Abstract
Objective: To investigate the protective effect and the mechanism of baicalein (Bai) in rats with renal ischemia–reperfusion injury (RIRI). Methods: Twenty-four male Sprague–Dawley rats were divided into three groups: sham, IR, and IR + Bai. Bai was administered by tail vein injection (30 mg/kg) 30 min before reperfusion in the IR + Bai group. The IR group and sham group received saline vehicle via the intravenous route. Results: Rats that underwent RIRI exhibited renal functional impairment, histological changes, significantly increased advanced oxidation protein product (AOPP) and malondialdehyde (MDA) levels (p < 0.01), and ICAM-1 and MCP-1 protein and mRNA expression were significantly upregulated (p < 0.01). Administration of Bai reduced AOPP and MDA levels, significantly inhibited expression of inflammatory factors (p < 0.05), and markedly improved renal function. Conclusion: Bai promotes the recovery of renal function in established acute RIRI, and alleviates kidney injury in a rat model.
Introduction
Renal ischemia–reperfusion injury (RIRI) is one among the pathogenic mechanisms responsible for acute kidney injury (AKI). The mechanisms underlying the acute tubular necrosis induced by renal ischemia–reperfusion have not yet been fully elucidated. To date, no drugs have been demonstrated to effectively prevent or alleviate AKI,Citation1 however the role of oxygen free radicals, inflammatory cytokines (including intercellular adhesion molecule-1, ICAM-1; and monocyte chemoattractant protein-1, MCP-1)Citation2,Citation3 and inflammatory cell infiltrationCitation4 in the development of RIRI has been recognized.
Baicalein (Bai, 5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one), a naturally occurring flavonoid, has demonstrated anti-oxidant and anti-inflammatory effects.Citation5–7 Baicalein treatment significantly inhibited cardiomyocyte apoptosis, inflammatory responses and oxidative stress in the heart after ischemia–reperfusion injury.Citation8 However, its effect on AKI and the molecular mechanisms involved remain unclear and warrant further investigation. Due to the potential antioxidant properties of Bai, we sought to examine the therapeutic effect in an in vivo model of acute RIRI and explore the possible mechanisms.
Materials and methods
Animals
All animal procedures were approved by the Animal Experiment Committee of Southern Medical University (Guangzhou City, China). Male Sprague–Dawley rats (initial weight, 200–250 g; Southern Medical University Animal Experiment Center, Guangzhou City, China) were maintained under standardized conditions and fed a standard rodent diet, with free access to tap water.
Establishment of an RIRI rat model
Animals were anesthetized with 3% sodium pentobarbital (30 mg/kg) by tail vein injection. Surgery was performed as described previously.Citation9 In order to establish RIRI, a median incision was made into the abdominal cavity and the right kidney was removed. The left kidney was exposed and perirenal fat and fascia were dissected. The left renal artery and vein were separated from their branches. The left renal artery was clamped with a non-invasive bulldog clamp for 60 min, then released. Establishment of RIRI was considered successful when the kidney was observed turning from pale to red. Baicalein was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
The treatment group, that is, the ischemia–reperfusion + Bia (IR + Bia) group of rats, was given single-dose intravenous injection Bia (30 mg/kg BW) 30 min before reperfusion. The control group (IR group) received saline vehicle via the intravenous route and underwent a clipping procedure and contralateral nephrectomy as described above. The sham group received saline treatment, both kidneys were exposed and perirenal tissues were separated. The right kidney was removed, but the left renal artery was not clamped.
After suturing the incision, the rats were maintained under normal conditions for 24 h, with free access to food and water. Specimens were taken at specified time intervals.
Specimen collection and processing
After surgery the rats were placed in metabolic cages, 24-h urine specimens were retained and the urinary production was calculated. After surgery (24 h), the rats were fully anesthetized by intraperitoneal injection of 3% sodium pentobarbital (30 mg/kg) and fixed on a rat board in supination. The abdominal wall was incised layer by layer. Blood specimens were collected from aorta abdominalis, and the left kidney was taken after tissue perfusion. Blood specimens were centrifuged at 3000 rpm and 4°C for 10 min within 30 min of collection, and serum specimens were retained for inspection. From the ventral half of the left kidney a wedge of approximately 0.3 cmCitation3 was resected and cut into 0.5–1 mmCitation3 pieces, then immediately placed in 2.5% glutaraldehyde in phosphate-buffered saline (PBS) for fixation; the remaining part of the ventral half of the left kidney was placed in 10% formaldehyde fixative. The other half of the left kidney was immediately frozen in liquid nitrogen.
Biochemical assays of urine and serum specimens
Urine and serum specimens were sent to the Clinical Laboratory in Nanfang Hospital (Guangzhou, China). Serum (Scr) and urinary creatinine (Ucr) were measured using an automatic biochemical analyzer (Toshiba, 7600-020+ID, Japan). The endogenous creatinine clearance rate (Ccr) was calculated by considering the urinary production, Ccr (mL/min) = [Ucr (µmol/L) × 24-h urinary production (mL)]/{Scr (µmol/L) × [1000/body weight (g)] × [60/86,400 s]}.Citation10
Light microscopy analysis of morphological changes in renal tissues
Fixed kidney tissue specimens were processed by conventional histologic section and hematoxylin and eosin (H&E) stain. The morphological characteristic changes of tubular necrosis were assessed by light microscopy. Tubular necrosis was scored according to a scale developed by Megyesi et al.Citation11 as follows: 0, no necrosis; 1, necrosis confined to 1/3 of the cortex, mainly in the S3 segment of the proximal tubule; 2, necrosis beyond 2/3of the cortex and 3, necrosis involving the whole cortex.
Measurement of advanced oxidation protein products
Plasma levels of advanced oxidation protein product (AOPP) were determined as described previously.Citation12 To exclude the interference of the turbidity of lipids upon light absorption, samples were diluted 1:5 in PBS and centrifuged (10,000 × g, 1 h, 4 °C). Samples below the lipid layer were used for AOPP measurement.
Measurement of malondialdehyde levels
The malondialdehyde (MDA) level in the serum was detected using a method described previouslyCitation13 and spectrophotometry (UV–Vis Spectrometer 6105; Jenway, Stone, UK). About 2.5 mL of trichloroacetic acid was added to 0.5 mL plasma and the tube was left to stand for 10 min at room temperature. After centrifugation at 3500 rpm for 10 min, the supernatant was decanted and the precipitate was washed with sulfuric acid. Then, 2.5 mL sulfuric acid and 3 mL thiobarbituric acid (TBA) in sodium sulfate were added to this precipitate. The coupling of lipid peroxide with TBA was carried out by heating in a boiling water-bath for 30 min. After cooling in cold water, the resulting chromogen was extracted with 4 mL of n-butyl alcohol by vigorous shaking. Separation of the organic phase was facilitated by centrifugation at 3000 rpm for 10 min and its absorbance at 530 nm was determined.
Real-time quantitative PCR assay of ICAM-1 and MCP-1 mRNA expression in renal tissues
Briefly, total RNA of the renal tissue samples was extracted with an RNA Extraction Agent (TaKaRa Bio, Shiga, Japan) following manufacturer instructions. cDNA was synthesized using a TaKaRa Bio Reverse Transcription kit following manufacturer instructions. Reverse-transcription products were used for quantitative PCR using a 7500 real-time PCR system (Applied Biosystems). The amplification and melting curves were obtained using the 2−ΔΔCt method. For MCP-1 mRNA expression analysis, two primers were used, sense primer 5′-ACGCTTCTGGGCCTGTTGTTCA-3′, and anti-sense primer 5′-TGGGGCATTAACTGCATCTGGCT-3′. For ICAM-1 mRNA expression analysis, the sense primer was 5′-GCGACCACGGAGCCAATTTCTCAT-3′, and the anti-sense primer was 5′-TCAGGACCCTAGTCGGAAGATCGAA-3′. The internal reference was GADPH, with the sense primer 5′-GGAGCGAGACCCCACTAACA-3′ and the anti-sense primer 5′-GGCGGAGATGATGACCCTT-3′.
Immunohistochemical detection of ICAM-1 and MCP-1 proteins in renal tissues
Expression of ICAM-1 and MCP-1 was assessed by immunohistochemical analyses as described previously.Citation14 Sections (thickness, 4 mm) were cut from paraffin blocks, mounted on polylysine-coated slides, and stained (H&E) for light microscopy. Sections were also dewaxed in xylene, rehydrated in a descending series of alcohols and blocked for endogenous peroxidase and avidin/biotin activities. After blocking with 3% bovine serum albumin (BSA) in PBS, sections were incubated with a rabbit polyclonal anti-mouse antibody against ICAM-1 and MCP-1 (1:150 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4 °C. Sections were then washed and incubated with a secondary antibody (goat anti-rabbit) using the Evision + Labeled Polymer kit (Dako, Glostrup, Denmark) for 30 min followed by incubation with avidin–biotin–peroxidase complex (Dako) and development with diaminobenzidine chromogen for 5 min. Finally, sections were rinsed in distilled water, counterstained with hematoxylin (Dako) and mounted on glass slides before evaluation under the microscope. Semi-quantitative analyses of immunohistochemistry were undertaken by image analysis software (Image Pro Plus; Media Cybernetics, Rockville, MD).
Statistical analysis
One-way analysis of variance (ANOVA) was used to compare the different mean values in the three experimental groups. The least-square difference or Dunnett’s T3 test (equal variance not assumed) was used to ascertain significant differences among the groups. Scr was analyzed by repeated-measures ANOVA followed by Dunnett’s T3 multiple comparison test. Tubular necrosis evaluation was assessed by the Kruskall–Wallis test followed by the Rank sum test for multiple comparisons. p < 0.05 was considered significant. Data are the mean ± SD. SPSS version 13.0 (SPSS, Chicago, IL) was used for all analyses.
Results
Renal function
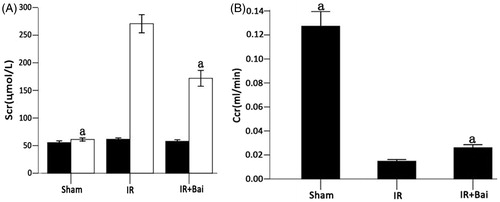
In comparison to the sham group, Scr increased and Ccr decreased following establishment of RIRI. After administration of Bai, the level of Scr was significantly lowered in rats in the Bai group (171.88 ± 40.96 μmol/L) than in the IR group (270.75 ± 46.84 μmol/L) (p < 0.05). On the contrary, the level of Ccr was significantly higher in rats in the Bai group (0.026 ± 0.0069 mL/min) than in the IR group (0.015 ± 0.004 mL/min, n = 8) (p < 0.05, ).
Figure 1. Changes in Scr before (▪) and 24 h after (□) ischemia–reperfusion injury and Ccr in rats after renal ischemia–reperfusion injury. (A) Ischemia–reperfusion injury and treatment with saline vehicle resulted in significant elevation of the Scr level after 24 h. Treatment with Bai after ischemia–reperfusion injury (IR + Bai) reduced the impairment of kidney function compared with the IR group (one-way ANOVA test; a p < 0.05; compared with the sham group; n = 8). A right nephrectomy without ischemia of the contralateral kidney (sham) did not increase the Scr level after 24 h. (B) IR-induced decreases in the Ccr. Treatment with Bai increased the Ccr (one-way ANOVA test; a p < 0.05, compared with the IR group; n = 8).
Renal tissue morphology
Light microscopic examination revealed that the renal tissues of rats in both the Bai group and IR group underwent pathological changes including loss of tubular epithelial cell brush border at the cortico-medullary junction, epithelial cell necrosis and exfoliation, tubular lumen formation, red blood cell overflow and basement membrane rupture. However, the lesions in rats in the Bai group were significantly less severe than those in the IR group (p < 0.01, ). Additionally, the renal tubule score was significantly lower in the Bai group than the IR group (p < 0.01, ).
Figure 2. Histomorphological changes in rat renal tubule after renal ischemia–reperfusion injury (H&E. Magnification, ×40 in A–C). (A) sham operation group, tubular epithelial cells with normal morphology; (B) simple ischemia–reperfusion operation group, tubular epithelial cells with loss of brush border, epithelial cell necrosis and exfoliation, tubular lumen formation, basement membrane rupture and interstitial inflammatory cell infiltration and (C) Bai group, various lesions obviously alleviated in comparison to the ischemia–reperfusion operation group.
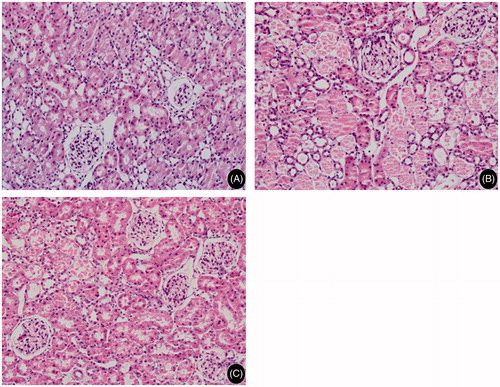
Table 1. Scoring of tubular necrosis in different experimental groups of rats after ischemia and reperfusion ( ).
).
Serum MDA
In comparison to the sham group, serum MDA was increased in the IR group of rats (p < 0.05, ). After administration of Bai, serum MDA in rats fell below that of the IR group (p < 0.05, ).
Table 2. Changes in serum malondialdehyde (MDA) in different experimental groups of rats after ischemia and reperfusion ( ).
).
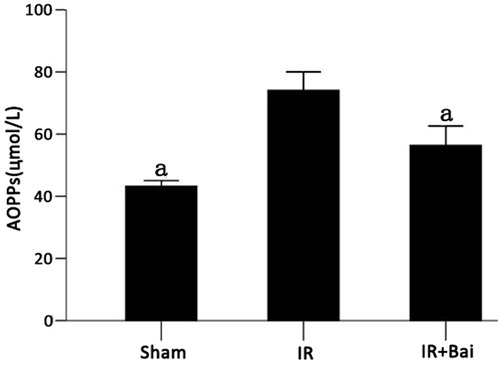
Serum AOPPs
Colorimetry analysis indicated that after RIRI, the level of AOPPs was dramatically increased. After the administration of Bai, AOPPs decreased by 14% (p < 0.05 for both comparisons, ).
ICAM-1 and MCP-1 expression in renal tissue
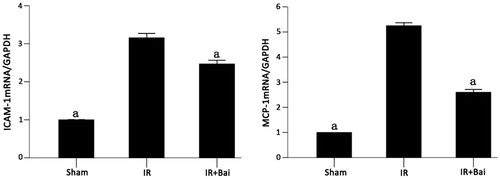
The expression of ICAM-1 and MCP-1 mRNA was higher in the renal tissues of rats in the IR group than the sham group (p < 0.05). Both parameters were substantially reduced after administration of Bai (p < 0.05, ).
Figure 4. Changes in ICAM-1 mRNA and MCP-1 mRNA levels of rat renal tissues after renal ischemia–reperfusion injury. Ischemia–reperfusion caused severe increase in expression of ICAM-1 and MCP-1 mRNAs. ATO reduced this upregulation (one-way ANOVA test; a p < 0.05; compared with the IR group; n = 8).
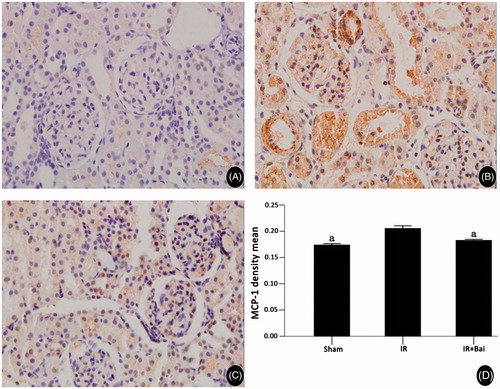
In the sham group, ICAM and MCP protein were expressed in rat renal tissues at low levels (0.24 ± 0.02 and 0.17 ± 0.004, respectively). In IR group animals, ICAM and MCP expression was clearly seen in renal tissue. The renal tubules, interstitial tissues and within proximal and distal convoluted tubules of the cortex medulla were positively stained, and significant MCP-1 expression was observed in glomeruli. ICAM and MCP staining of renal tissues was significantly weaker in the Bai group than the IR group (0.27 ± 0.12 vs. 0.34 ± 0.04, p < 0.05; and 0.18 ± 0.004 vs. 0.21 ± 0.01, p < 0.05, and ).
Figure 5. ICAM-1 expression in rat renal tissues of different experimental groups after renal ischemia–reperfusion injury and the results of semi-quantitative analyses. (Magnification, ×400 in A–C.) Expression of ICAM-1 after ischemia–reperfusion was increased and decreased after administration of Bai (one-way ANOVA test; a p < 0.05; compared with the IR group; n = 8).
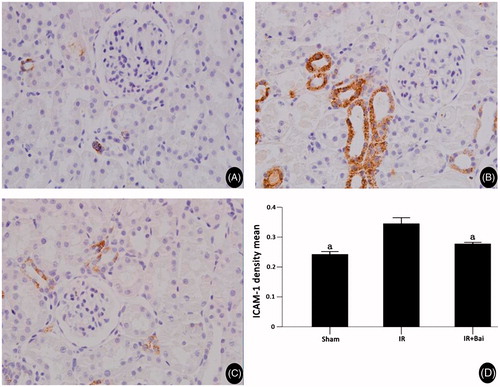
Figure 6. MCP-1 expression in rat renal tissues of different experimental groups after renal ischemia–reperfusion injury and the results of semi-quantitative analyses. (Magnification, ×400 in A–C.) Expression of ICAM-1 after ischemia–reperfusion was increased and decreased after administration of Bai (one-way ANOVA test; a p < 0.05; compared with the IR group; n = 8).
Discussion
In this study we sought to examine the therapeutic effect of the Bai, in an in vivo model of acute RIRI. Oxygen free radicals, cellular adhesion molecules and inflammatory cell infiltration are thought to contribute to the pathogenicity of RIRI,Citation2–4 and Bai has previously been implicated in antioxidant and free radical scavenging activities.Citation4–8
Oxygen free radicals can not only directly inhibit the reperfusion of renal tissue; but more importantly, they can induce and amplify inflammatory responses via oxidative stress, thereby increasing renal injury and apoptosis.Citation15 AOPPs are the products of oxidative stress which can induce respiratory burst of monocytes and neutrophils and induce the production of more active oxygen species by monocytes; additionally, AOPPs themselves can induce or aggravate oxidative stress.Citation16 It has been reported that in patients with chronic kidney disease, AOPPs act on redox-sensitive inflammatory pathways to promote renal fibrosis and accelerate disease progression.Citation17 In AKI such as that after coronary artery bypass graft surgery, AOPPs can be used as prognostic biomarkers to measure the recovery progress.Citation18
Additionally, ROS not only elicit injury to reperfused tissue directly, they also amplify the effect of injury of ROS through peroxidation (oxidative stress). This view is consistent with the notion that oxidative stress increases in critically ill patients with AKI.Citation19 Further, the resultant hydroperoxide products can induce protein oxidation, DNA mutation and even rupture, lipid oxidation or blistering of membranes, leading to cell damage. MDA is one aldehyde-type product of cellular lipid peroxidation which can reflect the oxidative status and the degree of cell damage in vivo.Citation20
In a rat model of RIRI we observed elevation of serum AOPPs and MDA, likely reflecting the increased level of oxidative stress during renal ischemia–reperfusion. RIRI likely induced generation of oxygen free radicals, inducing cell damage via lipid peroxidation. We observed that decreases in circulating Scr and Ccr levels coincided with the observed pathological changes in rat renal tissues and elevation of serum AOPPs and MDA. This result indirectly illustrates that RIRI-induced acute renal failure is at least partly due to cell damage caused by oxygen free radicals. In rats with RIRI, ICAM-1 and MCP-1 expression levels were significantly increased in renal tubules after 24 h reperfusion and ICAM-1 and MCP-1 mRNA expression levels increased renal tissues correspondingly. High expression of ICAM-1 and MCP-1 promoted the accumulation of inflammatory cells in the injured area, implicating the inflammatory response in the development of RIRI. Additionally, we observed changes in ICAM-1 and MCP-1 expression consistent with the Ccr decrease in rat renal tissues, indicating that the inflammatory response is involved in the process of ischemia–reperfusion and plays a role in promoting the formation and aggravation of acute renal failure.
In our study, application of Bai 30 min before reperfusion resulted in significantly lower levels of AOPPs and MDA in rats after reperfusion than the IR group. These results suggest that the protection afforded by Bai may be mediated by reducing oxidative stress, particularly by decreasing the production of oxygen free radicals and lipid peroxidation in cells. The most prominent injury to renal proximal tubular cells is likely to occur during the first 2–4 h of the reperfusion period. Sixty minutes of ischemia does not increase MDA levels, whereas 15 min of reperfusion results in a large increase in kidney lipid peroxidation and aggravates cell injury.Citation20,Citation21 Hence, Bai administration 30 min before reperfusion can protect the kidney.
Additionally, rats administered Bai had weaker ICAM-1 and MCP-1 expression in renal tubule and interstitial tissues of cortico-medullary junction and lower ICAM-1 and MCP-1 mRNA expression levels in renal tissues than the IR group. Regarding the renal junction, Scr levels were reduced and Ccr levels were increased in the Bai group, in comparison to the IR group. Together these results suggest that administration of Bai after AKI also alleviates RIRI and promotes the recovery of renal function. As for the protective mechanism, Bai likely blocks or inhibits intracellular oxidative stress and reduces production of oxygen free radicals, thereby reducing lipid peroxidation, alleviating cell damage, and inhibiting the synthesis and secretion of certain cytokines or adhesion molecules, ultimately inhibiting the inflammatory responses of the injured cells.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Kinsey GR, Li L, Okusa MD. Inflammation in acute kidney injury. Nephron Exp Nephrol. 2008;109(4):e102–e107
- Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66:486–491
- Li H, Nord EP. CD40 ligation stimulates MCP-1 and IL-8 production, TRAF6 recruitment, and MAPK activation in proximal tubule cells. Am J Physiol Renal Physiol. 2002;282:F1020–F1033
- Schrier RW, Wang W, Poole B, et al. Acute renal failure: Definitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004;114:5–14
- Jung EB, Lee CS. Baicalein attenuates proteasome inhibition-induced apoptosis by suppressing the activation of the mitochondrial pathway and the caspase-8- and Bid-dependent pathways. Eur J Pharmacol. 2014;730:116–124
- Gao D, Tawa R, Masaki H, Okano Y, Sakurai H. Protective effects of baicalein against cell damage by reactive oxygen species. Chem Pharm Bull (Tokyo). 1998;46(9):1383–1387
- Lin HY, Shen SC, Lin CW, Yang LY, Chen YC. Baicalein inhibition of hydrogen peroxide-induced apoptosis via ROS-dependent heme oxygenase 1 gene expression. Biochim Biophys Acta. 2007;1773(7):1073–1086
- Song L, Yang H, Wang HX, et al. Inhibition of 12/15 lipoxygenase by baicalein reduces myocardial ischemia/reperfusion injury via modulation of multiple signaling pathways. Apoptosis. 2014;19(4):567–580
- Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74:1156–1164
- Jianfang Fu, Yuan Li, Li Wang, et al. Paeoniflorin prevents diabetic nephropathy in rats. Comp Med. 2009;59:557–566
- Megyesi J, Andrade L, Vieira JM Jr, et al. Positive effect of the induction of p21WAF1/CIP1on the course of ischemic acute renal failure. Kidney Int. 2001;60:2164–2172
- Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–1313
- Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43
- Taal MW, Chertow GM, Rennke HG, et al. Mechanisms underlying renoprotection during renin-angiotensin system blockade. Am J Physiol Renal Physiol. 2001;280:F343–F355
- Chien CT, Lee PH, Chen CF, et al. De novo demonstration and co-localization of free-radical production and apoptosis formation in rat kidney subjected to ischemia/reperfusion. J Am Soc Nephrol. 2001;12:973–982
- Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–1313
- Li HY, Hou FF, Zhang X, et al. Advanced oxidation protein products accelerate renal fibrosis in a remnant kidney model. J Am Soc Nephrol. 2007;18:528–538
- Liang X, Chen Y, Zhuang J, et al. Advanced oxidation protein products as prognostic biomarkers for recovery from acute kidney injury after coronary artery bypass grafting. Biomarkers. 2012;17(6):507–512
- Lentini P, de Cal M, Cruz D, et al. The role of advanced oxidation protein products in intensive care unit patients with acute kidney injury. J Crit Care. 2010;25:605–609
- Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74:1156–1164
- Todorovic Z, Nesic Z, Stojanović R, et al. Acute protective effects of simvastatin in the rat model of renal ischemia-reperfusion injury: It is never too late for the pretreatment? J Pharmacol Sci. 2008;107:465–470