Abstract
Background: Renal involvement in type 2 diabetes is mostly due to diabetic nephropathy (DN), but a subset of diabetic patients could present with pure non-diabetic renal disease (NDRD) or NDRD superimposed on DN. We conducted a prospective cohort study to identify the underline renal pathology in type 2 diabetic patients with defined clinical criteria for renal biopsy. Methods: A total of 46 patients (27 female, mean age of 48.9 ± 11.9 years) with type 2 diabetes mellitus (DM) and atypical features of DN with unexpected proteinuria, hematuria, and/or renal impairment were enrolled in this study. Results: Of 46 patients with type 2 diabetes, 16 (34.8%) had DN, 20 (43.5%) had NDRD, and 10 (21.7%) had NDRD superimposed on DN. Membranous nephropathy (34%) was the most common NDRD. Patients with NDRD had a lower frequency of diabetic retinopathy (5%), shorter duration of diabetes, higher range of proteinuria, and better kidney survival. In multiple logistic regression analysis, only lack of diabetic retinopathy was independent predictor of NDRD. Positive and negative predictive value of diabetic retinopathy (DR) for diabetic nephropathy was 94 and 68%, respectively. Conclusion: Kidney biopsy is strongly recommended for patients with type 2 diabetes and atypical renal presentation for DN, particularly in the absence of DR. This approach could lead to diagnosis of NDRD with better renal survival.
Introduction
Diabetic nephropathy is a devastating complication of diabetes mellitus and leading cause of end stage renal disease (ESRD) worldwide.Citation1 Diabetic nephropathy (DN) from early stage is commenced by hyperfiltration followed by microalbuminuria and then macroalbuminuria that is usually continued by slowly progressive decline in renal function over time and ultimately ESRD.Citation2,Citation3 Type 2 diabetic patients with proteinuria and diabetic retinopathy (DR) most likely have DN, while high frequency of non-diabetic renal disease (NDRD) has reported in the absence of retinopathy.Citation4,Citation5 The reported prevalence of NDRD ± DN in diabetes patients is in the range of 17–85% depends upon given institution’s biopsy policy and geographic place.Citation5–12 The importance of NDRD diagnosis is appreciated by the reported better kidney survival following appropriate therapy.Citation10 The atypical clinical features of renal involvement, specially in short duration of diabetes which warrant renal biopsy, are determined due to the absence of diabetic retinopathy, rapidly decreasing renal function, briskly rising proteinuria or acute onset of nephrotic syndrome, and active urine sediment.Citation5–12
The present prospective study was carried out to estimate the frequency of NDRD ± DN in type 2 diabetes patients presented with atypical features of diabetic renal involvement. We also investigated the clinical course, renal outcome, and clinical predictors of NDRD.
Methods
We prospectively enrolled type 2 diabetes patients with atypical diabetic renal involvement for renal biopsy from 2008 to 2011 at two nephrology units of Shariati and Baharlo Hospitals in Tehran, Iran. The indications for biopsy were unexplained rapidly decreasing renal function or increasing proteinuria, acute onset of nephrotic syndrome, active urine sediment (more than 5–8 red blood cells with mostly acanthocyte shapes or RBC casts), and renal involvement in the absence of DR. Written informed consents were obtained from patients. Kidney biopsy samples were examined by light microscopy, immunofluorescence, and electron microscopy. Diabetic retinopathy was diagnosed based on the background ± proliferative changes on funduscopy and/or fluorescein angiography. A total of 46 patients were recruited. Mean age of the patients was 48.9 ± 11.9 (range: 27–68) years and 27 (59%) of them were female. Mean diabetic duration (from diagnosis of diabetes till biopsy time) was 7.2 ± 5.6 (range: 0.6–20) years (). Based on pathology reports, patients allocated in three groups: group I: pure DN, group II: pure NDRD, and group III: NDRD superimposed on DN. Patients followed for a mean of 29.7 ± 21.3 (range: 6–75) months. Demographic, clinical, and laboratories data at presentation and last follow-up were collected.
Table 1. Characteristics of 46 diabetic patients with clinically atypical renal involvement.
Statistical analysis
Numerical data are presented as mean ± SD. Differences between groups were compared with the t-test or ANOVA for continuous variables, and with the Pearson chi-square and Fisher’s exact test for categorical variables. Kaplan–Meier analysis was used to compare kidney survival. Independent predictors of NDRD were found out by multiple logistic regression analysis. Analyses were carried out with SPSS version 16 (SPSS Inc., Chicago, IL). Significant level was considered as p < 0.05.
Results
Of 46 type 2 diabetic patients, 16 (35%) had a pathologic diagnosis of DN, 20 (43%) had NDRD, and 10 (22%) had NDRD superimposed on DN (). Membranous nephropathy (MN) was the most common nephropathy in NDRD group (45%) followed by focal segmental glomerulopathy (FGS; 30%). Acute interstitial nephritis (AIN) was the most common lesion in group III (60%) followed by IgA nephropathy (20%; ).
Table 2. Pathologic diagnosis in 46 diabetic patients with clinically atypical renal involvement.
Indications for biopsies were as follows: unexplained rapidly increasing proteinuria in 31 cases (67.5%), unexpected serum creatinine in one patient (2.2%), active urine sediment and rising serum creatinine in 4 patients (8.7%), proteinuria and rising serum creatinine in 9 cases (19.7%), and all of them in 1 patient (2.2%). Urine sediment was active in 8 (17.4%) patients. Nephrotic range proteinuria was observed in 27 (58.7%) subjects.
No differences were noticed in age, sex, and rate of hematuria among three groups (). There was a significant difference in baseline proteinuria, serum creatinine, and estimated glomerular filtration rate (eGFR) among groups. There was a trend for higher nephrotic range proteinuria in NDRD group compared to pure DN patients. Duration of diabetes before biopsy was higher in pure DN patients compared to NDRD patients (9.8 ± 5.5 vs. 4.6 ± 3.7 years, p = 0.03). Seventeen of 26 patients in group 1 + 3 (DN ± NDRD) suffered from diabetic retinopathy, while one of the 20 patients in group 2 (pure NDRD) had diabetic retinopathy (p < 0.001). Multiple logistic regression analysis (included age, sex, diabetic duration, diabetic retinopathy, first serum creatinine, first GFR, first proteinuria, and nephrotic range proteinuria) disclosed that the absence of diabetic retinopathy was independent predictor for NDRD (p = 0.006, OR: 30.7, CI 95%: 2.7–349.5).
Table 3. Comparison of patients’ characteristics among three groups of kidney pathology.
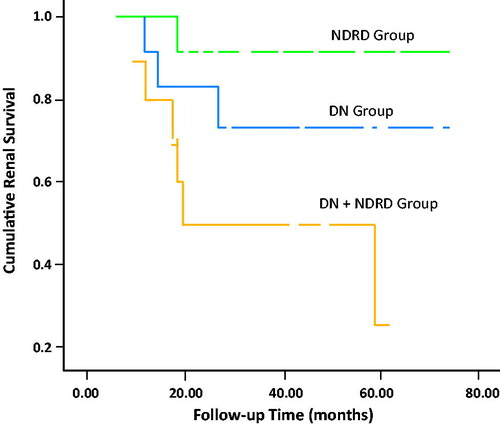
While the rate of proteinuria at baseline was significantly higher in pure NDRD patients, after appropriate immunosuppressive ± ACE/ARBs therapy, the last follow-up proteinuria did not differ among groups. Last estimated GFR was significantly higher in pure NDRD group. Stage 5 chronic kidney disease (ESRD) happened in 10 (21.7%) patients (19, 5, and 60% in groups I, II, and III, respectively, p = 0.03). In Kaplan–Meier Analysis, one-year kidney survival (percentage of the patients which did not develop ESRD by the last follow-up) for groups I (pure DN), II (pure NDRD), and III (DN + NDRD) was, 84% (SE: 0.11), 91% (SE: 0.08), and 75% (SE: 0.13), respectively. Three-year kidney survival for groups I, II, and III was 72% (SE: 0.14), 91% (SE: 0.08), and 50% (0.15), respectively. Five-year kidney survival was 72% (SE: 0.14), 91% (SE: 0.08), and 25% (SE: 0.19) for groups I, II, and III, respectively. Median survival (50%) for group III was 18 months (). Log rank test showed a significant difference in survival (p = 0.016).
Discussion
In this study, a prospective cohort study was designed to evaluate the prevalence of DN, NDRD, and NDRD superimposed on DN in type 2 diabetes patients with atypical clinical renal involvement for diabetic nephropathy. We also checked groups for clinical course and kidney survival.
Sixty-five percentages of biopsies revealed NDRD ± DN that is in the range of other reports (17–85%).Citation5–12 The most common pathologies in NDRD group were MN (45%) and FGS (30%), respectively. In the DN + NDRD group, although AIN (60%) was the most common lesion, IgA nephropathy (20%) was the prevalent glomerulopathy. This finding is in agreement with results of other studies.Citation8,Citation9,Citation13,Citation14 Overall, the common NDRD in both groups (II and III) were MN (34%), FGS (20.5%), AIN (20.5%), and IgA nephropathy (10%). These findings are in contrast to reports from Southeast Asia which IgA nephropathy (up to 65%) was the most common NDRD.Citation5,Citation6 Studies have shown that in the US FGSCitation14 and in India AINCitation7 were the prevail pathology. These contrasting reports in various regions are due to both distinctive common glomerular diseases and perhaps different criteria (and threshold) for kidney biopsy in type 2 diabetes patients with atypical renal presentations.
In this study, diabetic duration before biopsy was significantly shorter among NDRD patients. However, we found that after adjustments for other factors, only lack of diabetic retinopathy was the independent predictor of NDRD in this cohort. In literature review, studies mostly revealed that short duration of diabetes was an indicator of NDRD.Citation5,Citation10–12,Citation15
Likewise, NDRD patients had higher rate of baseline proteinuria and nephrotic syndrome, however, it did not differ with other groups after proper immunosuppressive therapy. Patients with NDRD ± DN were more likely to have hematuria (23%) compared to isolated DN patients (6%), but it did not reach to significant level.
In this study, baseline eGFR was lower in patients with NDRD superimposed on DN. It was expected as the common pathology of AIN happened on an about well-established diabetes. Stage 5 CKD (ESRD) happened in 10 (22%) of our patients including 19% in DN, 5% in NDRD, and 60% in DN + NDRD groups. One, three, and five year cumulative kidney survival was higher in NDRD patients compared to DN and DN + NDRD groups (p = 0.016; ). Despite the similar baseline eGFR in NDRD and DN groups, kidney survival was better in NDRD patients. We expected a better prognosis for NDRD patients, as their baseline serum creatinine level was acceptable and they were treated with aggressive immunosuppressive agents mostly for MN and FGS. The worst prognosis was endured for patients with NDRD superimposed on DN. It is postulated that the nature of NDRD (AIN) with unremitting course superimposed on a durable DN were main reasons for ominous prognosis in these patients.
In this study, diabetic retinopathy had a close correlation with the presence of DN (±NDRD), and the lack of DR was a good predictor of pure NDRD. Sensitivity of diabetic retinopathy for diabetic nephropathy (±NDRD) was 65% (CI: 0.46–0.81) with a specificity of 95% (CI: 0.75 to >0.99), and a positive predictive value of 94% (CI: 0.72 to >0.99) with a negative predictive value of 68% (CI: 0.49–0.82). False-positive rate of DR for DN was 5% (<0.0001 to 0.25) with a false-negative rate of 35% (0.19–0.54). One report suggested that the diagnostic specificity of DR for DN was 100%.Citation16 In a meta-analysis by Liang et al.,Citation15 DR was not observed in 23.6% of patients with biopsy-proven DN, while there was no evidence of DN in 17.6% of patients with DR. In general, the absence of DR is predictive of NDRD, but it could not rule out DN.
In summary, type 2 diabetes patients with pathology diagnosis of NDRD benefit from an early diagnosis followed by an appropriate disease specific therapy. We strongly recommend renal biopsy for type 2 diabetes with unusual presentation and course, particularly in the absence of DR. By this strategy, we could improve their kidney survival and potentially reduce the burden of CKD in this population.
Declaration of interest
The authors report no conflicts of interest.
References
- Ritz E, Rychlik I, Locatelli F, Halimi S. End-stage renal failure in type 2 diabetes: A medical catastrophe of worldwide dimensions. Am J Kidney Dis. 1999;34:795–808
- Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med. 1999;341:1127–1133
- Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003;63:225–232
- Christensen PK, Larsen S, Horn T, Olsen S, Parving HH. Causes of albuminuria in patients with type 2 diabetes without diabetic retinopathy. Kidney Int. 2000;58:1719–1731
- Zhou J, Chen X, Xie Y, Li J, Yamanaka N, Tong X. A differential diagnostic model of diabetic nephropathy and non-diabetic renal diseases. Nephrol Dial Transplant. 2008;23:1940–1945
- Lee EY, Chung CH, Choi SO. Non-diabetic renal disease in patients with non-insulin dependent diabetes mellitus. Yonsei Med J. 1999;40:321–326
- Soni SS, Gowrishankar S, Kishan AG, Raman A. Non diabetic renal disease in type 2 diabetes mellitus. Nephrology (Carlton). 2006;11:533–537
- Ghani AA, Al Waheeb S, Al Sahow A, Hussain N. Renal biopsy in patients with type 2 diabetes mellitus: Indications and nature of the lesions. Ann Saudi Med. 2009;29:450–453
- Lin YL, Peng SJ, Ferng SH, Tzen CY, Yang CS. Clinical indicators which necessitate renal biopsy in type 2 diabetes mellitus patients with renal disease. Int J Clin Pract. 2009;63:1167–1176
- Byun JM, Lee CH, Lee SR, et al. Renal outcomes and clinical course of non-diabetic renal diseases in patients with type 2 diabetes. Korean J Intern Med. 2013;28:565–572
- Tone A, Shikata K, Matsuda M, et al. Clinical features of non-diabetic renal diseases in patients with type 2 diabetes. Diabetes Res Clin Pract. 2005;69:237–242
- Huang F, Yang Q, Chen L, Tang S, Liu W, Yu X. Renal pathological change in patients with type 2 diabetes is not always diabetic nephropathy: A report of 52 cases. Clin Nephrol. 2007;67:293–297
- Chang TI, Park JT, Kim JK, et al. Renal outcomes in patients with type 2 diabetes with or without coexisting non-diabetic renal disease. Diabetes Res Clin Pract. 2011;92(2):198–204
- Pham TT, Sim JJ, Kujubu DA, Liu IL, Kumar VA. Prevalence of non-diabetic renal disease in diabetic patients. Am J Nephrol. 2007;27(3):322–328
- Liang S, Zhang XG, Cai GY, et al. Identifying parameters to distinguish non-diabetic renal diseases from diabetic nephropathy in patients with type 2 diabetes mellitus: A meta-analysis. PLoS One. 2013;8(5):e64184
- Parving HH, Gall MA, Skøtt P, et al. Prevalence and causes of albuminuria in non-insulin-dependent diabetic patients. Kidney Int. 1992;41:758–762