Abstract
Objective: Neutrophil gelatinase-associated lipocalin (NGAL) is produced in response to tubular injury. Contrast-induced acute kidney injury (CI-AKI) is associated with adverse outcomes in chronic kidney disease (CKD) patients. We sought to characterize blood NGAL level and the degree of kidney injury in CKD patients who underwent coronary angiography. Methods: This study was a prospective, blinded assessment of blood samples obtained from patients with estimated glomerular filtration rates (eGFRs) between 15 and 90 mL/min/1.73 m2 undergoing elective coronary angiography with iodinated contrast. Blood NGAL and serum creatinine were measured at baseline, 1, 2, 4, 6, 12, 24 and 48 h after contrast administration. Results: A total of 63 subjects with a mean eGFR of 48.17 ± 16.45 mL/min/1.73 m2 were enrolled. There was a graded increase in baseline NGAL levels across worsening stages of CKD (p = 0.0001). Post-procedure NGAL increased from baseline in each stage of CKD. Eight (12.7%) patients were diagnosed with CI-AKI by diagnostic criteria of 2012 KDIGO definition of CI-AKI, and seven (11.1%) patients developed subclinical CI-AKI defined by a twofold or greater rise in NGAL. There was no relationship between baseline eGFR and diabetes on the composite outcome of subclinical and clinical CI-AKI. Conclusions: Baseline and post-procedure NGAL are progressively elevated according to the baseline stage of CKD. Using a twofold rise in NGAL, 46.7% of composite CI-AKI is detected and complements the 53.3% of cases identified using KDIGO criteria. Traditional risk predictors were not independently associated with this composite outcome.
Introduction
Contrast-induced acute kidney injury (CI-AKI) is a common iatrogenic cause of hospital acquired acute kidney injury (AKI). Its incidence has been variedly reported from 2% to 25% according to the definition used in previous cohort studies.Citation1,Citation2 The occurrence of CI-AKI is associated with poor cardiovascular outcomesCitation3–7 and adverse renal events.Citation5,Citation6 Chronic kidney disease (CKD) has been recognized as a strong risk factor for both CI-AKI and cardiovascular events.Citation6 In addition, CKD patients who develop CI-AKI have higher rates of the subsequent rapid progression of CKD.Citation3,Citation8 Detecting acute-on-chronic kidney injury is therefore crucial to develop strategies to decrease morbidity and mortality from both cardiovascular and renal standpoints.
There are numerous proposed protocols and approaches for CI-AKI prophylaxis and treatment; however, there are no large-scale trials demonstrating an effective strategy.Citation9–12 While development of less toxic contrast agentsCitation13 is underway, early detection using novel biomarkers is a favorable platform for future trials of prophylaxis and treatment. Previous studies have shown that 41.1% of patients with AKI would be undiagnosed by measurement of serum creatinine (Cr) alone.Citation13–15 Thus, commercially available novel biomarkers help better inform clinicians of subclinical renal injury. In the past decade, various kidney injury biomarkers including neutrophil gelatinase-associated lipocalin (NGAL),Citation16 L-type fatty acid-binding protein (L-FABP)Citation17 and kidney injury molecule-1 (KIM-1),Citation18 have shown promising effects. Among these, NGAL is the protein encoded by lipocalin-2 gene and expressed at high levels in distal renal tubular cells and to a lesser extent in neutrophils, prostate cells and pneumocytes. Previous studies have shown that NGAL is secreted at a rapid rate into blood and urine within 2 h of AKICitation14,Citation19 and therefore has utility as a prognostic, diagnostic and severity marker in a variety of clinical settings.Citation20 It is important to understand that the NGAL gene is upregulated constitutively in the setting of CKD,Citation21 resulting in progressively higher chronic concentrations. Consequently, the diagnostic role of NGAL in acute-on-chronic kidney injury is complicated and is an issue worthy of additional studies. Currently, there is neither consensus on expected levels of blood NGAL in CKD patients nor are there diagnostic thresholds for elevation in the diagnosis of CI-AKI.
We recently demonstrated the value of baseline NGAL as an independent predictor of NGAL value at 48 h after the procedure irrespective of CKD stage and baseline eGFR.Citation21 In this analysis, we aimed to characterize both subclinical CI-AKI detected by NGAL and clinical CI-AKI heralded by Cr in patients who underwent non-urgent coronary angiography with or without percutaneous coronary intervention using intra-arterial iodinated contrast.
Methods
The ENCINO (neutrophil gelatinase-associated lipocalin (NGAL): a novel blood marker for determining the risk of developing contrast-induced nephropathy) studyCitation21 was a prospective, blinded, cohort study performed at three centers in Michigan (clinicaltrials.gov: NCT 00693329). Methods of the ENCINO study have been previously published by McCullough and coworkers.Citation21 The objective of analyzing the first 25% of 260 cases (from sample size calculation) was to understand the characteristics of the blood NGAL assay and determine the feasibility of calculating decision statistics for the outcome of CI-AKI. As the outcome by conventional definition was sufficiently rare, the study was stopped after the first analysis. Briefly, subjects over age 18 who were undergoing non-urgent coronary angiography with the intent of ad hoc PCI were screened and were considered eligible if the estimated glomerular filtration rate (eGFR) calculated by the four-variable Modification of Diet in Renal Disease equation was less than 90 mL/min/1.73 m2. The rationale for the eGFR exclusion criteria was to capture subjects with a reasonable probability of experiencing an excursion of NGAL after contrast exposure. Subjects were excluded if they were renal transplant recipients, treated with maintenance hemodialysis or if they had received intravascular contrast within the preceding 30 days. Blood specimens were obtained at baseline, 1, 2, 4, 6, 12, 24 and 48 h after contrast administration. The baseline plasma creatinine (Cr) and all follow-up values were measured by a core laboratory using isotope dilution mass spectrometry traceable methods which may not have been the same methods used for screening at the local sites. NGAL was measured in EDTA-anticoagulated plasma using the extended range Alere™ Triage® (San Diego, CA) NGAL immunoassay. This assay has a measurable range of 15–1300 ng/mL with a coefficient of variation of 13.9% using the replicate values from 51 samples with a mean concentration of 400.0 ± 100.0 ng/mL. NGAL and Cr results were not revealed to investigators or participants during the study. Clinical CI-AKI was defined by criteria of 2012 the Kidney Disease Improving Global Outcomes (KDIGO)’s guidelines on AKICitation22,Citation23: (1) increase in serum Cr by ≥0.3 mg/dL (≥26.5 μmol/L) within 48 h, or (2) increase in serum Cr to ≥1.5 times baseline, which is known or presumed to have occurred within the prior 7 days, or (3) urine volume <0.5 mL/kg/h for 6 h. Additionally, we defined “subclinical AKI” as increase in blood NGAL equal or more than 2 times of baseline (data referred from previous analysisCitation21) without serum Cr change that met criteria of AKI by 2012 KDIGO guideline. Univariate statistics were reported in counts and proportions and means ± standard deviations as appropriate. The Kolmogorov–Smirnov test was used to analyze normality of the baseline NGAL distribution before the use of parametric statistics. One-way analysis of variance was used to compare means of continuous variables stratified into stages of CKD according to the eGFR. Stepwise linear regression was used to identify independent predictors of the peak NGAL value. Binary logistic regression was used to evaluate eGFR and diabetes forced into the model as predictors of the composite outcome, which is subclinical plus clinical AKI. The Wilcoxon signed rank test was applied for comparison between baseline and peak of blood NGAL. The study was approved by IRB and conducted according to the ethical standards of 2000 Declaration of Helsinki. Informed consent was received from all participants in this study.
Results
There were 68 subjects recruited and consented from March 2008 to February 2009; however, five patients did not have baseline or follow-up blood testing because of scheduling and feasibility issues, and were excluded. A total of 63 subjects were evaluated with a mean age of 69.43 ± 9.32 years, 73% were male, 35% had diabetes, and a mean eGFR of 48.17 ± 16.45 mL/min/1.73 m2. Other baseline characteristics are shown in . The mean baseline NGAL was 191.32 ± 141.92 ng/mL with a range of 24.0–747.6 ng/mL; the Kolmogorov–Smirnov test suggested normality (p = 0.08). The baseline NGAL values were inversely related with baseline eGFR levels across CKD stages ( and ). We found 8/63 (12.7%) of subjects developed CI-AKI by criteria of 2012 KDIGO guidelines within 48 h of contrast administration. The mean baseline NGAL in these patients was 261.35 ± 186.48 ng/mL and the mean peak was 381.21 ± 253.27 ng/mL, p < 0.05. No patient required renal replacement therapy. Additional seven cases of subclinical CI-AKI were identified by our subclinical AKI criteria. Of note, we also found two CI-AKI patients met our increased NGAL criteria for subclinical AKI. In the patients who did not develop clinical acute-on-chronic kidney disease (n = 55), the mean baseline and peak of NGAL were 181.13 ± 133.36 ng/mL and 239.75 ± 140.71 ng/mL, respectively (, p > 0.05). Multivariate linear regression revealed that baseline NGAL (p = 0.025) and not eGFR (p = 0.993) was independently associated with the peak NGAL value; however, binary logistic regression which forced eGFR and diabetes into the model revealed no relationship between these established CI-AKI risk predictors and the composite outcome (). There were relatively few outlier data points, indicating a consistent measurement response to injury after exposure to contrast.
Figure 1. Pie chart of the clinical outcomes; no CI-AKI (n = 48), subclinical CI-AKI (n = 7), clinical KDIGO CI-AKI (n = 8), with two subjects meeting with subclinical and clinical criteria.
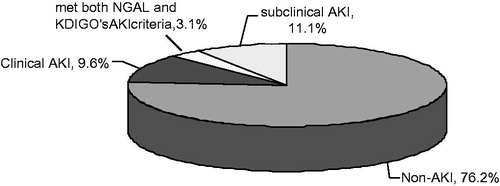
Figure 2. Baseline and peak value (mean ± standard deviation) of blood NGAL (ng/mL) according to the range of eGFR (mL/min/1.73 m2).
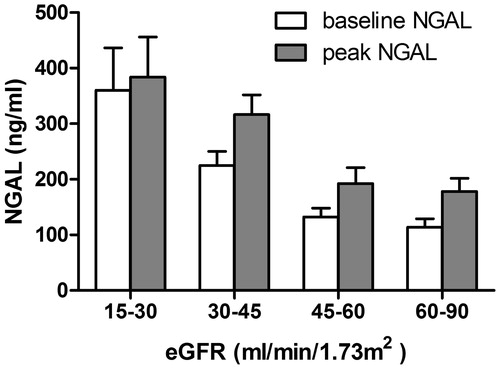
Figure 3. Baseline and peak value (mean ± standard deviation) of blood NGAL (ng/mL) in composite acute kidney injury group (combined KDIGO clinical and subclinical CI-AKI) and non-acute kidney injury group; *p < 0.05.
Table 1. Subject characteristics according to baseline eGFR (mL/min/1.73 m2). Data are expressed as mean ± standard deviation or count (proportion).
Table 2. Independent traditional predictors for the composite outcome of subclinical and clinical CI-AKI after contrast exposure by binary logistic regression.
Discussion
We found that the pre-procedure blood NGAL value increased in a graded manner across lower levels of eGFR and worsening stages of CKD. Also, it was baseline NGAL, and not eGFR that independently predicted the peak NGAL after contrast. There were only two cases who manifested both subclinical (by NGAL) and clinical CI-AKI. Interestingly, this may mislead that NGAL has limited utility in an early diagnosis of AKI because six out of eight AKI cases had no elevated NGAL at 48 h. In our study, this NGAL (−)/sCr (+) status represent all elderly patients who have diabetes. This finding is consistent with the previous report,Citation24 which can be explained by renal function loss without evidence of tubular injury or false negative NGAL. However, the mortality and renal replacement therapy rate remained significantly high in this group compared with NGAL (−)/sCr (−) status patients. Of note, NGAL (+)/sCr (−) status patients (seven patients in our study) have also showed significant increase of need for renal replacement therapy initiation, in-hospital mortality and length of ICU stay, compared with NGAL (−)/sCr (−) status patients in a large multicenter pooled analysis of prospective studies.Citation24 Due to our limitation of measurement at 48 h, this subclinical tubular injury plausibly preceded late-detected rising of serum creatinine which occurred after more than 50% glomerular functions have been lost. Also, creatinine may never rise significantly if glomerular filtration rate declines transiently. We showed that blood NGAL detected nearly an entirely new and equal sized group of subjects who experienced a significant renal injury from iodinated contrast. Thus, our findings suggest that NGAL can be a complementary marker to Cr in CI-AKI and can be a clinical addendum to our current methods of laboratory surveillance after contrast administration.Citation25,Citation26 In our previous analysis from ENCINO,Citation21 we suggested that the baseline NGAL is crucial for interpretation of NGAL change in subjects with eGFR < 90 mL/min/1.73 m2.Citation21 Thus, use of a doubling of NGAL allows a clinical utility of a variable level of baseline NGAL concentration and can be proposed as a reasonable conceptual starting point to understand subclinical CI-AKI.
The biologic response to toxic injury of kidney mediated by contrast is dependent on the relative nephron mass reflected by the eGFR.Citation21,Citation25,Citation27 In CKD, there is sustained up-regulation of NGAL production by viable distal renal tubular cells in an attempt to mitigate chronic oxidative stress catalyzed by intra- and extracellular poorly-liganded ironCitation21,Citation28; however, tubular NGAL expression alone may not entirely explain the distinction between baseline blood NGAL in various CKD stages. Rau et al. had showed that there was no difference in NGAL expression between anephric patients who underwent bilateral nephrectomy and dialysis patients who had intact kidneys; blood NGAL of 981 versus 838 ng/mL (p = 0.19), respectively,Citation25 creating a question on where the NGAL is being produced in the body. Furthermore, hemodialysis patients with residual renal function had significantly lower serum NGAL compared with those who were anuric.Citation29 When the literature is considered outside of nephrology, studies suggest that there are numerous sources of NGAL production,Citation21,Citation25 for example, cardiomyocytes, pneumocytes, prostate cells and leukocytes. Thus, it is possible that intravascular iodinated contrast is triggering the production and release of NGAL into the blood from sites outside and in addition to the kidney.Citation30–32
Previous trials have evaluated contrast volume and osmolality as well as antioxidants as preventive strategies for high risk patients.Citation33 While greater contrast volume and osmolality raise the risk for renal injury,Citation2 prophylactic N-acetylcysteine (NAC) has not been confirmed as the effective prevention.Citation1,Citation3,Citation34,Citation35 Nevertheless, none of these factors was associated with the peak of NGAL change in our study.
We identified eight patients who developed AKI according to 2012 KDIGO guidelines which call for an increase in serum Cr ≥ 0.3 mg/dLCitation36,Citation37 within 48 h of contrast exposure. The KDIGO definition allows for a longer detection window if a greater rise in serum Cr is detected or a sustained reduction in urine output is observed.Citation22,Citation23,Citation38–40 The emerging concept of subclinical AKI manifested by a rise in NGAL or other markers of structural kidney damage, without a sufficient rise in serum Cr to meet conventional definition, has confirmed to be associated with an increase in morbidity and mortality.Citation41–43 Our data suggest that blood NGAL could be used as a means to detect subclinical CI-AKI nearly doubling the case identification in CKD setting, and possibly expanding the possibilities for early prevention, treatment and monitoring.
Limitations
Our study has all the limitations of small prospective studies. We recruited only few women and African American subjects. Thus, our data of NGAL cannot be representative for these groups. Peak blood NGAL appeared to occur at 48 h post-catheterization, thus, additional measurements beyond this time frame would have been valuable. We recognize that NGAL is not specific to CI-AKI and has been described in a variety of AKI etiologies as well as other clinical contexts including sepsis and multiorgan system failure. The assay used in this study is unable to detect the difference between monomeric and dimeric NGAL and therefore cannot give exact concentrations of the single peptide. Lastly, we did not measure urine NGAL, or other novel markers of AKI such as kidney injury molecule-1, L-type fatty acid binding protein, interleukin-18, alpha/pi-glutathione S-transferase or cystatin-C, thus, we lacked an assessment of internal validity with respect to the degree of chronic and acute kidney disease assessed by these markers.Citation21
Conclusion
In summary, we characterized the baseline value of blood NGAL in various stages of CKD. In each stage, the post-procedure NGAL rose above the baseline level. Using NGAL in addition to KDIGO criteria identified larger numbers of patients considered to have an increased morbidity and mortality due to subclinical or clinical CI-AKI.Citation44–46 Future studies are needed using both novel markers for subclinical CI-AKI and serum Cr for conventional definitions of CI-AKI to evaluate the short- and long-term risk of clinical outcomes including acceleration of the progression of CKD, re-hospitalization, cardiac events and mortality.
Declaration of interest
The ENCINO Study was funded in part by Alere Inc, San Diego, CA. Alere also directly performed the plasma NGAL assay and directed the measurement of serum Cr at the central laboratory. David N. Stivers, PhD, is an employee and stockholder of Alere, Inc.
References
- Solomon R, Dauerman HL. Contrast-induced acute kidney injury. Circulation. 2010;122(23):2451–2455
- Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: Development and initial validation. J Am Coll Cardiol. 2004;44(7):1393–1399
- Tasanarong A, Vohakiat A, Hutayanon P, Piyayotai D. New strategy of alpha- and gamma-tocopherol to prevent contrast-induced acute kidney injury in chronic kidney disease patients undergoing elective coronary procedures. Nephrol Dial Transplant. 2013;28(2):337–344
- Guitterez NV, Diaz A, Timmis GC, et al. Determinants of serum creatinine trajectory in acute contrast nephropathy. J Interv Cardiol. 2002;15(5):349–354
- Solomon RJ, Mehran R, Natarajan MK, et al. Contrast-induced nephropathy and long-term adverse events: cause and effect? Clin J Am Soc Nephrol. 2009;4(7):1162–1169
- Brown JR, Malenka DJ, DeVries JT, et al. Transient and persistent renal dysfunction are predictors of survival after percutaneous coronary intervention: Insights from the Dartmouth Dynamic Registry. Catheter Cardiovasc Interv. 2008;72(3):347–354
- Gruberg L, Mintz GS, Mehran R, et al. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol. 2000;36(5):1542–1548
- Goldenberg I, Chonchol M, Guetta V. Reversible acute kidney injury following contrast exposure and the risk of long-term mortality. Am J Nephrol. 2009;29(2):136–144
- Mueller C, Buerkle G, Buettner HJ, et al. Prevention of contrast media-associated nephropathy: Randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002;162(3):329–336
- Aspelin P, Aubry P, Fransson SG, Strasser R, Willenbrock R, Berg KJ. Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med. 2003;348(6):491–499
- Stacul F, Adam A, Becker CR, et al. Strategies to reduce the risk of contrast-induced nephropathy. Am J Cardiol. 2006;98(6A):59K–77K
- Pannu N, Wiebe N, Tonelli M. Prophylaxis strategies for contrast-induced nephropathy. JAMA. 2006;295(23):2765–2779
- McCullough PA, Akrawinthawong K. Ascorbic acid for the prevention of contrast-induced acute kidney injury. J Am Coll Cardiol 2013;62(23):2176–2177
- Akrawinthawong K, Shaw MK, Kachner J, et al. Urine catalytic iron and neutrophil gelatinase-associated lipocalin as companion early markers of acute kidney injury after cardiac surgery: A prospective pilot study. Cardiorenal Med. 2013;3(1):7–16
- Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis. 2009;54(6):1012–1024
- Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231–1238
- Ivanisevic I, Peco-Antic A, Vulicevic I, et al. L-FABP can be an early marker of acute kidney injury in children. Pediatr Nephrol. 2013;28(6):963–969
- Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: A sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290(2):F517–F529
- Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: A prospective study. Clin J Am Soc Nephrol. 2008;3(3):665–673
- Alharazy SM, Kong N, Saidin R, et al. Serum neutrophil gelatinase-associated lipocalin and cystatin C are early biomarkers of contrast-induced nephropathy after coronary angiography in patients with chronic kidney disease. Angiology. 2014;65(5):436–442
- McCullough PA, Williams FJ, Stivers DN, et al. Neutrophil gelatinase-associated lipocalin: a novel marker of contrast nephropathy risk. Am J Nephrol. 2012;35(6):509–514
- Lameire N, Kellum JA. Contrast-induced acute kidney injury and renal support for acute kidney injury: A KDIGO summary (Part 2). Crit Care. 2013;17(1):205
- Fliser D, Laville M, Covic A, et al. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: Part 1: Definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant. 2012;27(12):4263–4272
- Haase M, Devarajan P, Haase-Fielitz A, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: A multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57(17):1752–1761
- Rau S, Habicht A, Kauke T, et al. Neutrophil gelatinase-associated lipocalin and end-stage renal disease: It is not all about the kidneys! Eur J Clin Invest. 2013;43(8):816–820
- Liu KD, Yang W, Anderson AH, et al. Urine neutrophil gelatinase-associated lipocalin levels do not improve risk prediction of progressive chronic kidney disease. Kidney Int. 2013;83(5):909–914
- Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007;71(10):967–970
- Bao G, Clifton M, Hoette TM, et al. Iron traffics in circulation bound to a siderocalin (Ngal)-catechol complex. Nat Chem Biol. 2010;6(8):602–609
- Malyszko J, Bachorzewska-Gajewska H, Poniatowski B, Malyszko JS, Dobrzycki S. Urinary and serum biomarkers after cardiac catheterization in diabetic patients with stable angina and without severe chronic kidney disease. Ren Fail. 2009;31(10):910–919
- Bolignano D, Lacquaniti A, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(2):337–344
- James MT, Ghali WA, Knudtson ML, et al. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation. 2011;123(4):409–416
- Wi J, Ko YG, Kim JS, et al. Impact of contrast-induced acute kidney injury with transient or persistent renal dysfunction on long-term outcomes of patients with acute myocardial infarction undergoing percutaneous coronary intervention. Heart. 2011;97(21):1753–1757
- Solomon RJ, Natarajan MK, Doucet S, et al. Cardiac Angiography in Renally Impaired Patients (CARE) study: A randomized double-blind trial of contrast-induced nephropathy in patients with chronic kidney disease. Circulation. 2007;115(25):3189–3196
- Trivedi H, Daram S, Szabo A, Bartorelli AL, Marenzi G. High-dose N-acetylcysteine for the prevention of contrast-induced nephropathy. Am J Med. 2009;122(9):874.e9–15
- Kshirsagar AV, Poole C, Mottl A, et al. N-acetylcysteine for the prevention of radiocontrast induced nephropathy: A meta-analysis of prospective controlled trials. J Am Soc Nephrol. 2004;15(3):761–769
- Barrett BJ, Parfrey PS. Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354(4):379–386
- Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: Updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011;21(12):2527–2541
- Davidson CJ, Hlatky M, Morris KG, et al. Cardiovascular and renal toxicity of a nonionic radiographic contrast agent after cardiac catheterization. A prospective trial. Ann Intern Med. 1989;110(2):119–124
- Hardiek KJ, Katholi RE, Robbs RS, Katholi CE. Renal effects of contrast media in diabetic patients undergoing diagnostic or interventional coronary angiography. J Diabetes Complicat. 2008;22(3):171–177
- Reddan D, Laville M, Garovic VD. Contrast-induced nephropathy and its prevention: What do we really know from evidence-based findings? J Nephrol. 2009;22(3):333–351
- Haase M, Kellum JA, Ronco C. Subclinical AKI – An emerging syndrome with important consequences. Nat Rev Nephrol. 2012;8(12):735–739
- Ronco C, Stacul F, McCullough PA. Subclinical acute kidney injury (AKI) due to iodine-based contrast media. Eur Radiol. 2013;23(2):319–323
- Ronco C, Kellum JA, Haase M. Subclinical AKI is still AKI. Crit Care. 2012;16(3):313
- McCullough PA, Shaw AD, Haase M, et al. Diagnosis of acute kidney injury using functional and injury biomarkers: Workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol. 2012;182:13–29
- McCullough PA, Bouchard J, Waikar SS, et al. Implementation of novel biomarkers in the diagnosis, prognosis, and management of acute kidney injury: Executive summary from the tenth consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol. 2013;182:5–12
- Pickering JW, Endre ZH. Linking injury to outcome in acute kidney injury: A matter of sensitivity. PLoS One. 2013;8(4):e62691
