Abstract
Methotrexate (MTX) is widely used in the treatment of various malignancies and nononcological diseases but its use has been limited by its nephrotoxicity. Silymarin (SLY), a natural flavonoid, has been reported to have antioxidant, anti-inflammatory and anti-apoptotic effects. This study was carried out to determine whether SLY exerts a protective effect against MTX-induced nephrotoxicity. Rats were divided into six groups: Group 1 (saline, i.p., single injection), Group 2 (0.5% carboxymethyl cellulose (CMC), by gavage once daily for five consecutive days), Group 3 (SLY, 300 mg/kg per day, i.p. for five consecutive days), Group 4 (MTX, 20 mg/kg, i.p., single injection), Group 5 (MTX + CMC similarly as groups 2 and 4) and Group 6 (MTX + CMC + SLY similarly as groups 2, 3 and 4). Histopathologic alterations including apoptotic changes of the kidney were evaluated. MTX injection exhibited dilated Bowman’s space, inflammatory cell infiltration, glomerular and peritubular vascular congestion and swelling of renal tubular epithelium cells. Apoptotic cell death was also markedly increased in renal tubules after MTX administration. SLY treatment resulted in statistically significant amelioration in the histological alterations and reduced the number of TUNEL-positive cells as compared with the MTX treated rats (p < 0.05). In conclusion, SLY treatment leads to a reduction on MTX-induced renal damage in rats. Since SLY is safe and acceptable for human consumption, further studies to define the exact mechanism of the protecting effect of SLY on MTX-induced nephrotoxicity and the optimum dosage of this compound would be useful.
Introduction
Methotrexate (MTX), a cytotoxic chemotherapeutic agent, is widely used to treat certain types of malignancies such as leukemia,Citation1 lymphoma,Citation2 breast cancer,Citation3 osteosarcoma,Citation4 head and neck cancers.Citation5 It is also used in the treatment of non-cancerous diseases, including rheumatoid arthritis,Citation6 psoriasisCitation7 and inflammatory bowel diseases.Citation8 Since more than 90% of MTX is excreted unchanged via the kidneys,Citation9 MTX treatment, particularly at high doses, may cause renal failure.Citation4 The MTX-induced nephrotoxicity is believed to be mediated by two primary mechanisms.Citation10 The first is MTX-induced crystal nephropathy, which occurs when MTX and its metabolites precipitate within the renal tubules.Citation11,Citation12 The second mechanism of MTX-induced renal injury is direct tubular toxicity; MTX causes the reactive oxygen radical overproduction in the kidney.Citation10,Citation13,Citation14 Several studies have revealed that MTX administration causes increased malondialdehyde (MDA) levels and myeloperoxidase (MPO) activity and decreased catalase activity, glutathione (GSH) levels and superoxide dismutase activity in the blood and kidney.Citation15–17 It has recently been reported that in addition to oxidative stress, abnormal generation of inflammatory mediators and neutrophil infiltration contribute to MTX-induced renal damage.Citation16
Silymarin (SLY) is a purified flavonoid extract isolated from the seeds of Silybum marinum (milk thistle).Citation18 SLY has antioxidative, anti-inflammatory, immunomodulatory and liver-regenerating properties.Citation19 It is acceptable for human consumption and widely used as a hepatoprotection and as a supportive therapy of liver disorders for more than three decades.Citation20 SLY has also hypolipidemic propertiesCitation21 and inhibitory effect on low density lipoprotein (LDL) oxidation in vitroCitation22 indicating it may be beneficial on the prevention of atherosclerosis. SLY has also antiviral properties; it has been shown that SLY displays both prophylactic and therapeutic effects against hepatitis C virus infection.Citation23 Anticarcinogenic effects of SLY have been shown in human breast cancer cells.Citation24 SLY has also antiulcer activity against experimentally-induced peptic ulcersCitation25 and induces pancreatic function recovery after alloxan induced pancreatic damage in rats.Citation26 SLY also exerts protective effects against nephrotoxicity induced by cisplatin,Citation27 adriamycinCitation28 and gentamicin.Citation29 However, as far as we know there are no reports on the estimation of the effects of silymarin against MTX-induced renal injury. Therefore, the present study aimed to evaluate the scavenging activity of SLY on MTX-induced nephrotoxicity in rat model.
Materials and methods
Experimental design
The study protocol was approved by the local ethics committee of the Firat University, Elazig, Turkey. Experiments involving the animals were conducted according to the policy of the European convention for the protection of vertebrate animals used for experimental and other scientific purposes in accordance with “Recommendations on the Establishment of Animal Experimental Guidelines”, and ethical procedures were conducted under Reduction, Replacement and Refinement. The study was carried out at the Experimental Research Unit of Firat University (FUDAM). Thirty six male Sprague–Dawley rats aged 8 weeks, weighing between 180 and 200 g were used in this study. Rats were fed with standard pellets and tap water, and 12 h light and dark cycles, standard temperature and humidity conditions were maintained. The rats were randomly divided into six groups, consisting of six animals per group. During 5 days of experimental stage, the rats treated as follows:
Group 1 (Control): Rats received saline intraperitoneally (i.p.) as a single dose on the first day and served as a control.
Group 2 (CMC): Rats received 0.5% carboxymethylcellulose (CMC) (Sigma, St. Louis, MO) by gavage daily for 5 days.
Group 3 (SLY): Rat received SLY (Sigma) (300 mg/kg per day, i.p.) for 5 days.
Group 4 (MTX): Rats received MTX at the dose of 20 mg/kg, i.p. as a single dose on the first day.
Group 5 (MTX + CMC): Rats received MTX at the dose of 20 mg/kg, i.p. as a single dose on the first day and then received 0.5% CMC by gavage daily for 5 days.
Group 6 (MTX + CMC + SLY): Rats received MTX at the dose of 20 mg/kg, i.p. as a single dose on the first day and then received SLY (300 mg/kg per day, i.p.) in 0.5% CMC by gavage for 5 days.
At the end of the experiment, the rats were decapitated under xylazine (10 mg/kg)–ketamine (75 mg/kg) anesthesia. Kidney tissues were removed and fixed with 10% neutralized formalin solution for histological examination and TUNEL assay.
Histological evaluation
Formalin-fixed tissues were embedded in paraffin blocks and the blocks were cut into 5 -μm thick sections, deparaffinized and hydrated in descending series of ethyl alcohol and distilled water and then stained with hematoxylin and eosin (H&E), dehydrated, cleared in xylene and mounted using Canada balsam. The slides were then examined using (NovelN-800 M, Ningbo, China) light microscope and microscopic scoring was done by an observer who was blinded to the treatment condition. Severity of kidney injury was semiquantitatively evaluated using the following alterations: (a) vascular congestion in glomerular and peritubular area, (b) inflammatory cell infiltration, (c) dilatation of Bowman’s space and (d) swelling of renal tubular epithelium cells. Scores were given as 0 = absent, 1 = weak, 2 = moderate and 3 = strong for each criteria. The microscopic score of each tissue was calculated as the sum of the scores given to each criterion, and at least five microscopic areas were examined under a 20× objective to score each specimen.
TUNEL assay
Parafin blocks were sectioned 5 µm and the sections were taken to slides with poly-l-lysine. ApopTag plus Peroxidase In Situ Apoptosis Detection Kit (Chemicon, Cat no: S7101, USA) was used for detection of apoptotic cells. According to the instructions of the manufacturer sections were deparaffinized in xylene, dehydrated through graded alcohol and washed in PBS. Tissues were incubated in a 0.05% proteinase K solution for 10 min. Then tissues were incubated with 3% hydrogen peroxide for five minutes to prevent endogenous peroxidase activity. Later washing with PBS, the tissues were placed in equilibration buffer for six minutes and in working solution (70% reaction buffer plus 30% TdT enzyme) at 37 °C under moist conditions for one hour. Stop/wash buffer were applied during for 10 min and then anti-digoxigenin-peroxidase for 30 min. Diaminobenzidine (DAB) substrate was used to show apoptotic cells. Cross-sections contrast-stained with Harrishematoxylin was sealed using a proper covering solution. Preparations were observed and evaluated using a research microscope (NovelN-800 M, Ningbo, China) and photographed. Cells with blue nuclei after TUNEL staining using Harrishematoxylin were considered normal, whereas cells with brown nuclei were considered apoptotic. At least 500 cells were counted on each field and the apoptotic index was calculated as a ratio of the TUNEL-positive cell number to the total cell number.
Statistical analyses
Results were expressed as the means ± standard error of the mean. Statistical significant difference was determined by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. Probability values (p) less than 0.05 were considered to be statistically significant. All analyses were performed using SPSS version 22.0 software.
Results
Renal histology
The results of the histological scoring of the renal tissue are shown in . Microscopic examination of kidney sections of control group showed normal morphology (). There were no differences in the CMC () and SLY () groups when compared with the control group (p > 0.05). MTX group revealed dilated Bowman’s space (), inflammatory cell infiltration (), glomerular and peritubular vascular congestion () and swelling of renal tubular epithelium cells () (p < 0.05). The findings of MTX + CMC group were similar to those of MTX group including dilatation of Bowman’s space (), inflammatory cell infiltration (), glomerular and peritubular vascular congestion () and swelling of renal tubular epithelium cells () (p > 0.05). SLY treatment resulted in significant amelioration of dilated Bowman’s space (), inflammatory cell infiltration (), glomerular and peritubular vascular congestion () and swelling of renal tubular epithelium cells () in the MTX + CMC + SLY group compared with MTX group (p < 0.05).
Figure 1. The histological scores of all the groups. Values are mean ± SD for six rats in each group. (a,b,c): Values with common superscripts are not statistically different, whereas values without common superscripts are statistically significantly different (p value <0.05).
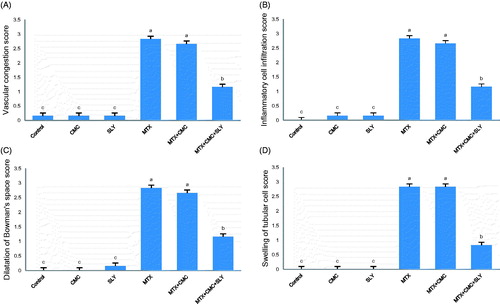
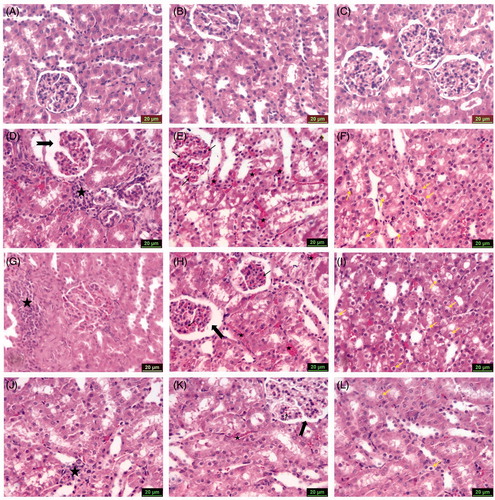
Figure 2. Photomicrographs of kidney sections stained with hematoxylin and eosin (scale bars = 20 μm), showing: (A) Group 1 (control), (B) Group 2 (CMC) and (C) Group 3 (SLY) show similarly undamaged kidney; (D) Group 4 (MTX) dilatation of Bowman’s space (arrow) and inflammatory cell infiltration (asterisk); (E) Group 4 (MTX) glomerular (arrows) and peritubular (asterisks) vascular congestion; (F) Group 4 (MTX) swelling of renal tubular epithelium cells (arrows); (G) Group 5 (MTX + CMC) inflammatory cell infiltration (asterisk); (H) Group 5 (MTX + CMC) dilatation of Bowman’s space (thick arrow) and glomerular (thin arrow), peritubular (asterisks) vascular congestion; (I) Group 5 (MTX + CMC) swelling of renal tubular epithelium cells (arrows); (J) Group 6 (MTX + CMC + SLY) inflammatory cell infiltration (asterisk); (K) Group 6 (MTX + CMC + SLY) glomerular (thin arrow) and peritubular (asterisk) vascular congestion, Bowman’s space (thick arrow); (L) Group 6 (MTX + CMC + SLY) swelling of renal tubular epithelium cells (arrows).
Evaluation of apoptosis in kidney tissues
The results of the apoptotic index are shown in . Using TUNEL assay to detect apoptotic renal tubular cells in the kidney sections, control (), CMC () and SLY () groups were similar and showed only a few TUNEL-positive cells. The number of TUNEL-positive cells markedly increased in the MTX () and the MTX + CMC () groups compared with the control group (p < 0.05). Treatment with SLY (MTX + CMC + SLY group) () reduced the number of TUNEL-positive cells as compared with the MTX group (p < 0.05).
Figure 3. The apoptotic index of all the groups. Values are mean ± SD for six rats in each group. (a,b,c): Bars with common superscripts are not statistically different, whereas values without common superscripts are statistically significantly different (p value <0.05).
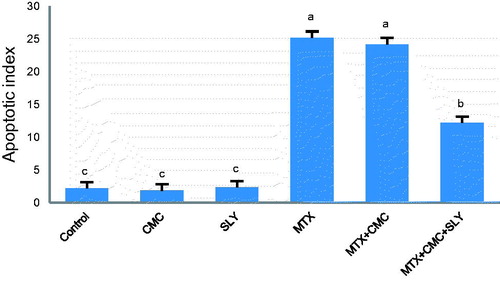
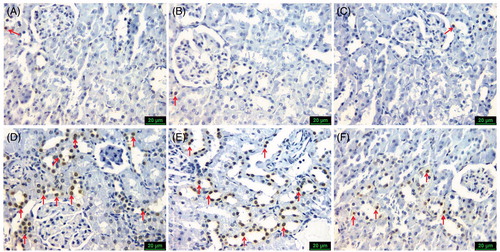
Figure 4. Representative photomicrographs of TUNEL staining in all six groups (scale bars=20 μm), showing: (A) Group 1 (control), (B) Group 2 (CMC) and (C) Group 3 (SLY) similarly only few TUNEL-positive cells (arrow); (D) Group 4 (MTX) and (E) Group 5 (MTX + CMC) similarly a lot of TUNEL-positive cells (arrows); (F) Group 6 (MTX + CMC + SLY) rare TUNEL-positive cells (arrows).
Discussion
Drugs used for cancer chemotherapy produce acute toxic side effects in multiple organ systems. MTX is one of the most widely used anticancer drugs and administration of high-dose MTX is an important component in the treatment of a variety of childhood and adult cancers.Citation30,Citation31 MTX-induced nephrotoxicity continues to occur despite the preventive measures such as administration of pharmacokinetically guided leucovorin, urinary alkalinization and intravenous hydration.Citation31,Citation32 MTX-induced renal toxicity can be life threatening because, it further leads to delayed elimination of MTX, and the resulting sustained, elevated circulating MTX level may lead to the development of other MTX-related toxicities.Citation30,Citation32 Treatment approaches including dialysis-based methods to remove MTX have limited effectiveness.Citation30
Several experimental therapeutic studies for the prevention of MTX-induced nephrotoxicity have been conducted. Abdel-Raheem and KhedrCitation16 reported that montelukast ameliorated MTX-induced histopathological alterations in the kidney tissues such as glomerular atrophy, tubular cystic dilatation, tubular degeneration, brush border disintegration and leucocyte cell infiltration. Abraham et al.Citation33 revealed that MTX treatment lead to severe glomerular and tubular damage in the kidney tissues and pretreatment with melatonin reduced MTX-induced damage to the kidney. Uzkeser et al.Citation34 indicated that MTX-induced histopathological changes including interstitial inflammation, swelling in the tubular epithelial cells and desquamated cells within the tubule lumen were healed with mirtazapine treatment. Sener et al.Citation35 reported that although severe glomerular congestion and degeneration, dilatation in Bowman’s space, inflammatory cell infiltration and tubular degeneration were determined in MTX-treated group, these changes were mild in MTX plus L-carnitine treated rats. In another study,Citation36 while severe glomerular congestion and degeneration, dilatation in Bowman’s space, inflammatory cell infiltration in interstitium and tubular degeneration were determined in the MTX-treated rats, there were mild glomerular and tubular degeneration and mild inflammatory cell infiltration in the interstitium in MTX plus pentoxifylline treated rats. In addition, pentoxifylline administration decreased the number of TUNEL-positive cells which were increased in MTX-treated rats. In the present study, the histopathological evidence of kidney injury such as dilated Bowman’s space, inflammatory cell infiltration, glomerular and peritubular vascular congestion and swelling of renal tubular epithelium cells has been observed in rats treated with MTX. In consistence with the previous reports, our findings indicate that SLY exerts a renoprotective effect against MTX-induced kidney damage in rats. In addition, the histopathological examination of kidneys of MTX-treated rats revealed increased apoptotic cells number in renal tubular epithelium cells. SLY treatment reduced the number of apoptotic cells as compared with the MTX group.
Several experimental studies have also highlighted the renoprotective properties of SLY against nephrotoxicity induced by different drugs such as cisplatin,Citation27 doxorubicin,Citation28 gentamicin,Citation29 cyclosporineCitation37 and chemicals such as arsenic,Citation38 manganese.Citation39 Similarly, mild nephroprotective action of SLY against MTX-induced renal injury was determined in the present study. The renoprotective effects of SLY have been mainly attributable to its antioxidant and free radical scavenging properties. Besides its antioxidant actions, SLY has also anti-inflammatory and immunomodulatory properties.Citation19 More recently, Manna et al.Citation40 reported that SLY has anti-inflammatory and cytoprotective effects via blocking TNF-induced activation of NF-kB and the kinases. They also indicate that SLY suppresses caspase activation leading to the inhibition of apoptosis and finally cytoprotection. Consequently, it seems likely that SLY could have diminished the detrimental effects of MTX by balancing oxidant-antioxidant status, regulation of immunomodulatory functions and inhibition of inflammation in the present study.
In conclusion, SLY treatment leads to a reduction on MTX-induced renal damage in rats. Since SLY is safe and acceptable for human consumption, further studies to define the exact mechanism of the protecting effect of SLY on MTX-induced nephrotoxicity and the optimum dosage of this compound would be useful.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This research was supported by Firat University Scientific Research Projects Management Unit (FUBAP) project number TF1152.
References
- Gorlick R, Goker E, Trippett T, Waltham M, Banerjee B, Bertino JR. Intrinsic and acquired resistance to methotrexate in acute leukaemia. N Engl J Med. 1996;335:1041–1048
- Joerger M, Huitema AD, Illerhaus G, Ferreri AJ. Rational administration schedule for high-dose methotrexate in patients with primary central nervous system lymphoma. Leuk Lymphoma. 2012;53:1867–1875
- Tannock IF, Boyd NF, DeBoer G, et al. A randomized trial of two dose levels of cyclophosphamide, methotrexate, and fluorouracil chemotherapy for patients with metastatic breast cancer. J Clin Oncol. 1988;6:1377–1387
- Widemann BC, Balis FM, Kempf-Bielack B, et al. High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma: Incidence, treatment, and outcome. Cancer. 2004;100:2222–2232
- Vermorken JB, Specenier P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol. 2010;21:252–261
- Weinblatt ME, Coblyn JS, Fox DA, et al. Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med. 1985;312:818–822
- Haustein UF, Rytter M. Methotrexate in psoriasis: 26 years' experience with low-dose long-term treatment. J Eur Acad Dermatol Venereol. 2000;14:382–388
- Helen STE, Schiano TD, Kuan SF, Hanauer SB, Conjeevaram HS, Baker AL. Hepatic effects of long term methotrexate use in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2000;95:3150–3156
- Henderson ES, Adamson RH, Oliverio VT. The metabolic fate of tritiated methotrexate. II. Absorption and excretion in man. Cancer Res. 1965;25:1018–1024
- Perazella MA, Moeckel GW. Nephrotoxicity from chemotherapeutic agents: Clinical manifestations, pathobiology, and prevention/therapy. Semin Nephrol. 2010;30:570–581
- Smeland E, Fuskevåg OM, Nymann K, et al. High-dose 7-hydroxymethotrexate: Acute toxicity and lethality in a rat model. Cancer Chemother Pharmacol. 1996;37:415–422
- Perazella MA. Crystal-induced acute renal failure. Am J Med. 1999;106:459–465
- Devrim E, Cetin R, Kiliçoğlu B, Ergüder BI, Avci A, Durak I. Methotrexate causes oxidative stress in rat kidney tissues. Ren Fail. 2005;27:771–773
- Kolli VK, Abraham P, Isaac B, Selvakumar D. Neutrophil infiltration and oxidative stress may play a critical role in methotrexate-induced renal damage. Chemotherapy. 2009;55:83–90
- Jahovic N, Cevik H, Sehirli AO. Melatonin prevents methotrexate induced hepatorenal oxidative injury in rats. J Pineal Res. 2003;34:282–287
- Abdel-Raheem IT, Khedr NF. Renoprotective effects of montelukast, a cysteinyl leukotriene receptor antagonist, against methotrexate-induced kidney damage in rats. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:341–353
- Oktem F, Yilmaz HR, Ozguner F, et al. Methotrexate-induced renal oxidative stress in rats: The role of a novel antioxidant caffeic acid phenethyl ester. Toxicol Ind Health. 2006;22:241–247
- Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61:2035–2063
- Pradhan SC, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res. 2006;124:491–504
- Wellington K, Jarvis B. Silymarin: A review of its clinical properties in the management of hepatic disorders. BioDrugs. 2001;15:465–489
- Metwally MAA, El-Gellal AM, El-Sawaisi SM. Effects of silymarin on lipid metabolism in rats. WASJ. 2009;6:1634–1637
- Locher R, Suter PM, Weyhenmeyer R, Vetter W. Inhibitory action of silibinin on low density lipoprotein oxidation. Arzneimittelforschung. 1998;48:236–239
- Polyak SJ, Morishima C, Shuhart MC, Wang CC, Liu Y, Lee DY. Inhibition of T-cell inflammatory cytokines, hepatocyte NF-kappaB signaling, and HCV infection by standardized Silymarin. Gastroenterology. 2007;132:1925–1936
- Zi X, Feyes DK, Agarwal R. Anticarcinogenic effect of a flavonoid antioxidant, silymarin, in human breast cancer cells MDA-MB 468: Induction of G1 arrest through an increase in Cip1/p21 concomitant with a decrease in kinase activity of cyclin-dependent kinases and associated cyclins. Clin Cancer Res. 1998;4:1055–1064
- Huilgol SV, Jamadar MG. Silymarin, an antioxidant bioflavonoid, inhibits experimentally-induced peptic ulcers in rats by dual mechanisms. Int J Appl Basic Med Res. 2012;2:63–66
- Soto C, Mena R, Luna J, et al. Silymarin induces recovery of pancreatic function after alloxan damage in rats. Life Sci. 2004;17:2167–2180
- Ninsontia C, Pongjit K, Chaotham C, Chanvorachote P. Silymarin selectively protects human renal cells from cisplatin-induced cell death. Pharm Biol. 2011;49:1082–1090
- El-Shitany NA, El-Haggar S, El-Desoky K. Silymarin prevents adriamycin-induced cardiotoxicity and nephrotoxicity in rats. Food Chem Toxicol. 2008;46:2422–2428
- Varzi H.N., Esmailzadeh S, Morovvati H, Avizeh R, Shahriari A, Givi ME. Effect of silymarin and vitamin E on gentamicin-induced nephrotoxicity. J Vet Pharmacol Therap. 2007;30:477–481
- Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694–703
- Widemann BC, Schwartz S, Jayaprakash N, et al. Efficacy of glucarpidase (carboxypeptidase g2) in patients with acute kidney injury after high-dose methotrexate therapy. Pharmacotherapy. 2014;34:427–439
- Rahiem Ahmed YAA, Hasan Y. Prevention and management of high dose methotrexate toxicity. J Cancer Sci Ther. 2013;5:106–112
- Abraham P, Kolli VK, Rabi S. Melatonin attenuates methotrexate-induced oxidative stress and renal damage in rats. Cell Biochem Funct. 2010;28:426–433
- Uzkeser H, Sener E, Bakan E, Hacimuftuoglu A. Preventive role of mirtazapine in methotrexate induced nephrotoxicity in rats. ScienceAsia. 2012;38:129–135
- Sener G, Ekşioğlu-Demiralp E, Cetiner M, et al. L-Carnitine ameliorates methotrexate-induced oxidative organ injury and inhibits leukocyte death. Cell Biol Toxicol. 2006;22:47–60
- Asvadi I, Hajipour B, Asvadi A, Asl NA, Roshangar L, Khodadadi A. Protective effect of pentoxyfilline in renal toxicity after methotrexate administration. Eur Rev Med Pharmacol Sci. 2011;15:1003–1009
- Zima TAS, Kamenikova L, Janebova M, Buchar E, Crkovska J, Tesar V. The effect of silibinin on experimental cyclosporine nephrotoxicity. Ren Fail. 1998; 20:471–479
- Prabu SM, Muthumani M. Silibinin ameliorates arsenic induced nephrotoxicity by abrogation of oxidative stress, inflammation and apoptosis in rats. Mol Biol Rep. 2012;39:11201–11216
- Chtourou Y, Garoui el M, Boudawara T, Zeghal N. Protective role of silymarin against manganese-induced nephrotoxicity and oxidative stress in rat. Environ Toxicol. 2014;29:1147–1154
- Manna SK, Mukhopadhyay A, Van NT, Aggarwal BB. Silymarin suppresses TNF-induced activation of NF-kappa B, c-Jun N-terminal kinase, and apoptosis. J Immunol. 1999;163:6800–6809
