Abstract
Objectives: The severity of acute kidney injury (AKI) has been a well-known predictor for in-hospital mortality. Whether AKI duration could predict in-hospital mortality is not clear. This study determines the association between the in-hospital mortality and AKI duration in patients after non-cardiac surgery. Materials and methods: Surgical patients who were admitted to the ICU were enrolled. AKI cases were defined using KDIGO guidelines and categorized according to the tertiles of AKI duration (1st tertile: 2 days, 2nd tertile: 3–6 days and 3rd tertile: 7 days). The adjusted hazard ratios (HRs) for in-hospital mortality are compared to those without AKI. The predictability of mortality is accessed by calculating the area under the curve (AUC) for the receiver operating characteristic (ROC) curve. Results: From a total of 318 postoperative patients, 98 developed AKI (1st tertile: 34 cases, 2nd tertile: 30 cases and 3rd tertile: 34 cases) and 220 had no AKI. The in-hospital mortality rates are 6.8% (non-AKI), 50% (1st tertile), 46.7% (2nd tertile) and 47% (3rd tertile). The HR’s for in-hospital mortality are 7.92, 6.68 and 1.68, compared to the non-AKI group (p = 0.006, 0.021 and 0.476). Cumulative in-hospital survival rates are significantly different for the non-AKI group and the AKI groups (p < 0.001). The AUC for AKI duration and stage together (0.804) is higher than that for AKI stage and AKI duration alone (0.803 and 0.777) (both ps < 0.001). Conclusion: In addition to severity, the duration of AKI may be a predictor of in-hospital mortality in patients, after non-cardiac surgery.
Introduction
Acute kidney injury (AKI) is a clinical syndrome characterized, when fully expressed, by decreased urine output and renal dysfunction, which results from a consequence of stressful events including cardiovascular surgery.Citation1 The defect can lead to expedition AKI affects costs, the length of hospitalization, comorbidity, and mortality.Citation2–5 Mortality has been shown in previous studies ∼60%.Citation4,Citation5 The incidental approaches described in the postoperative AKI are decreasing. One study generally provided information about perioperative hemodynamic stabilization in the association with reduction of consequences of AKI.Citation6 However, a complete review of many insights that have resulted from postoperative AKI is far beyond the mortality rate based on the diagnosis and classification of AKI. Several distinguishing predictors in postoperative AKI are represented by liver disease, high body mass index, a high-risk operation, advanced age, emergent operation, chronic obstructive pulmonary disease, and peripheral venous occlusion disease.Citation7 A study group consisting of members of the Acute Dialysis Quality Initiative (ADQI) and the Acute Kidney Injury Network (AKIN) are important classifications and stages for AKI according to RIFLE classification and AKIN criteria.
The most difference in AKIN criteria is the involvement of a small change in serum creatinine of >0.3 mg/dL, which increases the sensitivity for the detection of AKI, in comparison with the RIFLE classification.Citation8,Citation9 The association of new guideline recommendations with the definition of AKI was proposed by Kidney Disease Improving Global Outcomes (KDIGO) in accordance with previous two criteria recently.Citation10 AKI stages have been validated in the prognosis of mortality rate. However, AKI criteria gathered from changes in the serum creatinine and/or urine output is not a reliable predictor of mortality. The essential information of AKI duration is further prognosis. Based on recent studies, it was proposed that postoperative AKI duration and mortality in patients may have correlated, but their results lacked data regarding to urine output and exclude critically ill surgical patients.Citation11,Citation12 Nevertheless, Han et al.Citation13 postulated that one of mortality risks in critically ill patients was AKI duration. In this study, <5% of the patients had not been described their surgical categories.Citation13
Our study focusing on the biochemical components, clinical parameters, and organ scoring systems of critically ill patients following non-cardiac surgery revealed the association between the duration of AKI and in-hospital mortality. However, no in-depth studies of the effects of AKI duration on the non-cardiovascular surgery have been reported. Therefore, this study evaluated the effects of AKI stages and AKI duration on the 8-year in-hospital mortality rate of critically ill patients undergoing non-cardiovascular surgery in Taiwan.
Materials and methods
Ethics
This study was conducted in accordance with the Declaration of Helsinki (2000) by the World Medical Association. The protocol was approved by the institutional review board of Mackay Memorial Hospital (12MMHIS017) and the need for informed consent was waived. Mackay Memorial Hospital is a tertiary referral center for kidney disease. This study used a single center and all the patients enrolled for the study were directly diagnosed and followed up at Mackay Memorial Hospital.
Materials
Patients older than 18 years who underwent major surgery and were admitted to the ICU department of Taipei Mackay Memorial Hospital from January 2004 to December 2012 were reviewed for this retrospective observational study. Exclusion criteria included patients who underwent chronic dialysis, patients who started renal replacement therapy (RRT) before surgery (n = 9) or during admission (n = 30), patients with an ICU stay of <48 h (n = 15), patients who underwent any radiology study using intravenous contrast within the first seven postoperative days (n = 5), patients in whom AKI developed later than 7 days after surgery (n = 7), patients without urine output record during AKI or baseline renal function (n = 9) and those who initially admitted for other medical problems, such as congestive heart failure (n = 15), respiratory failure (n = 28), and hepatic failure (n = 23). It is inappropriate to assume that the risk factors are similar for subjects receiving cardiac and non-cardiac surgery, so those who underwent cardiac surgery were excluded (n = 272) ().Citation7 Finally, a total of 318 eligible patients were included for analysis.
Methods
Disease severity was assessed by using the Acute Physiology and Chronic Health Evaluation II (APACHE II) score, the Multiple Organ Dysfunction Score (MODS) and the Sequential Organ Failure Assessment (SOFA) score.Citation14,Citation15 Demographic data, biochemical data, clinical characteristics, underlying disease, the length of ICU stay and hospitalization, the severity of sepsis, surgery categories, urine output during the AKI period, serial renal function, the AKI duration, the use of diuretics and vasoactive drugs were also recorded. The use of vasoactive drugs was recorded as inotrope infusion with inotrope equivalent (IE) score.Citation16 The AKI was defined and classified according to urine output and the increment of serum creatinine, based on the KDIGO stages ().Citation10 The patients’ last serum creatinine obtained before admission at the outpatient department, the serial serum creatinine during ICU admission and hospitalization, and duration of AKI and severity of peak AKI were also recorded for KDIGO classification. Patients with AKI were stratified according to the maximum KDIGO class (KDIGOmax). The duration of AKI is defined by the number of days, from the first day to the end of AKI, that subjects at least meet the criteria for KDIGO stage 1. The end of AKI is determined when it does not conform to the criteria for AKI. As in a previous report, the duration of AKI is divided into three subgroups, according to the tertiles of the AKI duration; no more than 2 days (first tertile), 3–6 days (second tertile) and at least 7 days (third tertile).Citation12 Patients who required RRT during admission were excluded because RRT can confound AKI duration. The definitions of severe sepsis and septic shock are based on the 2012 International Guidelines for the Management of Severe Sepsis and Septic Shock.Citation17 Emergent surgery is defined as the anesthesiologist indicates an emergent code as part of the American Society of Anesthesiology physical status code.Citation12 Respiratory failure with ventilator dependency is defined as hypoxic and/or hypercapnic respiratory failure requiring a ventilator. In-hospital mortality is recorded as a primary outcome. Complete renal recovery, defined as a serum creatinine level of no more than 0.2 mg/dL higher than the baseline serum creatinine level at the end of AKI, is also examined.Citation12
Table 1. Definition and stage for AKI, according to the KDIGO classification.
Statistical analysis
Continuous variables with a normal distribution are summarized as mean ± SEM, unless otherwise stated. Variables with a non-normal distribution are expressed as a median [interquartile range (IQR)]. Patients without AKI were used as a reference group. Analysis of variance (ANOVA), chi-squared, Mann–Whitney U or Kruskal–Wallis tests are used to determine the differences in the demographic data, the laboratory variables, the length of hospitalization and recovery from AKI between patients with postoperative AKI (three subgroups) and the non-AKI group. The in-hospital mortality rate is calculated using the Kaplan–Meier method. Cumulative survival curves for postoperative patients with different KDIGO stages and AKI duration use the log-rank test. The hazard ratios (HRs) and 95% confidence intervals (CIs) for in-hospital mortality are calculated using the Cox proportional hazard model, with adjustment for age, gender, the need for a ventilator, the organ scoring systems, the surgery category, underlying diseases, emergent operation, the use of vasoactive drugs (IE score) and diuretics, the severity of sepsis, the baseline renal function and KDIGOmax. The discriminative ability of the criteria to predict mortality is determined using the area under the curve (AUC) for the receiver operating characteristic (ROC) curve. All the statistical analyses were performed using SPSS software (version 17.0, SPSS Inc., Chicago, IL) and a p value of <0.05 was considered statistically significant.
Results
In total, 318 patients who underwent surgery were analyzed. None of these patients received organ transplantation. All of the subjects were Asian descent. The mean age was 65.6 years. The ages of the included patients ranges from 18 to 89 years. There were 225 (70.7%) males and 93 (29.2%) females. AKI cases were diagnosed using serum creatinine criteria (76.1%), urine output criteria (23.9%) or both (16%). The urine output of most of the patients (72%) was recorded hourly. Estimated hourly urine output was used for those whose urine output was recorded every 2–4 h (n = 89). Among the subjects with AKI, the proportions in each AKI stage were 34.7%, 21.4% and 43.8% (from 1st stage to 3rd stage). The majority of AKI case could be determined by serum creatinine criteria alone (82.4%, 90% and 97.1% from 1st tertile to 3rd tertile), where as 5.8% (1st tertile), 6.6% (2nd tertile) and 11.7% (3rd tertile) of AKI cases could be determined by urine output criteria alone. The overall incidence of postoperative AKI was 30.8% (98 cases) and the in-hospital mortality rate of the subjects with AKI was higher than that of those without AKI [47.9% (47 cases) vs. 6.8% (15 cases), p < 0.001]. Most of the patients underwent abdominal surgery (39%), trauma (10.1%) and other surgeries, such as soft tissue debridement (12.2%). Baseline demographical, clinical, scoring systems, surgical categories and laboratory characteristics for non-AKI and AKI subgroups are shown in . The patients’ data shown in is divided according to KDIGO stages and is subdivided using the tertiles for AKI duration. Various parameters, such as the serum creatinine at baseline and peak AKI, the length of hospitalization, the length of ICU stay, prothrombin time and the proportions of the AKI stages show a significant difference for four groups. The durations of AKI, KDIGOmax, and the organ scoring systems, such as APACHE II score, MODS, SOFA score, are also more severe in the AKI groups than in the non-AKI group. However, the age, gender, body mass index, cigarette smoking, the need for a ventilator, mean arterial pressure, the severity of sepsis, surgical categories, underlying diseases, inotropes equivalent (IE) score, the use of diuretics and the least daily urine output were no difference between two groups.
Table 2. Patients’ demographic data and clinical characteristics.
Table 3. Patients’ data divided according to KDIGO stages and subdivided by tertiles of the AKI duration.
Among scoring systems, APACHE II and KDIGOmax were strong predictors of in hospital mortality [the adjusted HRs (95% CIs) were 1.101 (1.034–1.172) and 2.447 (1.769–3.385), both p < 0.01]. shows the in-hospital survival curve for different groups, according to their AKI stages and the different tertiles of AKI in each of the KDIGO stages, using a log rank test. The multivariate-adjusted HRs (95% CIs) for in-hospital mortality, compared to those for non-AKI, are as follows: KDIGO stage 1: 4.843 (2.221–10.551); KDIGO stage 2: 4.278 (1.804–10.135) and KDIGO stage 3: 7.179 (3.776–13.664) (all ps < 0.001). The mean duration of AKI in the AKI group was 7.07 days (median, 3.5 days; IQR, 1–10 days). A 1-day increase in AKI duration produces an insignificant increase (2.7%) in hospital mortality, after multivariate adjustment (p = 0.083). However, there is a significant (2.5%) increase in hospital mortality for each extra day of AKI duration for those with a duration of <2 weeks (p = 0.041). The AKI group is subdivided into three subgroups, according to the duration of AKI: 1st tertile (n = 34): ≤2 days; 2nd tertile (n = 30): 3–6 days; 3rd tertile (n = 34): ≥7 days. The mortality rates are higher for AKI groups than the non-AKI group. The unadjusted HRs are 9.82 (1st tertile), 7.75 (2nd tertile) and 3.14 (3rd tertile) (all ps < 0.05). The HRs are still significant in the 1st tertile and the 2nd tertile, after adjustment for confounding factors, such as age, gender, the need for a ventilator, the baseline renal function, underlying diseases, emergent operation (model 2), surgical category, the use of vasoactive drugs and diuretics, severity of sepsis (model 3), APACHE II, MODS, SOFA score and the KDIGOmax (model 4) (). The adjusted HRs for in-hospital mortality are 7.92, 6.68 and 1.68, compared to the non-AKI group (p = 0.006, 0.021 and 0.476). The cumulative survival curve for the tertiles of AKI and the different AKI stages in each of the tertiles of AKI are shown in . The length of hospitalization is shorter for the non-AKI group (mean, 28.2 days; median, 27 days) than for the AKI groups (1st tertile: mean, 25.2 days, median, 22 days; 2nd tertile: mean, 31.8 days; median, 29 days; 3rd tertile: mean, 44.2 days, median, 42 days) (p < 0.05 in the 2nd and 3rd tertile groups). The proportions of recovery from AKI are significantly different for the AKI groups (1st tertile: 73.2%, 2nd tertile: 30.4%, 3rd tertile: 26.5%) (p < 0.001). The AUC (95% CI) of the ROC for in-hospital mortality are 0.777 (0.710–0.845), according to the tertiles of AKI duration. When the duration of AKI is considered as a continuous variable, the AUC is 0.780 (0.711–0.847). The AUC of the ROC for in-hospital mortality is greater when the AKI duration and stage are considered together [0.804 (0.735–0.873)] than the AUC calculated using the AKI stage with KDIGO classifications [0.803 (0.735–0.872)] (all ps < .001) (). The multivariate adjusted HRs (95% CI) for in hospital mortality in the APACHE II score and age are 1.023 (1.006–1.140) and 1.115 (1.052–1.182) (both ps < 0.01).
Figure 2. Cumulative survival rate for 318 postoperative patients based on their AKI stages and the different tertiles of AKI in each of the KDIGO stages. Cumulative survival curve according to AKI stages (A) and the different tertiles of AKI in each of the KDIGO stages (B–D). The overall in-hospital survival rates were significantly in (A), (B) and (D) (p < 0.001 by log-rank test).
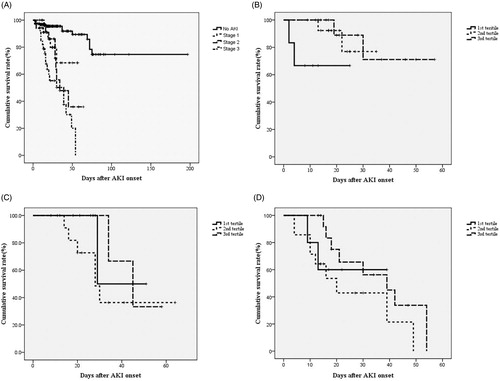
Figure 3. Cumulative survival rate for 318 postoperative patients based on the tertiles of AKI and the different AKI stages in each of the tertiles. Cumulative survival curve according to tertiles of AKI stages (A) and the different stages of AKI in each of the tertiles (B–D). *p < 0.05 by log-rank test in (A) and (C).
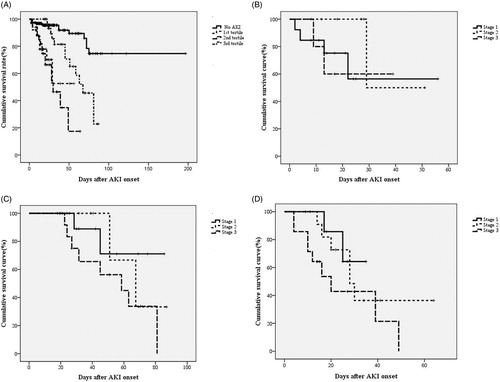
Figure 4. The AUC of the ROC curve for in-hospital mortality according to AKI stage, duration and stage together with duration.
Table 4. Hazard ratios for in-hospital mortality, for different durations of AKI.
Discussion
Our retrospective-observational research cited here provided that the duration of postoperative AKI predicted in-hospital mortality in patients undergoing non-cardiac surgery after adjustment for clinical characteristics, biochemical data, underlying diseases, surgical categories, scoring system, and prescriptions in AKI onset in surgical patients. The validity of the AKI stage, defined by either the AKIN criteria or the RIFLE classification, is a well-know predictor for mortality.Citation9,Citation18 However, several lines of evidence support the definition of AKI was unoptimized. Two studies used variance component analysis to determine the association of AKI with mortality. The conclusion revealed that some participants with AKI were undiagnosed and no proportional increase between mortality rate and AKI stages.Citation19,Citation20 Following the insufficiency of serum creatinine responsible for AKI, the next major quantum of deficiency in the field arose from the understanding that the limitations of the urine output criteria and inappropriately identifying prognostic factors of mortality in patients with AKI.Citation21,Citation22
Because the factors including volume status and nutrition status that affect creatinine, serum creatinine was found to be subnormal standard biomarker for AKI. One report describe that plasma neutrophil gelatinase-associated lipocalin (NGAL) and serum cystatin C were better prognostic factors of AKI following cardiac surgery compared to serum creatinine.Citation23 One of the AKI criteria includes urine output being very common when compared to previous studies; hence, the definition of AKI appears to be related to more comprehensive criteria.Citation11,Citation12 Han and his colleagues reported that the percentage of AKI diagnosed by urine output criteria alone occurred between 2.9% and 25.7%, and their research reached 7.9%.Citation13,Citation22 AKI has been well described in geriatric population, and an increased incidence of AKI becomes higher than previous studies (2–25%).Citation24 The proportion of AKI in these patients is 33.6% due to the use of angiotensin converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) prior to surgical intervention. The association between preoperative management of ACEIs and/or ARBs and cardiovascular surgery should increase the incidence of AKI ∼27.2%.Citation25 In addition to previous parameters of AKI, the duration of AKI in the presence of prognostic factor of in-hospital mortality exists and presents with varying surgical complications. The current definition of AKI, as proposed by the RIFLE, AKIN and KDIGO classifications, does not involve any component along with the duration of AKI. In this setting, the significance based on the length of AKI duration, and the 1-day increase in AKI duration may be relatively free of the significant value. There is valuable increase (2.5%) in those AKI patients within 2 weeks after multivariate adjustment, and the result suggests that the longer than 2 weeks of AKI duration is not an accurate predictor of in-hospital mortality. The AKI duration indicates a surrogate for recuperative potential from injured kidney.
The AKI duration is positively linked between the severity and the treatment resistance of AKI patients. AKI not only impacts the kidneys but also affects the function of multiple organs. Hence, the longer duration of AKI may lead to the more severe illness or extra-renal organ dysfunction. Conditions that allow for rapid recovery of renal medullary blood flow and quick regained renal function subsequently based on the age and renal volume.Citation12,Citation26 Previous publications separated AKI duration into several groups including transient azotemia (<3 days) and acute tubular necrosis (>4 days); short (≤2 days), medium (3–6 days) and long (≥7 days); the 1st, 2nd and 3rd tertiles of AKI duration and a renal replacement therapy group.Citation12,Citation13,Citation27 However, we must be aware of potential bias or confounding in our study because the initiation of renal replacement therapy may fail to control the internal validity of AKI duration, and patients with AKI undergoing renal replacement therapy are excluded.Citation28 Uchino and his coworkers addressed that the majority of AKI duration was <15 days.Citation27 Nevertheless, 14 (14.3%) of 98 patients suffered >2 weeks of AKI. Recurrent AKI or patients’ compliance to the impaction on AKI might occur in the group. This phenomenon might explain why the AKI duration is not a good predictor of in-hospital mortality. According to and , the study demonstrated the duration of AKI is a predictor of in-hospital mortality in those with moderate AKI after non-cardiac surgery. It is less predictive in those with mild or severe AKI. The predictability for in-hospital mortality of AKI stages is stronger than those of AKI duration.
This study has several limitations. Initially, the study population is restricted to one hospital, so the result is difficult to extrapolate to all the populations. The outcome and the duration of AKI are different due to various therapeutic strategies of AKI. Many factors that predispose the AKI duration have been described. The failure of therapeutic regimen is putatively associated with an increased AKI duration as the outcome of poorly controlled AKI. Diuretics can elevate the amount of urine output but the discriminative function of urine output criteria is not changed for the survival rate calculations.Citation22 Second, those patients who were excluded due to no data of urine output probably made a selection bias. Not all the patients had the complete record of hourly urine output. The estimated hourly urine output is easy to lead to underestimate of the severity of the AKI according to the urine output criteria only. Third, the predictive accuracy of the Cox proportional hazard model has its own limitations based on biased regression coefficients. Finally, there may be residual confounding factors that could attenuate the estimated selection probabilities between the duration of AKI and in-hospital mortality. In conclusions, the duration of postoperative AKI, especially AKI duration <7 days, predicts in-hospital mortality of patients undergoing non-cardiac surgery after adjustment for the variables involving the baseline and clinical characteristics, organ scoring system, underlying disease, emergent intervention, surgical category, AKI stages, and severity of infection. The combination of AKI duration with AKI stage is better in predicting in-hospital mortality than AKI stage alone, estimating the area under the ROC curve. The duration and severity of AKI are both important parameters for surgical candidates. This study is limited by its retrospective nature, so the therapeutic strategy and subsequent outcomes are difficult to be valid. Further multicenter longitudinal cohort-based studies are warranted to approve the relationship between the duration of AKI and in-hospital mortality.
Acknowledgments
Special thanks to Y.-W.C. in statistical analysis.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Heringlake M, Knappe M, Vargas HO, et al. Renal dysfunction according to the ADQI-RIFLE system and clinical practice patterns after cardiac surgery in Germany. Minerva Anestesiol. 2006;72:645–654
- Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, lengths of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370
- Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961–973
- Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA. 2005;294:813–818
- Liano F, Pascual J. Epidemiology of acute renal failure: A prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int. 1996;50:811–818
- Brienza N, Giglio MT, Marucci M, Fiore T. Does perioperative hemodynamic optimization protect renal function in the surgical patient: A meta-analytic study. Crit Care Med. 2009;37:2079–2090
- Kheterpal S, Tremper K, Englesbe M, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology. 2007;107:892–902
- Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure – Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212
- Kidney Disease: Improving Global Outcomes (KDIGO) acute kidney injury work group: KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138
- Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31
- Brown JR, Kramer RS, Coca SG, Parikh CR. Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann Thorac Surg. 2010;90:1142–1148
- Coca SG, King JT Jr, Rosenthal RA, Perkal MF, Parikh CR. The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney Int. 2010;78:926–932
- Han SS, Kim S, Ahn SY, et al. Duration of acute kidney injury and mortality in critically ill patients: A retrospective observational study. BMC Nephrol. 2013;14:133
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-relatedOrgan Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710
- Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: A reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652
- Chen YS, Ko WJ, Lin FY, et al. Preliminary result of an algorithm to select proper ventricular assist devices for high-risk patients with extracorporeal membrane oxygenation support. J Heart Lung Transplant. 2001;20:850–857
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228
- Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: A comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292–1298
- Joannidis M, Metnitz B, Bauer P, et al. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009;35:1692–1702
- Lopes JA, Fernandes P, Jorge S, et al. Acute kidney injury in intensive care unit patients: A comparison between the RIFLE and the Acute Kidney Injury Network classifications. Crit Care. 2008;12:R110
- Waikar SS, Betensky RA, Bonventre JV. Creatinine as the gold standard for kidney injury biomarker studies? Nephrol Dial Transplant. 2009;24:3263–3265
- Han SS, Kang KJ, Kwon SJ, et al. Additional role of urine output criterion in defining acute kidney injury. Nephrol Dial Transplant. 2012;27:161–165
- Haase-Fielitz A, Bellomo R, Devarajan P, et al. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery – A prospective cohort study. Crit Care Med. 2009;37:553–560
- Chao CT, Wu VC, Lai CF, et al. Advanced age affects the outcome-predictive power of RIFLE classification in geriatric patients with acute kidney injury. Kidney Int. 2012;82:920–927
- Arora P, Rajagopalam S, Ranjan R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol. 2008;3:1266–1273
- Schmitt R, Coca S, Kanbay M, Tinetti ME, Cantley LG, Parikh CR. Recovery of kidney function after acute kidney injury in the elderly: A systematic review and meta-analysis. Am J Kidney Dis. 2008;52:262–271
- Uchino S, Bellomo R, Bagshaw SM, Goldsmith D. Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol Dial Transplant. 2010;25:1833–1839
- Seabra VF, Balk EM, Liangos O, Sosa MA, Cendoroglo M, Jaber BL. Timing of renal replacement therapy initiation in acute renal failure: A meta-analysis. Am J Kidney Dis. 2008;52:272–284