Abstract
This study was designed to investigate the protective effects of sitagliptin on renal damage induced by renal ischemia reperfusion (I/R) in rats. For this, rats were randomly divided into four groups (n = 8): (1) sham group, in which the rats only underwent right nephrectomy; (2) right nephrectomy and left kidney ischemia (1 h) and reperfusion (24 h) group (I/R); (3) 5 mg/kg sitagliptin administrated group, per-oral once a day for two weeks; (4) 5 mg/kg sitagliptin administrated group, per-oral once a day for two weeks before left kidney I/R (n = 8). Sitagliptin-treated rats that underwent renal I/R demonstrated significant decrease in the serum urea nitrogen and creatinine and also, lipid peroxidation, total oxidant status and malondialdehyde level in the renal tissue when compared to the renal I/R group. Additionally, reduced glutathione, glutathione peroxidase, superoxide dismutase, catalase and total antioxidative capacity were significantly increased after renal I/R in sitagliptin-treated rats. Our histopathological findings were in accordance with these biochemical results. In sum, in the current study all of our results indicated that sitagliptin treatment ameliorated renal damage induced by renal I/R in rats.
Introduction
Ischemic renal injury can occur as a result of renal transplantation,Citation1 partial nephrectomy,Citation2 revascularization of renal artery,Citation3 traumaCitation4 and hydronephrosisCitation5 among other reasons. Renal ischemia may result in many unwanted situations, especially hypertension and chronic renal failure.Citation6 Although restoration of blood flow is the only way to save renal tissue from eventual damage, reperfusion often exacerbates kidney dysfunction induced by reperfusion injury, a situation that is called the oxygen paradox. Clinical and experimental studies have provided evidence that ischemia-reperfusion (I/R) injury is mediated by reactive oxygen species (ROS) and nitric oxide (NO).Citation7–10 These free radicals can attack a wide variety of cellular components, including DNA, proteins and membrane lipids.Citation11
Glucagon-like peptide-1 (GLP-1) is secreted mainly by the entero-endocrine cells of the intestine in response to the presence of nutrients. Native GLP-1 has a short half-life of minutes, being rapidly degraded by dipeptidyl peptidase IV (DPP-IV). Therefore, to assess the roles of intact GLP-1, it is necessary to use of DPP-IV inhibitor for preventing its degradation. It facilitates glucose-induced insulin release and therefore analogs of GLP-1 are of potential interest as a possible approach for the treatment of diabetes.Citation12 Thus, the U.S. Food and Drug Administration approved sitagliptin, the first oral DPP-IV inhibitor for the treatment of type 2 diabetes on 17 October 2006.Citation13 Interestingly, recent data have suggested that GLP-1 can exert a direct cytoprotective effect via inhibition of apoptosis either directly in target cells expressing the GLP-1 receptor or possibly activation of survival factors.Citation14 Also, GLP-1 is one of the best studied incretin hormones,Citation15 exerts insulinotropic and insulinomimetic properties via the G-protein-coupled, GLP-1 receptor, which has been reported to be expressed in the renal tissue.Citation16 Recently, Bose et al.Citation17 reported that GLP-1 can directly protect the heart against I/R injury.
All of the investigations mentioned above encouraged us to plan this experimental design. Since clinical and experimental studies have pointed that renal I/R injury is mediated by free radical species; in the current experimental study, we focused to investigate possible antioxidative and protective effect of sitagliptin against ischemia-reperfusion-induced renal injury in light of biochemical and histopathological analyses.
Materials and methods
Animals and experimental protocol
This animal experimental study was designed according to the ARRIVE guidelines.Citation18 This study was approved by Animal Ethics Committee (Reference Number: 2012/A-31) and was conducted in accordance with the “Animal Welfare Act and the Guide for the Care and Use of Laboratory animals (NIH publication No. 5377-3, 1996), Animal Ethics Committee.”
Healthy, 32 Wistar albino female rats aged weighing 250–300 g were obtained from Inonu University Laboratory Animals Research Center and placed in a temperature (21 ± 2 °C) and humidity (60 ± 5%) controlled room in which a 12:12-hour light:dark cycle was maintained. The rats were fed standard commercial pellets and water ad libitum. The rats were randomly divided into four groups: (1) sham group, in which the rats only underwent right nephrectomy (n = 8); (2) right nephrectomy and left kidney ischemia (1 h) and reperfusion (24 h) group (I/R) (n = 8); (3) 5 mg/kg sitagliptin administrated orally once a day for two weeks (n = 8); (4) 5 mg/kg sitagliptin administrated orally once a day for two weeks before I/R (n = 8). The dosage and treatment time of sitagliptin were selected according to the levels used successfully in the renal I/R model. In this study, authors tried to solve apart from our investigation, the probing effects of sitagliptin against renal I/R by using diabetic rats.Citation19
Surgical procedure
The rats were anesthetized with ketamine (75 mg/kg) and xylazine (8 mg/kg) administrated intraperitoneally (ip) and for blood glucose measurements, blood samples were obtained from the tail vein of each rat. Plasma glucose levels were measured before the operation by the glucose oxidase method using a commercial strip (Glucometer Elite, Bayer, Germany). All rats were performed right nephrectomy through their right dorso-lateral incisions and the left renal artery and vein were isolated for 30 min with no further surgical intervention to allow for circulatory readjustment after the right nephrectomy; then, the left renal vessels were occluded for 1 h. Following this procedure, left kidney reperfusion was achieved during 24 h reperfusion by removing the clamp. Both right nephrectomy model and duration of renal I/R were selected according to our previous renal I/R studies in rat.Citation20,Citation21
Sham-operated (first group) and sitagliptin-administrated group (third group) animals only underwent right nephrectomy. I/R groups (second and fourth groups) both underwent right nephrectomy and I/R procedure. After controlling the bleeding, the skin and skin textures were sutured again. At the end of the surgical procedure, all rats were sacrificed with high doses of the anesthesia mixture and the kidneys were quickly removed, decapsulated and placed into liquid nitrogen and stored at −70 °C until assayed for malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), reduced glutathione (GSH), total antioxidant capacity (TAC) and total oxidant status (TOS). Also, trunk blood was extracted to evaluate serum levels of blood urea nitrogen (BUN) and creatinine (Cr) using an Olympus Autoanalyzer (Olympus Instruments, Tokyo, Japan).
Biochemical determination
The MDA contents of homogenates were determined spectrophotometrically by measuring the presence of thiobarbituric acid reactive substances (TBARS).Citation22 Three milliliters of 1% phosphoric acid and 1 mL 0.6% thiobarbituric acid solution were added to 0.5 mL of plasma pipetted into a tube. The mixture was heated in boiling water for 45 min. After the mixture cooled, the color was extracted into 4 mL of n-butanol. The absorbance was measured by a spectrophotometer (UV-1601; Shimadzu, Kyoto, Japan) at 532 nm. The amount of lipid peroxides was calculated as TBARS of lipid peroxidation. The results are expressed in nmol/g tissue.
Total (Cu-Zn and Mn) SOD (EC 1.15.1.1) activity was determined according to the method of Sun et al.Citation23 The principle of this method is the inhibition of nitroblue tetrazolium reduction by the xanthine-xanthine oxidase system as a superoxide generator. One unit of SOD was defined as the enzyme amount causing 50% inhibition in the nitroblue tetrazolium reduction rate. SOD activity was expressed as units per milligram protein (U/g protein).
CAT (EC 1.11.1.6) activity was determined according to Aebi’s method.Citation24 The principle of the assay is based on the determination of the rate constant (k, s−1) or the H2O2 decomposition rate at 240 nm. Results were expressed as k per gram protein (k/g protein).
Determination of GPx activity (EC 1.6.4.2) was measured by the method of Paglia and Valentine.Citation25 An enzymatic reaction in a tube containing NADPH, GSH, sodium azide and glutathione reductase was initiated by the addition of H2O2, and the change in absorbance at 340 nm was monitored by a spectrophotometer. The results were expressed in units per gram protein (U/mg protein).
The GSH content in the lung tissue as non-protein sulfhydryl was analyzed following a previously described method.Citation26 Aliquots of tissue homogenate were mixed with distilled water and 50% trichloro-acetic acid in glass tubes and centrifuged at 3000 rpm for 15 min. The supernatants were mixed with Tris buffer (0.4 M, pH 8.9) and 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB, 0.01 M) was added. After shaking the reaction mixture, its absorbance was measured at 412 nm within 5 min of the addition of DTNB against blank with no homogenate. The absorbance values were extrapolated from a glutathione standard curve and expressed as GSH (µmol/g tissue).
Measurement of TAC
Total antioxidant capacity levels were determined using a novel automated colorimetric measurement method developed by Erel.Citation27 In this method, the hydroxyl radical, the most potent biological radical, is produced by the Fenton reaction and reacts with the colorless substrate O-dianisidine to produce the dianisyl radical, which is bright yellowish-brown in color. Upon the addition of sample, the oxidative reactions initiated by the hydroxyl radicals present in the reaction mix are suppressed by the antioxidant components of the sample, preventing the color change and thereby providing an effective measure of the total antioxidant capacity of the sample. The assay has excellent precision values, which are lower than 3%. The results were expressed as mmol Trolox equivalent/L.
Measurement of TOS
TOS was determined using a novel automated measurement method, developed by Erel.Citation28 Oxidants present in the sample oxidize the ferrous ion–O-dianisidine complex to ferric ion. The oxidation reaction is enhanced by glycerol molecules, which are abundantly present in the reaction medium. The ferric ion makes a colored complex with xylenol orange in an acidic medium. The color intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. The assay was calibrated with hydrogen peroxide and the results were expressed in terms of mmol H2O2 equivalent/L.
Measurement of oxidative stress index
The percentage ratio of the TOS to TAC yields the oxidative stress index (OSI), an indicator of the degree of oxidative stress. The OSI value for the renal samples was also calculated as OSI (arbitrary unit) = TOS (mmol H2O2 equivalent/g protein)/TAC (mmol Trolox equivalent/g protein).
Renal histopathology
After decapitation of rats, kidney tissue samples were placed in 10% neutral buffered formalin solution and processed routinely by embedding in paraffin. Five-micrometer thick sections were cut, deparaffinized, hydrated and stained with hematoxylin and eosin (H&E). The renal sections from all treatments were examined in blind fashion for tubular cell swelling, cellular vacuolization, pyknotic nuclei, medullary congestion and moderate-to-severe necrosis. All area of each kidney slide were examined and a score from 0 to 3 was given for each tubular profile involving an intersection: 0, normal histology; 1, tubular cell swelling, brush border loss, nuclear condensation, with up to one-third of tubular profile showing nuclear loss; 2, as for score 1, but greater than one-third and less than two-thirds of tubular profile showing nuclear loss; and 3, greater than two-thirds of tubular profile shows nuclear loss. There was also shrinkage of nearly all the glomeruli with enlargement of Bowman’s space in the score 3.Citation29,Citation30
Statistical analysis
For detecting even minor effects, the required sample sizes used in this experiment were identified using statistical power analysis. The sample sizes necessary for a power of 0.80 were estimated using NCSS software (Kaysville, UT). All data were analyzed with a commercially available statistics software package (SPSS for Windows v. 15.0, Chicago, IL). Distributions of the groups were analyzed with the Kolmogorov-Smirnov test. As all groups showed a normal distribution except GPx, parametric statistical methods were used to analyze the data. After a significant Kruskal–Wallis H-test, a Conover test was also performed for GPx results. The values are given as median (min–max). For other parameters, a one-way ANOVA was performed with Tukey’s post-hoc test. Results were presented as mean ± SD. p < 0.05 was regarded as statistically significant in all data.
Results
Blood glucose levels and kidney weight
As shown in , the kidney weights of the I/R rats were significantly higher than the sham group, whereas sitagliptin-treated group reduced the kidney weight significantly. Because of the sitagliptin pharmacological features, the serum glucose levels of sitagliptin-treated group rats were significantly lower than the other groups.
Table 1. The serum levels of glucose and body kidney weight.
Effect of sitagliptin on antioxidant and oxidative stress status
As shown in , renal tissue MDA levels were found to be significantly higher in the renal I/R group (172.46 ± 56.74 vs. 102.72 ± 19.32 nmol/g tissue), whereas the activities of SOD and CAT were lower when compared to the sham group (0.59 ± 0.09 vs. 0.91 ± 0.17 U/g protein; 139.40 ± 35.04 vs. 184.33 ± 24.10 k/g protein, respectively). Sitagliptin treatment reduced the level of end product of lipid peroxidation, MDA (117.78 ± 18.21 nmol/g tissue), and elevated the SOD and CAT enzyme activities (0.81 ± 0.13 U/g protein and 159.25 ± 21.37 k/g protein, respectively). Also, according to the sham group, I/R led to a reduction in GPx and GSH levels [0.77 (0.65–1.08) vs. 1.18 (1.16–1.66) U/mg protein; 3.10 ± 0.56 vs. 4.09 ± 0.64 µmol/g tissue, respectively] in the kidney tissue. Sitagliptin-treated group elevated the GPx and GSH levels [1.07 (0.95–1.30) U/mg protein and 4.08 ± 0.57 µmol/g tissue, respectively).
Table 2. The levels of MDA‚ SOD‚ CAT, GSH and GPx in kidney tissue.
Ischemia reperfusion resulted due to elevated TOS levels in the renal tissue when compared to the sham group whereas sitagliptin treatment augmented the TOS production. Also, the results of OSI levels were in accordance with the TOS levels. In the TAC levels, there were no statistical differences among the groups ().
Table 3. The levels of TOS, TAC and OSI in kidney tissue.
Effect of sitagliptin on the serum parameters
As shown in , the serum levels of BUN and Cr were significantly higher in the I/R group (161 ± 18.24 and 2.35 ± 0.574 mg/dL, respectively) when compared to the sham group (32.62 ± 3.38 and 0.58 ± 0.021 mg/dL, respectively). Although I/R + sitagliptin treatment slightly reduced the elevated BUN and Cr levels, however, it did not reach to the statistically significant level.
Table 4. The serum levels of BUN and Cr.
Effect of sitagliptin on renal histology
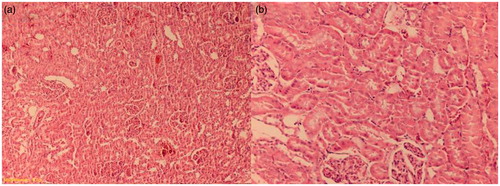
There was a normal kidney histological appearance in the sham and sitagliptin-treated groups (). Renal I/R group had a score 3 of tubular damage. Also, there were a lot of glomeruli with an enlargement of Bowman’s space in this group (). Tubular damage was clearly decreased in the sitagliptin-treated group (). The renal score of the groups are presented in .
Figure 2. (a) Frank tubular necrosis, edema and increase of Bowman’s space (I/R group). (b) The large area of tubular necrosis (I/R group). H&E × 100.
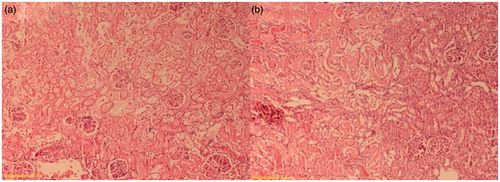
Figure 3. Decreased tubular necrosis, with desquamations of tubular epithelium (sitagliptin-treated group). H&E × 200.
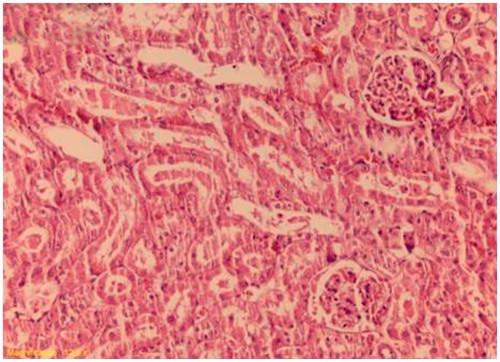
Table 5. Renal histopathologic score.
Discussion
Renal I/R injury occurs both intracellular injury and inflammatory reactions, which are related to each other.Citation31 Sepsis, shock, hypotension,Citation32 some renal surgeries, including renal stone surgery, partial nephrectomy, renal transplantation, and renal artery revascularization, or cardiothoracic surgeryCitation33,Citation34 can cause renal ischemia and these states are the beginning of the injury process and begin with a reduction of energy production in the mitochondria. Therefore, cell membrane permeability is increased due to the cellular ion imbalance, increase of activity of proteases and phospholipases existence. Depending on the long ischemic period, a necrotic type of cell death can occur.Citation31 During reperfusion, the provided molecular oxygen is consumed as an electron acceptor and used for the formation of two superoxide anions, hydrogen peroxide and the hydroxyl radical which are believed to cause the actual injury induced by I/R.Citation35 Indeed, clinical and experimental studies have shown that I/R injury is mediated by ROS.Citation36–38 It has been demonstrated that ROS-mediated cellular damage occurs when oxygen is supplied to the tissue by reperfusion and tissue detoxification capacity is deficient for ROS formation.Citation39 Hence, oxidation of cell protein and membrane lipid peroxidation causes cell death and damage of DNA helix.Citation31,Citation40,Citation41 Similarly, reperfusion period initiates inflammatory response and actualizes irreversible tissue damage.Citation40,Citation42 Research's efforts have focused on ameliorating renal I/R injury by the pharmacological inhibition of free radical injury. Since more recently, Bose et al.Citation17 reported that GLP-1 can directly protect the heart against I/R injury, in the present study, we focused on whether to test the protective effect of sitagliptin in renal I/R injury.
Sitagliptin is a potentially identical antidiabetic agent.Citation43,Citation44 Beside to its incretin action, GLP-1 has also reduced pancreatic B-cell apoptosis.Citation44,Citation45 GLP-1 is mediated by the pro-survival kinases PI3K/Akt, p42/44 PKA and P70s6; in that, treatment caused an increase in expression of active caspase 3 was reduced. It has also been reported that GLP-1 receptor mimetics can protect I/R injury. In the available literatures about GLP-1 and sitagliptin, the effects on the renal I/R injury are really limited. Vaghasiya et al.Citation19 showed that sitagliptin ameliorated renal damage induced by renal I/R in diabetic rats. However, in the mentioned article, the effects of sitagliptin against renal I/R injury in non-diabetic rats were not investigated. Also, Wang et al.Citation46 reported that DPP-4 inhibitor attenuates toxic effects of indoxyl sulfate on kidney tubular cells. Joo et al.Citation47 reported that DPP-4 IV inhibitor attenuates kidney injury in rat remnant kidney. However, according to our knowledge, there is no study regarding the pure effect of sitagliptin on the renal I/R injury.
As shown in , sitagliptin treatment in the I/R group (group 4) reduced serum glucose level, whereas in group 3, sitagliptin administrated to normal animals, the serum glucose level was found to be high. In fact, we did not find any explanation and come across any literature regarding this unexpected result. We thought that may be a beneficial clue for the readers associated with further studies. Further, molecular and mechanistic investigations are needed to explore this result. May be renal I/R triggers the production of some mediators, which interact with dipeptidyl peptidase IV and therefore, sitagliptin treatment exerts beneficial effects in this group apart from normal rats.
In our study, we found that I/R caused an increase in MDA and a decrease in SOD, CAT activity and GSH levels in the renal tissue. However, administering of 5 mg/kg oral sitagliptin to the I/R group reduced the MDA levels, while increasing the CAT, SOD activity and GSH contents. One of the main consequences of I/R injury is lipid peroxidation. In this way, lipid peroxidation is the main pathway of oxidative stress, regardless of the source of free radicals. MDA is a major end product of lipid peroxidation and is used for monitoring cellular lipid damage.Citation48 Possibly, blocking this pathway is an effective strategy to prevent ROS-mediated kidney damage.Citation49 SOD, GSH and CAT are the antioxidant enzymes, which are the defence mechanism against ROS activities. Unfortunately, antioxidant enzyme levels reduce on the renal I/R injury.Citation50,Citation51 At the same time, it was shown that ischemia has potency to increase mRNA level of CAT and SOD, whereas, reperfusion decreases mRNA level of CAT. On the other hand, manganese-SOD remains high during reperfusion.Citation52 Also, the elevation in the level of SOD in renal I/R injury was reported in other studies.Citation53,Citation54 In the current study, sitagliptin treatment tended to increase SOD activity and GSH levels. These effects were statistically significant.
Our other important biochemical finding is that I/R caused an elevation in TOS, a novel index oxidative stress, in the renal tissue when compared to the sham group. Sitagliptin treatment showed beneficial effects on TOS production. Also, the result of OSI, which is a novel and very important index of oxidative stress, were in accordance with the TOS levels. However, there were no statistical differences among the groups for TAC levels, (). These findings seem to be related to ROS production and imbalance between oxidant and antioxidant status in the renal tissue.
Herein, renal I/R caused elevation of BUN and Cr levels in serum when compared to the sham group. I/R+sitagliptin treatment did not reduce the elevated BUN and Cr levels (). Elevation of serum BUN and Cr levels is a sign of deterioration of glomerular function, which causes kidney failure over time.Citation52 Recently, it has been reported that for humans, serum Cr, in association with certain other clinical characteristics, may be a more accurate measure of GFR than Cr clearance. This impairment in glomerular function was accompanied by an increase in BUN. In the earlier phases of kidney disease, serum Cr concentration is more significant than the BUN level. On the other hand, in the earlier phases of kidney diseases, BUN level begins to rise only after a marked renal parenchymal injury occurs. These findings are compatible with the ischemic lesion related to renal damage.Citation55
We also observed the kidney weights of the I/R rats were significantly higher than those of the control group. Although, sitagliptin-treated group reduced kidney weight significantly, it could not come close to the level of the control group. Also, we evaluated histopathologic changes in the rat kidneys in terms of histological damage. Many studies have shown that renal I/R injury causes changes in the renal blood flow due to the microvascular injury and impaired renal vascular reactivity, and as a consequence, infiltration of inflammatory cells, tubular epithelial cell injury, glomerular damage, apoptosis and fibrosis occur.Citation56 In accordance with earlier studies in the current study, I/R caused frank tubular necrosis, a lot of glomeruli with an enlargement of Bowman’s space and edema in the renal tissue. In the current study, we showed that the sitagliptin-treated group is significantly ameliorated this histological damage.
As conclusion, according to our biochemical and histopathological results, sitagliptin, an oral DPP-IV inhibitor, showed a protective effect against I/R-induced renal injury at least via its antioxidant and free radical scavenger effects in rats. Our results are in accordance with earlier reports mentioning that GLP-1 receptor which is necessary to use DPP-IV inhibitor exerts antiapoptotic and antioxidant properties, such as in I/R injury. In the current study, we focused especially on the antioxidant properties of sitagliptin against I/R-induced renal injury apart from detailed mechanistic distinguish. However, further experimental and clinical studies are required to confirm these findings before conducting clinical applications for treating renal I/R injury.
Declaration of interest
The authors have no conflicts of interest to disclose.
This study was supported by a grant from The Scientific and Technological Research Council of Turkey (project number: 2209/A-2012, belongs to Ms Nuransoy).
References
- Lin Y, Manning PT, Jia J, et al. CD47 blockade reduces ischemia-reperfusion injury and improves outcomes in a rat kidney transplant model. Transplantation. 2014;98(4):394–401
- Gao Y, Chen L, Ning Y, et al. Hydro-Jet-assisted laparoscopic partial nephrectomy with no renal arterial clamping: A preliminary study in a single center. Int Urol Nephrol. 2014;46(7):1289–1293
- Landry GJ, Lau IH, Liem T, et al. Adjunctive renal artery revascularization during juxtarenal and suprarenal abdominal aortic aneurysm repairs. Am J Surg. 2010;199:641–645
- Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142
- Stravodimos KG, Koritsiadis G, Lazaris AC, et al. Hydronephrosis promotes expression of hypoxia-inducible factor 1 alpha. Urol Int. 2009;82(1):38–42
- Yamamoto S, Hagiwara S, Hidaka S, et al. The anti-oxidant EPC-K1 attenuates renal ischemia-reperfusion injury in a rat model. Am J Nephrol. 2011;33:485–490
- Mohamed Ael H, Lasheen NN. Comparative study on the protective role of vitamin C and L-arginine in experimental renal ischemia reperfusion in adult rats. Int J Physiol Pathophysiol Pharmacol. 2014;6(3):153–165
- Paller MS. Acute renal failure: Controversies, clinical trials, and future. Semin Nephrol. 1998;18:482
- Akcetin Z, Busch A, Kessler G, et al. Evidence for only a moderate lipid peroxidation during ischemia-reperfusion of rat kidney due to its high antioxidative capacity. Urol Res. 1999;27:280–284
- Defraigne JO, Detry O, Pincemail J, et al. Direct evidence of free radical production after ischemia and reperfusion and protective effect of desferrioxamine: ESR and vitamin E studies. Eur J Vasc Surg. 1994;8:537–543
- Reiter RJ, Guerrero JM, Garcia JJ, et al. Reactive oxygen intermediates molecular damage and aging. Relation to melatonin. Ann NY Acad Sci. 1998;854:410–424
- Deacon CF, Hughes TE, Holst JJ, et al. Dipeptidyl peptidase IV inhibition potentiates the insulinotropic effect of glucagon-like peptide 1 in the anesthetized pig. Diabetes. 1998;47:764–769
- White JR. Dipeptidyl peptidase-IV inhibitors: Pharmacological profile and clinical use. Clin Diabetes. 2008;26(2):53–57
- Redondo A, Trigo MV, Acitores A, et al. Cell signalling of the GLP-1 action in rat liver. Mol Cell Endocrinol. 2003;204:43–50
- Wei Y, Mojsov S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: Brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 1995;358:219–224
- Vila Petroff MG, Egan JM, Wang X, et al. Glucagon-like peptide-1 increases cAMP but fails to augment contraction in adult rat cardiac myocytes. Circ Res. 2001;89:445–452
- Bose AK, Mocanu MM, Carr RD, et al. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–151
- Colak C, Parlakpinar H. Animals in research: reporting in vivo experiments: ARRIVE guidelines – Review. J Turgut Ozal Med Cent. 2012;19(2):128–131
- Vaghasiya J, Sheth N, Bhalodia Y, et al. Sitagliptin protects renal ischemia reperfusion induced renal damage in diabetes. Regul Pept. 2011;166(1–3):48–54
- Tasdemir C, Tasdemir S, Vardi N, et al. Protective effect of infliximab on ischemia/reperfusion-induced damage in rat kidney. Renal Failure. 2012;34(9):1144–1149
- Altintas R, Parlakpinar H, Beytur A, et al. Protective effect of dexpanthenol on ischemia-reperfusion-induced renal injury in rats. Kidney Blood Press Res. 2012;36(1):220–230
- Uchiyama M, Mihara M. Determination of MDA precursor in tissue by TBA test. Anal Biochem. 1978;36:271–278
- Sun USY, Oberley LW, Li Y, et al. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500
- Aebi H. Catalase. In: Bergmeyer HU, ed. Methods of Enzymatic Analysis. New York: Academic Press; 1974:673–677
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–170
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77
- Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–285
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111
- Karaman A, Turkmen E, Gursul C, et al. Prevention of renal ischemia/reperfusion-induced injury in rats by leflunomide. Int J Urol. 2006;13(11):1434–1441
- Altintas R, Polat A, Vardi N, et al. The protective effects of apocynin on kidney damage caused by renal ischemia/reperfusion. Exp Endourolog. 2013;27:617–624
- de Groot H, Rauen U. Ischemia-reperfusion injury: Processes in pathogenetic networks: A review. Transplant Proc. 2007;39:481–484
- Sheridan AM, Bonventre JV. Pathophysiology of ischemic acute renal failure. Contrib Nephrol. 2001;32:7–21
- Chatterjee PK. Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: A comprehensive review. Naunyn Schmiedebergs Arch Pharmacol. 2007;376:1–43
- Landry GJ, Lau IH, Liem T, et al. Adjunctive renal artery revascularization during juxta renal and suprarenal abdominal aortic aneurysm repairs. Am J Surg. 2010;199:641–645
- Holmberg SR, Cumming DV, Kusoma Y, et al. Reactive oxygen species modify the structure and function of the cardiac sarcoplasmic reticulum calcium-release channel. Cardioscience. 1991;2:19–25
- Kurcer Z, Parlakpinar H, Vardi N, et al. Protective effects of chronic melatonin treatment against renal ischemia/reperfusion injury in streptozotocin-induced diabetic rats. Exp Clin Endocrinol Diabetes. 2007;115(6):365–371
- Weight SC, Bell PRF, Nicholson ML, et al. Renal ischemia-reperfusion injury. Br J Surg. 1996;83:162–170
- Locatelli F, Pozzoni P, Del Vecchio L, et al. Renal replacement therapy in patients with diabetes and end-stage renal disease. J Am Soc Nephrol. 2004;15:S25–S29
- Erdogan H, Fadillioglu E, Yagmurca M, et al. Protein oxidation and lipid peroxidation after renal ischemia reperfusion injury: Protective effects of erdosteine and N-acetylcysteine. Urol Res. 2006;34:41–46
- Hayes JD, McLellan LI. Glutathione and glutathione dependent enzymes represent a coordinately regulated defence against oxidative stress. Free Radic Res. 1999;31:273–300
- Lien YH, Lai LW, Silva AL, et al. Pathogenesis of renal ischemia/reperfusion injury: Lessons from knockout mice. Life Sci. 2003;74:543–552
- Filho DW, Torres MA, Bordin AL, et al. Spermatic cord torsion, reactive oxygen and nitrogen species and ischemia-reperfusion injury. Mol Aspects Med. 2004;25:199–210
- Nauck MA Heimesaat MM, Behle K, et al. Effects of glucagon-like-peptide1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87:1239–1246
- Urosova IA, Farilla L, Hui H, et al. GLP-1 inhibition of pancreatic islet cell apoptosis. Trends Endocrininol Metab. 2004;15:27–33
- Li Y, Hansotia T, Yusta B, et al. Glucagon-like-peptide 1 receptor signaling modulates beta cell apoptosis. J Biol Chem. 2003;278:471–478
- Wang WJ, Chang CH, Sun MF, et al. DPP-4 Inhibitor attenuates toxic effects of indoxyl sulfate on kidney tubular cells. PLoS One. 2014;9(4):e93447
- Joo KW, Kim S, Ahn S, et al. Dipeptidyl peptidase IV inhibitor attenuates kidney injury in rat remnant kidney. BMC Nephrology. 2013;14:98
- Ozdemir R, Parlakpinar H, Polat A, et al. Selective endothelin A (ET A) receptor antagonist (BQ 123) reduces both myocardial infarct size and oxidant injury. Toxicology. 2006;219:142–149
- Sahna E, Parlakpinar H, Ozturk F, et al. The protective effects of physiological and pharmacological concentrations of melatonin on renal ischemia-reperfusion injury in rats. Urol Res. 2003;3:188–193
- Parlakpinar H, Olmez E, Acet A, et al. Beneficial effects of apricot-feeding on myocardial ischemia-reperfusion injury in rats. Food Chem Toxicol. 2009;49:802–808
- Walker EM, Gale GR. Methods of reduction of cisplatin nephrotoxicity. Ann Clin Lab Sci. 1981;11:397--410
- Singh I, Gulati S, Orak JK, et al. Expression of antioxidant enzymes in rat kidney during ischemia-reperfusion injury. Mol Cell Biochem. 1993;125:97–104
- Erdogan H, Fadillioglu E, Yagmurca M, et al. Protein oxidation and lipid peroxidation after renal ischemia-reperfusion injury: Protective effects of erdosteine and N-acetylcysteine. Urol Res. 2006;34:41–46
- Tenorio-Velázquez VM, Barrera D, Franco M, et al. Hypothyroidism attenuates protein tyrosine nitration, oxidative stress and renal damage induced by ischemia and reperfusion: Effect unrelated to antioxidant enzymes activities. BMC Nephrol. 2005;6:12
- Parlakpinar H, Tasdemir S, Polat A, et al. Protective role of caffeic acid phenethyl ester (CAPE) on gentamicin-induced acute renal toxicity in rats. Toxicology. 2005;207:169–177
- Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–2210