Abstract
Background: The reports on the efficacy of statins for the prevention of contrast-induced acute kidney injury (CIAKI) remain controversial. The objective of this meta-analysis was to assess the effect of statins for the prevention of CIAKI. Methods: Comprehensive literature searches for randomized controlled trials (RCTs) of periprocedural statin treatment for prevention of CIAKI were performed using MEDLINE, EMBASE, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials Systematic Reviews and clinicaltrials.gov from inception until May 2014. The primary outcome was the incidence of CIAKI. Results: Thirteen prospective RCTs were included in our analysis. Of 5803 patients with contrast exposures, 304 patients (5.2%) had CIAKI. Patients in the statin group had an overall lower incidence of CIAKI (3.6%) compared to the control group (6.9%). Intravenous (IV) fluid hydration was used in both groups of all included studies for prevention of CIAKI. There was a significant protective effect of periprocedural statins on the incidence of CIAKI when compared to the control group [risk ratios (RRs): 0.49; 95% CI: 0.37–0.66, I2 of 25%]. Conclusions: Our study demonstrates a statistically significant protective effect of statin treatment during procedures with contrast exposures. This finding suggests the use of statins in addition to standard IV crystalloid hydration may be beneficial in the prevention of CIAKI.
Introduction
Contrast-induced nephropathy or contrast-induced acute kidney injury (CIAKI) is a well-recognized complication of radiological interventions, cardiac catheterization or minimally invasive procedures that require iodinated contrast administration. It is a common cause of acute kidney injury (AKI) in an inpatient setting.Citation1 Despite current prophylactic strategies with hydration and low contrast volume, the incidence of CIAKI has been reported from 2% in the general population without risk factors to more than 40% in high-risk patients.Citation2–7 The overall incidence of CIAKI is approximately 150,000 patients each year worldwide.Citation8 Patients with CIAKI have also been demonstrated to have longer hospitalizations and higher long-term adverse event and mortality rates.Citation2,Citation9
In addition to the improvement of lipid profiles and cardiovascular effects, statins ameliorate endothelial function and reduce oxidative stress and inflammation which underlies the pathogenesis of CIAKI.Citation10,Citation11 However, the beneficial effects of statins on prevention of CIAKI are still controversial. Several studies have demonstrated a protective effect of statins for CIAKI prevention.Citation12–20 Conversely, a few studies showed no significant beneficial effect of statins for prevention of CIAKI.Citation21–28
To assess whether statins had beneficial effects on prevention of CIAKI, we performed this meta-analysis to investigate the effect of statins on the incidence of CIAKI between statin and control groups.
Methods
Search strategy
Two investigators (WC and CT) independently searched published RCTs indexed in MEDLINE, EMBASE, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials Systematic Reviews and clinicaltrials.gov from inception to May 2014 using the search strategy described in Appendix. A manual search for additional relevant studies using references from retrieved articles was also performed. Conference abstracts and unpublished studies were excluded.
Inclusion criteria
The inclusion criteria was as follows: (1) RCTs published as original studies to evaluate the effect of periprocedural statins for the prevention of CIAKI in patients undergoing elective cardiac catheterization or any radiological procedures with contrast exposures, (2) data for analysis for relative risks, hazard ratios, standardized incidence ratio with 95% confidence intervals (CI) were provided, (3) reference groups composed of participants who were not prescribed statins or used lower dose of statins compared to interventional group and (4) only studies in humans.
Study eligibility was independently determined by the two investigators noted above. Differing opinions were resolved by mutual consensus. The quality of each study was independently evaluated by each investigator using Jadad quality assessment scale.Citation29 The studies with Jadad quality assessment scale ≥2 were included in the analysis for the validity of our study results.
Data extraction
A standardized data collection form was used to extract the following information: last name of the first author, study design, year of study, country of origin, year of publication, sample size, characteristics of included participants, randomization techniques, blinding status, statin dose, type of statin, type of contrast, definition of CIAKI, outcome ascertainment and relative risk with 95% CI. The two investigators mentioned above independently performed this data extraction.
Statistical analysis
Review Manager 5.2 software from the Cochrane Collaboration was used for data analysis. Point estimates and standard errors were extracted from individual studies and were combined by the generic inverse variance method of DerSimonian and Laird.Citation30 Given the high likelihood of between study variances, we used a random-effect model rather than a fixed-effect model. Statistical heterogeneity was assessed using the Cochran's Q test. This statistic is complemented with the I2 statistic, which quantifies the proportion of the total variation across studies that is due to heterogeneity rather than chance. A value of I2 of 0–25% represents insignificant heterogeneity, 26–50% low heterogeneity, 51–75% moderate heterogeneity and > 75% high heterogeneity.Citation31 The presence of publication bias was assessed by funnel plots of the logarithm of odds ratios versus their standard errors.Citation32
Results
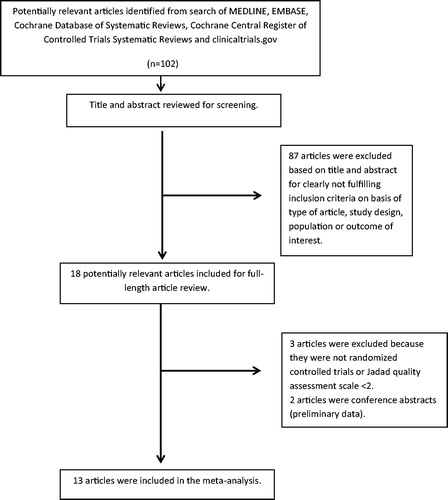
Our search strategy yielded 102 potentially relevant articles. Eighty-seven articles were excluded based on title and abstract for clearly not fulfilling inclusion criteria on the basis of the type of article, study design, population or outcome of interest. Eighteen articles underwent full-length-article review. Five articles were subsequently excluded (three articles were not RCTs or Jadad quality assessment scale <2 and two articles were conference abstracts with preliminary results). Thirteen prospective RCTs were identified and included in the data analysis.Citation14,Citation16–20,Citation22–28 outlines our search methodology and selection process. describes the detailed characteristics and quality assessment of the included studies.
Table 1. Main characteristics of the studies included in this meta-analysis.
The incidence of CIAKI
Of 5803 patients who had elective cardiac catheterization or radiological procedures with contrast exposures, 304 patients (5.2%) developed CIAKI. Patients in the statin group had overall lower incidence of CIAKI (3.6%) compared to control group (6.9%).
The risk of CIAKI in statin and control groups
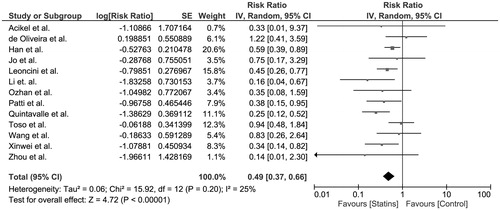
The pooled risk ratio (RR) of CIAKI in the periprocedural statin group versus control group was 0.49 (95% CI, 0.37–0.66). Intravenous (IV) fluid hydration was used in both groups of all included studies for prevention of CIAKI. The statistical heterogeneity was insignificant with an I2 of 25%. shows the forest plot of the included studies.
Figure 2. Forest plot of the included studies comparing risk of CIAKI in patients who used statins and those who did not; square data markers represent risk ratios (RRs); horizontal lines, the 95% CIs with marker size reflecting the statistical weight of the study using random-effects meta-analysis. A diamond data marker represents the overall RR and 95% CI for the outcome of interest.
Evaluation for publication bias
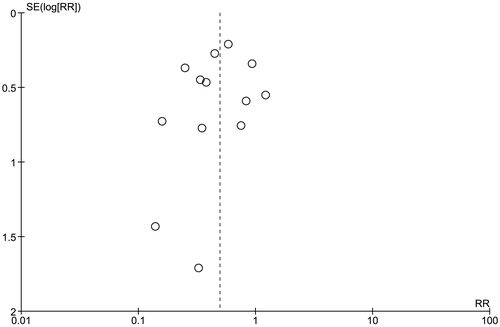
A funnel plot to evaluate publication bias for the risk of CIAKI in patients receiving statins versus control group is summarized in . The graph is fairly asymmetric and, thus, providing a suggestion to the presence of publication in favor of negative studies.
Discussion
Our meta-analysis showed an overall incidence of CIAKI of 5.2% in patients with contrast exposures. Patients in the statin group had a lower incidence of CIAKI (3.6%) when compared to the control group (6.9%). Based on the pooled incidence of CIAKI in the statin and control groups, the number needed to treat (NNT) (the number of patients) to prevent a CIAKI event using statin is 31. Our study also demonstrated a significant protective effect of periprocedural statins on the incidence of CIAKI.
Statins, potent lipid-lowering agents, have been widely prescribed for primary and secondary prevention of coronary artery disease. Studies have demonstrated the cholesterol-independent or “pleiotropic” effects of statins including amelioration of endothelial function, stabilization of atherosclerotic plaques, reduction of oxidative stress and inflammation and inhibition of thrombogenic process.Citation33 Statins have also been found to up-regulate inhibitors of transforming growth factor-beta signaling and decreases renal fibrosis.Citation34 Recently, a meta-analysis of statin use on renal function demonstrated their renoprotective effects on slow progression of chronic kidney disease (CKD) and reduction of proteinuria.Citation35
The underlying pathogenesis of CIAKI is not completely understood. However, studies have suggested that a combination of direct tubular injury, hemodynamic changes of renal blood flow mediated by increase in free radicals and vasoconstriction might play important roles.Citation11,Citation36 The pleiotropic effects of statins might potentially reduce vascular endothelial inflammation and free radicals that are caused by contrast media nephrotoxicity. Although a previous meta-analysis on this topic showed inconclusive data, it showed a trend of beneficial effects of statins in reducing the incidence of CIAKI.Citation37 Our studies included newer studies that used lower volume of contrast media, which caused less tubular toxicities.Citation16–20,Citation27 Therefore, our meta-analysis successfully reveals the benefit of statins for the prevention of CIAKI.
The strengths of our study include insignificant heterogeneity among included studies. All RCTs in this meta-analysis used the same definition of CIAKI (an increase in serum creatinine ≥0.5 mg/dL within 48–72 h of contrast exposure). Thus, this meta-analysis provides reliable results on the effects of statins for the prevention of CIAKI.
Although almost all included studies were of moderate to high qualityCitation14,Citation16–20,Citation23,Citation25,Citation28 (as evaluated by Jadad quality assessment scale), there are some limitations. Firstly, there is publication bias in favor of negative studies in this meta-analysis. Despite this, our study demonstrates the positive effect of statins for CIAKI prevention. Secondly, although all included studies used IV fluid hydration for CIAKI prevention in both statin and control groups, rate and type of IV fluid used in studies were different and these differences could potentially affect the incidence of CIAKI among included studies. In addition, the effective dose of statins for the prevention of CIAKI is still not well established. Only two included RCTs compared high dose statin to low dose statin and they showed different results. Almost all studies revealing the beneficial effect of statins, however, used moderately high dose statins ().
In summary, our study demonstrates a statistically significant protective effect of statin treatment during procedures with contrast exposures for the prevention of CIAKI. This finding suggests the use of statins in addition to standard IV crystalloids hydration may be beneficial in the prevention of CIAKI.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- de Freitas do Carmo LP, Macedo E. Contrast-induced nephropathy: Attributable incidence and potential harm. Crit Care. 2012;16:127
- Marenzi G, Lauri G, Assanelli E, et al. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44:1780–1785
- Briguori C, Airoldi F, D'Andrea D, et al. Renal insufficiency following contrast media administration trial (remedial): A randomized comparison of 3 preventive strategies. Circulation. 2007;115:1211–1217
- Marenzi G, Assanelli E, Marana I, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354:2773–2782
- Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180–184
- Mitchell AM, Jones AE, Tumlin JA, Kline JA. Incidence of contrast-induced nephropathy after contrast-enhanced computed tomography in the outpatient setting. Clin J Am Soc Nephrol. 2010;5:4–9
- Huber W, Eckel F, Hennig M, et al. Prophylaxis of contrast material-induced nephropathy in patients in intensive care: Acetylcysteine, theophylline, or both? A randomized study. Radiology. 2006;239:793–804
- Feldkamp T, Kribben A. Contrast media induced nephropathy: Definition, incidence, outcome, pathophysiology, risk factors and prevention. Minerva Med. 2008;99:177–196
- Solomon RJ, Mehran R, Natarajan MK, et al. Contrast-induced nephropathy and long-term adverse events: Cause and effect? Clin J Am Soc Nephrol. 2009;4:1162–1169
- Farmer JA. Pleiotropic effects of statins. Curr Atheroscler Rep. 2000;2:208–217
- Katholi RE, Woods WT Jr, Taylor GJ, et al. Oxygen free radicals and contrast nephropathy. Am J Kidney Dis. 1998;32:64–71
- Khanal S, Attallah N, Smith DE, et al. Statin therapy reduces contrast-induced nephropathy: An analysis of contemporary percutaneous interventions. Am J Med. 2005;118:843–849
- Patti G, Nusca A, Chello M, et al. Usefulness of statin pretreatment to prevent contrast-induced nephropathy and to improve long-term outcome in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2008;101:279–285
- Xinwei J, Xianghua F, Jing Z, et al. Comparison of usefulness of simvastatin 20 mg versus 80 mg in preventing contrast-induced nephropathy in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Am J Cardiol. 2009;104:519–524
- Yoshida S, Kamihata H, Nakamura S, et al. Prevention of contrast-induced nephropathy by chronic pravastatin treatment in patients with cardiovascular disease and renal insufficiency. J Cardiol. 2009;54:192–198
- Han Y, Zhu G, Han L, et al. Short-term rosuvastatin therapy for prevention of contrast-induced acute kidney injury in patients with diabetes and chronic kidney disease. J Am Coll Cardiol. 2014;63:62–70
- Leoncini M, Toso A, Maioli M, Tropeano F, Villani S, Bellandi F. Early high-dose rosuvastatin for contrast-induced nephropathy prevention in acute coronary syndrome: Results from the prato-acs study (protective effect of rosuvastatin and antiplatelet therapy on contrast-induced acute kidney injury and myocardial damage in patients with acute coronary syndrome). J Am Coll Cardiol. 2014;63:71–79
- Patti G, Ricottini E, Nusca A, et al. Short-term, high-dose atorvastatin pretreatment to prevent contrast-induced nephropathy in patients with acute coronary syndromes undergoing percutaneous coronary intervention (from the ARMYDA-CIN [atorvastatin for reduction of myocardial damage during angioplasty – contrast-induced nephropathy] trial). Am J Cardiol. 2011;108:1–7
- Quintavalle C, Fiore D, De Micco F, et al. Impact of a high loading dose of atorvastatin on contrast-induced acute kidney injury. Circulation. 2012;126:3008–3016
- Li W, Fu X, Wang Y, et al. Beneficial effects of high-dose atorvastatin pretreatment on renal function in patients with acute st-segment elevation myocardial infarction undergoing emergency percutaneous coronary intervention. Cardiology. 2012;122:195–202
- Kandula P, Shah R, Singh N, Markwell SJ, Bhensdadia N, Navaneethan SD. Statins for prevention of contrast-induced nephropathy in patients undergoing non-emergent percutaneous coronary intervention. Nephrology (Carlton). 2010;15:165–170
- Jo SH, Koo BK, Park JS, et al. Prevention of radiocontrast medium-induced nephropathy using short-term high-dose simvastatin in patients with renal insufficiency undergoing coronary angiography (promiss) trial – A randomized controlled study. Am Heart J. 2008;155:499.e491–499.e498
- Zhou X, Jin YZ, Wang Q, Min R, Zhang XY. Efficacy of high dose atorvastatin on preventing contrast induced nephropathy in patients underwent coronary angiography. Zhonghua Xin Xue Guan Bing Za Zhi. 2009;37:394–396
- Wang ZCH, Pan YD. Effect of atorvastatin on renal function in patients given contrast medium after interventional procedures. Med J Chin People's Armed Police Forces. 2009;20:919–922
- Toso A, Maioli M, Leoncini M, et al. Usefulness of atorvastatin (80 mg) in prevention of contrast-induced nephropathy in patients with chronic renal disease. Am J Cardiol. 2010;105:288–292
- Ozhan H, Erden I, Ordu S, et al. Efficacy of short-term high-dose atorvastatin for prevention of contrast-induced nephropathy in patients undergoing coronary angiography. Angiology 2010;61:711–714
- de Oliveira MSA-MK, Ribamar-Costa J Jr, Abizaid A, et al. Impact on renal function of rosuvastatin preload prior to elective percutaneous coronary intervention in chronic statin users. Rev Bras Cardiol Invasiva. 2012;20:303–308
- Acikel S, Muderrisoglu H, Yildirir A, et al. Prevention of contrast-induced impairment of renal function by short-term or long-term statin therapy in patients undergoing elective coronary angiography. Blood Coagul Fibrinolysis. 2010;21:750–757
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560
- Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–872
- Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118
- Chade AR, Zhu XY, Grande JP, Krier JD, Lerman A, Lerman LO. Simvastatin abates development of renal fibrosis in experimental renovascular disease. J Hypertens. 2008;26:1651–1660
- Geng Q, Ren J, Song J, Li S, Chen H. Meta-analysis of the effect of statins on renal function. Am J Cardiol. 2014;114:562–570
- Gami AS, Garovic VD. Contrast nephropathy after coronary angiography. Mayo Clin Proc. 2004;79:211–219
- Zhang T, Shen LH, Hu LH, He B. Statins for the prevention of contrast-induced nephropathy: A systematic review and meta-analysis. Am J Nephrol. 2011;33:344–351
Appendix
Searches: MEDLINE, EMBASE, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials Systematic Reviews and clinicaltrials.gov until May, 2014
exp hydroxymethylglutaryl-CoA Reductase Inhibitors/
hydroxymethylglutaryl-CoA Reductase Inhibitors$.mp
exp statin/
statin$.mp
simvastatin$.mp
pravastatin$.mp
lovastatin$.mp
atorvastatin$.mp
fluvastatin$.mp
pitavastatin$.mp
hydroxymethylglutaryl-coa reductase inhibitor$.mp
hmg coa reductase inhibitor$.mp
hydroxymethylglutaryl coenzyme a reductase inhibitor$.mp
hmg co a reductase inhibitor$.mp
mevinolin$.mp
pravachol$.mp
lipitor$.mp
zocor$.mp
mevacor$.mp
lescol$.mp
exp contrast nephropathy/
contrast nephropathy$.mp
exp contrast medium/
contrast medium$.mp.
exp contrast-induced nephropathy/
contrast-induced nephropathy$.mp.
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20
21 or 22 or 23 or 24 or 25 or 26
27 and 28
limit 30 to humans
limit 31 to randomized controlled trial