conclusion should be treated with some more caution.
Some limitations existing in this meta-analysis should be addressed, and these include the following aspects. First, our result was based on unadjusted estimates; therefore, more precise analysis should be performed if individual data were attainable, which would allow for the adjustment by other factors, including age, ethnicity, family history, and environmental factors. Second, the controls were not uniformly defined. Although all the controls were healthy populations, most of them were general populations; some controls were hospital-based while other controls were population-based. Hence, non-differential misclassification bias is possible. Third, in the subgroup analysis, only two published studies for Caucasians was included in the meta-analysis, which makes the stratified analysis not so reliable. The results should be interpreted with caution. Four, due to the lack of the original data of the included studies, it is limited that we further evaluate the potential interactions among gene-environment. Finally, our study only included these papers published in English and Chinese. There may be some language bias.
Overall, a systematic review of the association of TGF-β1gene-509C/T polymorphism with IgA nephropathy risk is more powerful than any single study. This meta-analysis does not support the hypothesis that TGF-β1gene-509C/T polymorphism is a risk factor for the development of IgA nephropathy. However, this does not exclude the fact that this or other polymorphisms of TGF-β1 gene may be associated with the progression or other features of IgA nephropathy and that this study investigated only its impact on the susceptibility of IgA nephropathy. It is necessary to carry out further researches with a larger number of worldwide researches in standardized and unbiased ways to better estimate the association of other variables with the susceptibility of IgA nephropathy.
Declaration of interest
All authors wish to declare that they have no conflict of interests in this study or its publication, financial or otherwise.
This work was supported by Hunan Province Science and Technology Project (2014SK2013), fundamental research funds for the central universities of Central South University (2014zzts069).
References
- Strippoli GF, Maione A, Schena FP, Tognoni G, Craig JC. IgA nephropathy: A disease in search of a large-scale clinical trial to reliably inform practice. Am J Kidney Dis. 2009;53:5–8
- Barratt J, Eitner F, Feehally J, Floege J. Immune complex formation in IgA nephropathy: A case of the ‘right' antibodies in the ‘wrong' place at the ‘wrong' time? Nephrol Dial Transplant. 2009;24:3620–3623
- Glassock RJ. IgA nephropathy: Challenges and opportunities. Cleve Clin J Med. 2008;75:569–576
- Amoroso L, De Sanctis L, Cappelli P, et al. Management of IgA nephropathy. G Ital Nefrol. 2011;28:622–632
- Wyatt R, Julian B. IgA nephropathy. N Engl J Med. 2013;368:2402–2414
- Kalliakmani P, Nakopoulou L, Tsakas S, et al. Urinary interleukin-6 (IL-6) and transforming growth factor (TGF-beta) levels in corticosteroid treated patients with IgA nephropathy. Clin Nephrol. 2011;76:144–150
- Lepenies J, Eardley KS, Kienitz T, et al. Renal TLR4 mRNA expression correlates with inflammatory marker MCP-1 and profibrotic molecule TGF-beta(1) in patients with chronic kidney disease. Nephron Clin Pract. 2011;119:97–104
- Ito Y, Goldschmeding R, Kasuga H, et al. Expression patterns of connective tissue growth factor and of TGF-beta isoforms during glomerular injury recapitulate glomerulogenesis. Am J Physiol Renal Physiol. 2009;299:545–558
- Brabcova I, Tesar V, Honsova E, et al. Association of advanced vasculopathy and transforming growth factor-beta1 gene expression with immunoglobulin A nephropathy progression. Nephrol Dial Transplant. 2011;26:573–579
- Wang C, Liu X, Peng H, et al. Mesangial cells stimulated by immunoglobin A1 from IgA nephropathy upregulates transforming growth factor-beta1 synthesis in podocytes via renin-angiotensin system activation. Arch Med Res. 2010;41:255–260
- Ye XF, Yorioka N, Oda H, Taniguchi Y, Yamakido M. Role of intercellular adhesion molecule-1, lymphocyte function-associated antigen-1, and macrophages in ddY mouse nephropathy. Hiroshima J Med Sci. 1997;46:75–80
- Border WA, Okuda S, Nakamura T, Languino LR, Ruoslahti E. Role of TGF-beta 1 in experimental glomerulonephritis. Ciba Found Symp. 1991;157:178–193
- Braga M, Bhasin S, Jasuja R, Pervin S, Singh, R. Testosterone inhibits transforming growth factor-beta signaling during myogenic differentiation and proliferation of mouse satellite cells: Potential role of follistatin in mediating testosterone action. Mol Cell Endocrinol. 2002;350:39–52
- Mi XH, Luan S, Ma XY, et al. Effects of Artesunate on MCP-1 and MCP-1 mRNA expression of renal tissue in the rat IgA nephropathy model. Sichuan Da Xue Xue Bao Yi Xue Ban. 2009;40:821–825
- Buckova D, Izakovicova HL, Benes P, Znojil V, Vacha J. TGF-beta1 gene polymorphisms. Allergy. 2001;56:1236–1237
- Valladares-Salgado A, Angeles-Martinez J, Rosas M, et al. Association of polymorphisms within the transforming growth factor-beta1 gene with diabetic nephropathy and serum cholesterol and triglyceride concentrations. Nephrology (Carlton). 2010;15:644–648
- Lim CS, Kim YS, Chae DW, et al. Association of C-509T and T869C polymorphisms of transforming growth factor-beta1 gene with susceptibility to and progression of IgA nephropathy. Clin Nephrol. 2005;63:61–67
- Li Y, Liu FY, Peng YM, et al. the relationship between the TGF-beta1 gene −509C/T polymorphism and tubulointerstitial damage resulting from primary nephrotic syndrome. Ren Fail. 2010;32:420–427
- Chootrakool H, Shi JQ, Yue R. Meta-analysis and sensitivity analysis for multi-arm trials with selection bias. Stat Med. 2001;30:1183–1198
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558
- DerSimonian R. Meta-analysis in the design and monitoring of clinical trials. Stat Med. 1996;15:1237–1252
- Shi-liang Li, Lin-Xu, Jie Wang. Relationship of transforming growth factor β1 gene-509C/T polymorphism with IgA nephropathy in Western Guangxi. China J Modern Med. 2011;21:1607–1610
- Qin Wei, Zhang Ying-Juan, Tan Chun-Yu. Association of TGF-β1 gene polymorphism with IgA nephropathy. West China Med J. 2008;23:65–69
- Xue Chao, Li You-Ji, Li Cai-Xia. Relationship of TGFβ1-509C/T polymorphism with IgA nephropathy in Han Nationality of Chinese population. J Sun Yat-Sen Univ (Medical Sciences). 2005;26:261–263
- Lim CS, Kim YS, Chae DW, et al. Association of C-509T and T869C polymorphisms of transforming growth factor-beta1 gene with susceptibility to and progression of IgA nephropathy. Clin Nephrol. 2005;63:61–67
- Vuong MT, Lundberg S, Gunnarsson I, et al. Genetic variation in the transforming growth factor-beta1 gene is associated with susceptibility to IgA nephropathy. Nephrol Dial Transplant. 2009;24:3061–3067
- Sato F, Narita I, Goto S, et al. Transforming growth factor-beta1 gene polymorphism modifies the histological and clinical manifestations in Japanese patients with IgA nephropathy. Tissue Antigens. 2004;64:35–42
- Brezzi B, Del PD, Lupo A, et al. Primary IgA nephropathy is more severe in TGF-beta1 high secretor patients. J Nephrol. 2009;22:747–759
- Kim SK, Lee JY, Jeong P, et al. Association between lymphotoxin Beta receptor gene polymorphisms and IgA nephropathy in Korean children. Immunol Invest. 2012;41:447–457
- Woo KT, Lau YK, Chan CM, et al. ACE gene sequence and nucleotide variants in IgA nephropathy. Singapore Med J. 2011;52:824–834
- Bantis C, Heering PJ, Siekierka-Harreis M, et al. Impact of aldosterone synthase gene C-344T polymorphism on IgA nephropathy. Ren Fail. 2011;33:393–397
- Lee JS, Suh KT, Kim JI, Lim JM, Goh TS. Association of estrogen receptor gene polymorphism in patients with degenerative lumbar spondylolisthesise. J Korean Neurosurg Soc. 2011;50:415–419
- Woo KT, Lau YK, Zhao Y, et al. Urotensin 2 and retinoic acid receptor alpha (RARA) gene expression in IgA nephropathy. Ann Acad Med Singapore. 2010;39:705–709