Abstract
Background: Ischemic postconditioning (IPoC) is defined as a series of intermittent interruptions of blood flow in the early phase of reperfusion that mechanically alters the hydrodynamics of reperfusion and it attenuates renal damage after ischemia/reperfusion (I/R) injury in vivo. But all of these data had been obtained in adult populations and whether this protection was maintained in aging kidneys was unknown. The objective of this study was to establish whether the efficacy of IPoC is maintained in aging kidneys. Materials and methods: The aged (24-month-old) and young (3-month-old) Wistar rats were used. Rats were subjected to 45 min of renal ischemia, both with and without treatment with IPoC. Serum urea nitrogen and creatinine levels, histological examination and apoptosis were assessed at 24 h. Oxidative stress was evaluated and apoptosis-related molecules were studied by Western blotting. Results: In young rat kidneys, IPoC significantly attenuated the renal dysfunction and cell apoptosis induced by I/R. In contrast, IPoC failed to limit renal dysfunction, possibly a consequence of increased apoptosis in aged rat kidneys. Conclusions: Our data indicated that IPoC was ineffective in aged rat kidneys. These findings may have major implications in that severe apoptosis in aged kidneys might be refractory to anti-apoptotic effect by IPoC.
Introduction
Renal ischemia, whether caused by shock or during surgery or transplantation, is a major cause of acute renal failure (ARF). Although reperfusion is essential for the survival of ischemic tissue, there is good evidence that reperfusion itself causes additional injury.Citation1 Ischemic postconditioning (IPoC) is defined as a series of intermittent interruptions of blood flow in the early phase of reperfusion that mechanically alters the hydrodynamics of reperfusion and it attenuated renal damage after I/R injury in vivo. IPoC has been studied comprehensively in animal models of renal ischemia/reperfusion (I/R) injury and studies indicated that IPoC attenuated renal damage after I/R injury in vivo.Citation2–12
However, a recent disappointed clinical report demonstrated that IPoC has no benefit in terms of reduced delayed graft function (DGF) or better renal function after kidney transplantation, although IPoC is feasible and appears safe.Citation13 One explanation is that healthy young animals were used in most experimentation on animals. In this clinical report donors were older and obviously had some kind of comorbidity.Citation13 It remained to be determined whether the lost protective effects of IPoC were associated with aging in vivo. The major purpose of this study was to determine whether kidneys from older rat were refractory to protection induced by IPoC.
Materials and methods
Animal preparation
Young adult (3-month-old) and aged (24-month-old) male Wistar rats were from the Center of Experimental Animal in Medical College, Wuhan University. This project was approved by the committee of experimental animals of Wuhan University (NO. 42000500002258), and the procedures were carried out according to the routine animal-care guidelines. All experimental procedures were complied with the Guide for the Care and Use of Laboratory Animals. Animal preparation was performed as previously described.Citation2,Citation3 Briefly, rats were anesthetized with pentobarbital (45 mg/kg) and placed on a homeothermic table to maintain core body temperature at 37 °C. The midline laparotomy was made and the left kidney was subjected to 45 min of ischemia followed by reperfusion after right nephrectomy.
Experimental protocols
Based on the randomized block design, young and aged male Wistar rats were divided into three groups: (1) sham-operated control group (sham), (2) ischemia/reperfusion group (I/R): kidneys were subjected to 45 min of ischemia followed by reperfusion, and (3) ischemic postconditioning group (IPoC): kidneys were subjected to 6 cycles of 10 s of reperfusion followed by 10 s ischemia immediately after 45 min of ischemia.Citation2–4,Citation7,Citation11,Citation12 Rats were killed at 24 h after I/R injury. Sham operations were performed in a similar manner, except that the renal vessels were clamped. Each group comprised 12 rats.
Preservation of kidneys
The left kidney was removed under fully maintained anesthesia. After removal, the kidney was fixed in 10% phosphate-buffered formalin or immediately frozen, and stored at −80 °C for different determinations.
Serum assays
To assess Cr and BUN, blood samples were collected, centrifuged and kept at −20 °C until analyses, adopting standard techniques using an Olympus AU 2700 Analyzer (Olympus Optical Co. Ltd, Tokyo, Japan).
Histological examination
The kidney was fixed in 10% neutral-buffered formalin, paraffin embedded and sectioned at 4 um thick according to the standard procedure. The sections were deparaffinized and hydrated gradually, and examined by HE staining. Morphological assessment was performed by an experienced renal pathologist who was unaware of the treatment. A grading scale of 0–4, as outlined by Jablonski,Citation14 was used for the histopathological assessment of ischemia/reperfusion-induced damage of the proximal tubules.
Measurement of MDA and SOD
The renal tissue malondialdehyde (MDA) and superoxide dismutase (SOD) were measured using commercialized chemical assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Results were determined according to the manufacturer’s guide. All protein concentrations of renal tissue homogenate samples (n = 6 for each group) were determined with Coomassie blue method (assay kit was purchased from Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Caspase-3 activity assay
Activities of caspase-3 were measured using commercialized caspase-3 activity kit (Beyotime Institute of Biotechnology, Shanghai, China). In brief, renal cortices (n = 6 for each group) were homogenized in lysis buffer. The lysate was centrifuged at 20,000 g for 10 min at 4 °C, and supernatants were incubated for 1 h at 37 °C with 10 µL caspase-3 substrate (Ac-DEVDpNA) (2 mM). Substrate cleavage was measured with a spectrofluorometer at 405 nm.
Western blot analysis
The cytosolic protein samples for Western blot analysis were prepared as described with some modifications.Citation3,Citation15 Whole-cell lysates were obtained by homogenizing the renal sample (n = 6 for each group) with a homogenizer in five volumes of buffer (20 mM Hepes, 1.5 mM MgCl2, 10 mM KCl, 1 mM EDTA, 1 mM EGTA, 250 mM sucrose, 0.1 mM PMSF, 1 mM dithiothreitol [DTT] and proteinase inhibitor cocktail tablets; pH 7.9). Samples were further centrifuged at 750 g at 4 °C for 15 min to separate the sample into supernatant A and pellet A. Supernatant A, containing the cytosolic/mitochondrial protein, was further centrifuged at 16,000 g for 30 min at 4 °C to separate supernatant B from pellet B. Supernatant B was used as the cytosolic fraction. The primary antibodies and concentrations were as follows: Bcl-2, cytochrome c, and Bax (Santa Cruz Inc., Santa Cruz, CA; 1:200), caspase-3 and caspase-9 (Santa Cruz Inc., Santa Cruz, CA; 1:500), β-actin (Santa Cruz Inc., Santa Cruz, CA; 1:500). After extensive rinsing with TBST buffer, the membranes were incubated with secondary antibodies (Santa Cruz Inc., Santa Cruz, CA; 1:2000) for 1 h at room temperature and then developed with the use of an enhanced chemiluminescence system (ECL kit, Pierce Biotechnology Inc., Rockford, IL).
Statistical analyses
All data are expressed as means ± SEM. The Kolmogorov–Smirnov test was applied to test for a normal distribution. The means of the different groups were compared using one-way ANOVA Student–Newman–Keuls test. All statistical analyses were performed with the SPSS statistical package (SPSS 13.0 for Windows; SPSS, Inc., Chicago, IL). Significant differences were accepted when p values were less than 0.05.
Results
Renal function
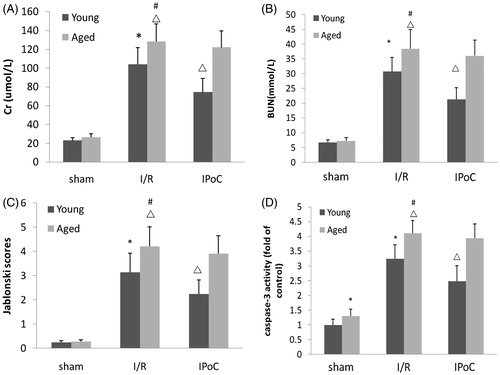
The renal functional parameter in sham-operated rats was similar between young and aged rats. Rats subjected to I/R injury showed significant increases in BUN and Cr compared with sham-operated rats. Moreover, the BUN and Cr were higher in aged rats than in young rats. The renal function changes induced by I/R were significantly improved by IPoC treatment in young IPoC group compared with young I/R group and it had no difference in aged rats ().
Figure 1. Effect of IPoC on the renal functional. (A) The effects of IPoC on the serum Cr concentrations after 45 min ischemia. (B) Effect of IPoC on the serum BUN concentrations after 45 min ischemia. (C) Jablonski scores for histological appearance of acute tubular necrosis from sham, I/R and IPoC groups. (D) Caspase-3 activity. Notes: Bars represent means ± SE (n = 6). *p < 0.05 versus young sham-operated group, #p < 0.05 versus aged sham-operated group, △p < 0.05 versus young I/R group.
Renal histology
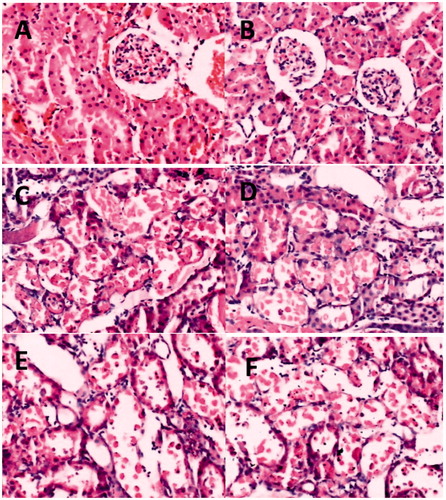
Renal tissue morphology in sham-operated rats was similar between young and aged rats. Forty-five minutes of renal ischemia followed by 24 h reperfusion resulted in significant renal injury as evidenced by tubular necrosis, medullary hemorrhage, congestion and development of proteinaceous casts (). According to Jablonski scores, quantitative analysis showed a dramatically increased score in the aged and the young I/R group. Meanwhile these damages were more severe in aged rats than in young rats. In contrast, the score was significantly reduction in the young IPoC group compared with the young I/R group and there was no significant improvement in score in the aged IPoC group compared with the aged I/R group ().
Figure 2. Histological evaluations of renal tissues. (A–F): Representative kidney sections obtained 24 h after sham surgery or I/R. (A) Section from young sham-operated rats. (B) Section from aged sham-operated rats. (C) Section from young rats subjected to I/R. (D) Section from aged rats subjected to I/R. (E) Section from young rats subjected to I/R and IPoC. (F) Section from aged rats subjected to I/R and IPoC. Note: All Hematoxylin and Eosin ×400.
Oxidative stress
The MDA level which is an index of lipid peroxidation was higher in aged sham-operated group compared with young sham-operated group. Renal I/R injury significantly increased MDA level in both aged and young rats. Moreover, the MDA level was higher in aged I/R group than in young I/R group. There was significant reduction in MDA level in young IPoC group compared with young I/R group. But there were no significant differences in MDA level in aged IPoC group compared with aged I/R group ().
Table 1. Effect of IPoC on the renal content of MDA and SOD at 24 h after reperfusion.
Aged rats showed decreased SOD activities compared with young rats. Renal I/R injury significantly decreased SOD activities in both aged and young rats. Meanwhile SOD activity was lower in aged I/R group than in young I/R group. SOD activity increased strongly in young IPoC group compared with young I/R group and there was no significant improvement in the aged IPoC group compared with the aged I/R group ().
Apoptosis and apoptosis-related molecules
Apoptosis was evaluated by caspase-3 proteolytic activity. The aged sham-operated rats had increased caspase-3 proteolytic activity compared with young sham-operated rats. Renal I/R injury significantly up-regulated the caspase-3 proteolytic activity in kidneys of both aged and young rats. Moreover, the caspase-3 activity was higher in aged rats than in young rats. However, the caspase-3 activity was decreased strongly in young IPoC group compared with young I/R group and there were no significant differences in aged IPoC group compared with aged I/R group ().
Western blot analysis showed that aged sham-operated rats had increased active caspase-3 expression compared with young sham-operated rats. But the active caspase-9 expression had no significant differences in aged sham-operated rats compared young sham-operated rats. Renal I/R injury significantly up-regulated the levels of active caspase-3 and caspase-9 expression in kidneys of both aged and young rats. The levels of active caspase-3 and caspase-9 expression were higher in aged I/R group than in young I/R group. IPoC treatment inhibited the expression of active caspase-3 and caspase-9 in young rats, but it had no effects in aged rats ().
Figure 3. Apoptosis-related molecules expressions at 24 h after reperfusion. (A) Representative blots showing the effect of IPoC treatment on the active caspase-3, active caspase-9, cytochrome c, Bax and Bcl-2 expressions in the cytoplasm. (B) The relative band densities of active caspase-3 to the mean value of the control. (C) The relative band densities of active caspase-9 to the mean value of the control. (D) The relative band densities of cytochrome c to the mean value of the control. (E) The relative band densities of Bax to the mean value of the control. (F) The relative band densities of Bcl-2 to the mean value of the control. Notes: Bars represent means ± SE (n = 6) *p < 0.05 versus young sham-operated group, #p < 0.05 versus aged sham-operated group, △p < 0.05 versus young I/R group.
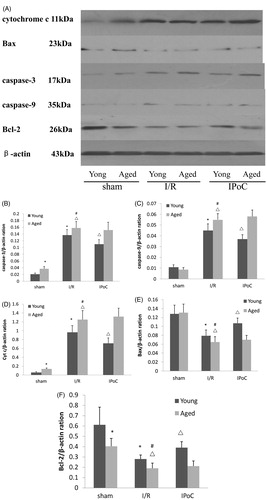
Expression of cytochrome c in the cytosol was significantly increased in aged sham-operated group compared with young sham-operated group. Renal I/R injury up-regulated cytosolic cytochrome c protein expression in kidneys of both aged and young rats. Moreover the up-regulation was more obvious in aged rats than in young rats. This up-regulation of cytochrome c was significantly attenuated in young IPoC group compared with young I/R group and there was no significant improvement in aged IPoC group compared with aged I/R group. On the contrary, the levels of Bax in the cytosol had no significant differences in aged sham-operated group compared with young sham-operated group. Renal I/R injury down-regulated cytosolic Bax protein expression in kidneys of both aged and young rats. The aged rats displayed lower Bax level in the cytosol than did the young rats. IPoC treatment restored cytosolic Bax level in young IPoC group compared with young I/R group. But there was no significant improvement in the aged IPoC group compared with the aged I/R group. In addition, aged rats showed decreased Bcl-2 levels compared with young rats. Renal I/R injury significantly reduced cytosolic Bcl-2 levels in kidneys of both aged and young rats and the reductions were higher in aged I/R group than in young I/R group. IPoC treatment restored the levels of Bcl-2 in young rats, but it had no effects in aged rats ().
Discussion
Although IPoC is powerful against I/R injury intervention and appears safe, its feasibility needs to be discussed. Our study showed that IPoC proved to be an effective defense against I/R in young groups, but it was ineffective in aged groups. Moreover, we demonstrated for the first time that the protective effects of IPoC treatment was lost in aged rats after renal I/R injury.
The early moments of reperfusion are important in the pathogenesis of postischemic injury and that manipulation of this early reperfusion phase reduced I/R injury. IPoC provides a new tool to protect organ from I/R injury, for example, heart,Citation16,Citation17 brainsCitation18 and kidney.Citation2–12 However, healthy young animals were used in most of the experimentations on animals. A few reports had also shown that aging mouse hearts were refractory to cardioprotection by IPoC.Citation19,Citation20 A recent clinical report demonstrated that IPoC has no benefit in terms of reduced DGF or better renal function after kidney transplantation.Citation13 In this clinical report, donors were older and obviously had some kind of comorbidity. This was the first report of IPoC in human kidney transplantation. Whether such a protective phenomenon of IPoC is invalided in the aged I/R models needs to be elucidated. In our study, renal I/R injury induced more severe renal dysfunction, morphology changes and oxidative stress in aged rats. Meanwhile, the protective effects of IPoC are lost in aged rats, which were proved by renal function, HE, MDA levels and SOD activity.
The kidneys undergo involutional changes with age. Elderly patients have an increased susceptibility to ischemic ARF. Apoptosis plays a role in this process. Recent studies demonstrated that there were more severe reperfusion-induced injuries and apoptotic tubular cells in the kidneys of aged rats after I/R injury.Citation21–23 Although the previous study showed that IPoC attenuated apoptosis after I/R injury,Citation3,Citation8 we speculated that the more severe apoptosis may contribute to the lost protective effects of IPoC. Our results are compatible with these studies, which showed that renal I/R injury induced more severe apoptosis in aged rats which was proved by caspase-3 activity. Despite IPoC had an anti-apoptotic effect in young rats, aged rat kidneys were refractory to anti-apoptotic effect by IPoC.
Renal apoptosis is an important factor in the development of ARF after I/R injury.Citation24 In response to oxidase load in the mitochondria, the outer membrane of mitochondria becomes permeabilized, resulting in the anslocation of Bax from cytosol to the mitochondri and the release of cytochrome c normally confined to the mitochondrial intermembrane space. The pro-apoptotic proteins translocation was controlled by the Bcl-2 family proteins.Citation25 Release of cytochrome c into the cytosol leads to the formation of the apoptosome, a complex comprised of apoptotic protease-activating factor-1 (Apaf-1), procaspase-9 and ATP. The apoptosome permits the autoactivation of procaspase-9, which is followed by the activation of procaspase-3.Citation26 Active caspase-3 activates the caspase activated DNase, leading to DNA fragmentation. In previous studies, the mitochondrial pathway was an important target for IPoC.Citation3,Citation7,Citation8 In order to further clarify the reason of lost, we investigated the expressions of key apoptotic-related molecules. This study supported previous findings and further supported our opinion. Our study showed that IPoC increased the levels of anti-apoptotic Bcl-2 protein and inhibited Bax translocation to the mitochondria and cytochrome c release, thus attenuating the downstream caspase activation in young rats. However, these effects were weak in aged rats. Therefore, we speculated that in aged rats, the severe apoptosis might be refractory to anti-apoptotic effect by IPoC.
In our study, we tested only aged rats. Whether IPoC is invalid in the some kind of comorbidity rats needs additional investigation, for example, hypertension, hyperlipemia, and diabetes. On the other hand, it is unclear whether IPoC is invalid in large aged animals. Because large animals such as dogs and pigs are often regarded as better human surgical models compared with rodents. Second, only a 24-h period of survival was assessed. Our former study documented that IPoC protected rats against I/R damage after 12 weeks and has beneficial effects on renal fibrosis.Citation4 It is possible that IPoC treatment might only leads to long-term protection in aged rats. Thus, the long-term consequences of IPoC may need to be investigated in further study. Third, despite we observed that IPoC could not decrease apoptosis in aged rat kidneys, it is not known whether this effect was the result or the cause of failure. Thus, further research is needed. Furthermore, we observed an inherent interconnection between the effects of IPoC treatment on tissue salvage and the protein signals in vivo study. To confirm these results in vitro studies are needed.
In conclusion, our study demonstrated that kidneys from older rat were refractory to protection induced by IPoC. The lost protective effects of IPoC were associated with severe apoptosis in aged rats.
Declaration of interest
This study was supported in part by grants from the National Natural Science Foundation of China (nos. 30901494 and 30901552).
References
- Wang HJ, Varner A, AbouShwareb T, et al. Ischemia/reperfusion-induced renal failure in rats as a model for evaluating cell therapies. Ren Fail. 2012;34:1324–1332
- Liu X, Chen H, Zhan B, et al. Attenuation of reperfusion injury by renal ischemic postconditioning: The role of NO. Biochem Biophys Res Commun. 2007;359:628–634
- Chen H, Xing B, Liu X, et al. Ischemic postconditioning inhibits apoptosis after renal ischemia/reperfusion injury in rat. Transpl Int. 2008;21:364–371
- Weng X, Shen H, Kuang Y, et al. Ischemic postconditioning inhibits the renal fibrosis induced by ischemia-reperfusion injury in rats. Urology. 2012;80:484e1–484e7
- Jiang B, Liu X, Chen H, et al. Ischemic postconditioning attenuates renal ischemic/reperfusion injury in mongrel dogs. Urology. 2010;76:1519e1–1519e7
- Jiang B, Chen Q, Liu X, et al. Ischemic postconditioning protects renal function after 24 hours of cold preservation in a canine autotransplantation model. Transplant Proc. 2012;44:1776–1781
- Wang W, Tang T, Zhang P, et al. Postconditioning attenuates renal ischemia-reperfusion injury by preventing DAF down-regulation. J Urol. 2010;183:2424–2431
- Zhang WL, Zhao YL, Liu XM, et al. Protective role of mitochondrial K-ATP channel and mitochondrial membrane transport pore in rat kidney ischemic postconditioning. Chin Med J (Engl). 2011;124:2191–2195
- Serviddio G, Romano AD, Gesualdo L, et al. Postconditioning is an effective strategy to reduce renal ischemia/reperfusion injury. Nephrol Dial Transplant. 2008;23:1504–1512
- Eldaif SM, Deneve JA, Wang NP, et al. Attenuation of renal ischemia-reperfusion injury by postconditioning involves adenosine receptor and protein kinase C activation. Transpl Int. 2010;23:217–226
- Yun Y, Duan WG, Chen P, et al. Ischemic postconditioning modified renal oxidative stress and lipid peroxidation caused by ischemic reperfusion injury in rats. Transplant Proc. 2009;41:3597–3602
- Yun Y, Duan WG, Chen P, et al. Down-regulation of cyclooxygenase-2 is involved in ischemic postconditioning protection against renal ischemia reperfusion injury in rats. Transplant Proc. 2009;41:3585–3589
- Van den Akker EK, Hesselink DA, Manintveld OC, et al. Ischemic postconditioning in human DCD kidney transplantation is feasible and appears safe. Transpl Int. 2014;27:226–234
- Jablonski P, Howden BO, Rae DA, et al. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983;35:198–204
- Matsumori Y, Hong SM, Aoyama K, et al. Hsp70 overexpression sequesters AIF and reduces neonatal hypoxic/ischemic brain injury. J Cereb Blood Flow Metab. 2005;25:899–910
- Staat P, Rioufol G, Piot C, et al. Postconditioning the human heart. Circulation. 2005;112:2143–2138
- Hausenloy DJ, Yellon DM. The therapeutic potential of ischemic conditioning: An update. Nat Rev Cardiol. 2011;8:619–629
- Xing B, Chen H, Zhang M, et al. Ischemic postconditioning inhibits apoptosis after focal cerebral ischemia/reperfusion injury in the rat. Stroke. 2008;39:2362–2369
- Boengler K, Buechert A, Heinen Y, et al. Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res. 2008;102:131–135
- Przyklenk K, Maynard M, Darling CE, et al. Aging mouse hearts are refractory to infarct size reduction with post-conditioning. J Am Coll Cardiol. 2008;51:1393–1398
- Kusaka J, Koga H, Hagiwara S, et al. Age-dependent responses to renal ischemia-reperfusion injury. J Surg Res. 2012;172:153–158
- Qiao X, Chen X, Wu D, et al. Mitochondrial pathway is responsible for aging-related increase of tubular cell apoptosis in renal ischemia/reperfusion injury. J Gerontol A Biol Sci Med Sci. 2005;60:830–839
- Xu X, Fan M, He X, et al. Aging aggravates long-term renal ischemia-reperfusion injury in a rat mode. J Surg Res. 2014;187:289–296
- Padanilam BJ. Cell death induced by acute renal injury: A perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol. 2003;284:608–627
- Shimizu S, Narita M, Tsujimoto Y, et al. Bcl-2 family pro-teins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487
- Li P, Nijhawan D, Budihardio I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489

