Abstract
Insidious progressive renal damage caused by type 1 diabetes mellitus (T1DM) begins in childhood before it becomes manifest in adult ages. Heat shock proteins (HSPs) regulate the cell response to any hazardous factors to prevent cell structure. The aim of the study is to determine whether urine levels of HSPs increase in diabetic children with time and indicate a progressive renal injury in T1DM. Thirty-three patients with T1DM and 24 healthy children were enrolled in the study. Renal function was normal in all patients. Urine levels of HSP27, HSP40, HSP60, HSP70, and HSP90 were measured by enzyme-linked immunosorbent assay at two consecutive years (2012 and 2013). The results were evaluated as urine HSP/creatinine ratios (uHSP/Cr). Mean urine HSP27/Cr, HSP40/Cr, HSP60/Cr, HSP70/Cr, HSP90/Cr in patient group were significantly higher than in controls in 2012 (uHSP27/Cr 460.12 ± 217.64 versus 270.02 ± 136.83 pg/mgCr; uHSP40/Cr 180.89 ± 118.59 versus 99.44 ± 62.49 pg/mgCr; uHSP60/Cr 114.40 ± 64.91 versus 70.50 ± 43.70 pg/mgCr; uHSP70/Cr 41.17 ± 28.42 versus 16.47 ± 7.32 pg/mgCr; uHSP90/Cr 175.64 ± 102.22 versus 107.61 ± 75.85 pg/mgCr) (p < 0.05). In 2013, uHSP70/Cr level increased significantly (51.08 ± 27.72 pg/mgCr; p = 0.001), whereas uHSP60/Cr level decreased and uHSP27/Cr, uHSP40/Cr, uHSP90/Cr levels remained stable (p > 0.05). Area under the curve (AUC) for uHSP70/Cr (0.957) was significantly higher than the others. Using a cutoff 22.59 pg/mgCr for uHSP70/Cr to predict of diabetic damage, sensitivity and specificity were 85% and 96%, respectively. Our results suggest that uHSP70/Cr increases over time and may indicate early phases of progressive kidney damage in diabetic children.
Introduction
Diabetic nephropathy (DN) is one of the major causes of end-stage renal disease (ESRD) in adults.Citation1,Citation2 Although DN is considered to be a disease of adult population, nowadays it may emerge in childhood because the incidence of type 1 diabetes mellitus (T1DM) has been increasing in children and the age of onset has been decreasing over the last decade.Citation2 Progression of DN consists of five stages.Citation1,Citation2 The first stage, hyperfiltration and hyperperfusion phase, encompasses 0–2 years of T1DM and is characterized by slightly increased glomerular filtration rate (GFR).Citation1,Citation2 The second stage which is identified with basement membrane thickening develops 2–5 years after diagnosis of T1DM.Citation1,Citation2 There are no clinical and laboratory abnormalities during these two periods.Citation1,Citation2 The first sign of DN is accepted to be microalbuminuria in third stage which occurs 6–15 years after the DM diagnosis.Citation1,Citation2 A progressive increase in albumin excretion results in overt proteinuria in fourth stage and nephropathy progresses to ESRD in fifth stage. In other words, diabetic kidney injury emerges before onset of microalbuminuria.Citation1,Citation2 Consequently, biomarkers are needed to diagnose diabetic kidney injury in earlier stages of the disease in order to prevent ESRD in this patient group.
Heat shock proteins (HSPs) are intracellular chaperones which are categorized as protein families according to their molecular weight such as small HSPs (16–40 kDa), HSP60 (60 kDa), HSP70 (70 kDa), HSP90 (90 kDa).Citation3,Citation4 The main function of these chaperones is contributing to preserve cell survival by restoring protein homeostasis.Citation3 HSPs are involved in correct folding of proteins, refolding of misfolded proteins and degradation of irrecoverable proteins to protect structure of cytoskeleton.Citation3–6 They are essentially expressed under normal conditions and can be induced by various stressors such as thermal, oxidative, osmotic, ischemic, toxic injury.Citation3,Citation7,Citation8
Although the functions of HSPs are not completely understood, it is considered that some of HSPs are related to specific functions.Citation3 HSP27 is a member of small HSPs family.Citation3 In condition of oxidative stress, it acts as an antioxidant by increasing intracellular glutathione and decreasing intracellular iron resulted in decreased reactive oxygen species.Citation3 Moreover, HSP27 prevents apoptosis by blocking various pathways and can regulate actin structure of cytoskeleton via stimulating actin polymerization.Citation3,Citation9 HSP47, one of the members of HSP40 family, is reported to be associated with the procollagen and to play a significant role in fibrosis of vital organs.Citation10 HSP60 primarily presents in mitochondrial matrix and assists to mitochondrial HSP70 in refolding of damaged proteins under stress conditions.Citation3 HSP70 family is the most abundant HSPs in the cells. HSP73 has been reported to be associated with tubular reabsorption of protein in experimental nephrotic syndrome and HSP72 has been found to be a cryoprotectant protein in ischemic kidney injury.Citation3 HSP90/70 complex contributes to maturation of steroid hormone receptors.Citation3
Some studies suggest that HSPs may be used as biomarkers to diagnose colonic or hepatocellular cancers.Citation11,Citation12 Besides there are some reports about that HSPs are changed in experimental model of diabetes mellitus.Citation5,Citation13,Citation14 The aim of the study is to determine whether urine levels of HSPs increase in diabetic children with time and indicate a progressive renal injury in T1DM.
Patients and methods
Thirty-three consecutive patients with T1DM (18 male and 15 female) who applied to outpatient clinic of pediatric endocrinology department for routine follow-up in 2012 and 24 healthy children (15 male and 9 female) were enrolled in the study. Mean age of the patients and control groups were 11.73 ± 3.82 and 12.05 ± 3.17 years, respectively (p = 0.78). Gender distribution was not significantly different between the two groups (p > 0.05). The mean follow-up duration was 40.6 ± 25.5 (6.4–93.9). All patients were on intensive insulin treatment or insulin pump treatment. Patient characteristics were given in .
Table 1. Patient characteristics.
After informed consent was obtained, patients underwent standard physical examination and blood samples were drawn for biochemical analysis. The body mass index (BMI) was evaluated based on percentile curves of Turkish children and obesity was defined as BMI higher than 95th percentile.Citation15 The mean BMI of the patients was 19.61 ± 3.52. Hypertension was defined as systolic and/or diastolic blood pressure to be higher than the 95th percentile for age and gender.Citation16 Normal blood pressure was observed in all patients.
The mean estimated GFR was 157.46 ± 34.61 mL/min/1.73 m2 (107.25–303.32) according to Schwartz formula.Citation17 Hyperfiltration was defined as eGFR exceeding 134 and 149 mL/min/1.73 m2 for males and females, respectively.Citation18 Twenty-five of our patients (75.7%) had hyperfiltration. A complete urinalysis and a urine culture were performed and urinary tract infection was excluded in all patients. None of our patients had urinary tract infection or urolithiasis or history of nephrotoxic drug usage in last three months. Patients were considered microalbuminuric if they had a urine microalbumin/creatinine ratio (uMA/Cr) of above 30 mg/g Cr in at least two out of three urine samples during outpatient follow-up.Citation19 Microalbuminuria was established in only 3 patients. The mean uMA/Cr was 20.17 ± 47.51 (1.28–239.41) in our patient group.
Urine samples were obtained to measure urine levels of HSP27, HSP40, HSP60, HSP70, HSP90, microalbumin, and creatinine. The results were evaluated as urine HSP/creatinine ratios (uHSP/Cr). The same measurements were repeated after one year (in 2013) to determine whether urine levels of HSPs increase in diabetic children with time. Since our results showed that uHSP70/Cr is the best indicator for diabetic kidney injury, we also considered to evaluate serum and urine levels and also fractional excretion rate of HSP70 to form an idea whether the source of uHSP70 was kidney or not. We invited the same patients to our outpatient clinic in 2014 again and 13 of them accepted to join further analysis. Blood and urine samples were obtained from these patients to measure HSP70 by enzyme-linked immunosorbent assay (ELISA) and fractional excretion of HSP70 was calculated.
Urine samples
Urine samples immediately were centrifuged for 10 min at 2000 × g. Aliquots of urine supernatant were stored at −80 °C for assaying. Urinary levels of HSP70, HSP60, HSP40, and HSP27 were assessed by ELISA technique. Urine HSP70, HSP60, HSP40, HSP27 were analyzed using Human HSP70, 60(HSP60), 40(HSP40), 27(HSP27) ELISA Kit (Cat no.: 20959, 20989, 20960, 23324, respectively) purchased from Bio Medical Assay (BMASSAY, Beijing, China) following the manufacturer’s instructions. Their levels were expressed as ng/mL. The detection and quantification limits were set at <0.05 ng/mL for HSP70, HSP60, HSP40 and <0.1 ng/mL for HSP27. The ELISA kit shows no cross-reactivity with any of the cytokines. The intra-assay coefficient of variations (CV) of HSP70, HSP60, HSP40, HSP27 were 5.2%, 6.4%, 4.9%, 7.1% and the inter-assay CV were 8.2%, 9.3%, 7.9%, 10.1% respectively. HSP90 ELISA Kit (Cat no.: 20958) the detection and quantification limit was set at <0.05 ng/mL for HSP90. The intra-assay CV of HSP90 was 4.9%, and the inter-assay CV was 7.9%. The Abbott Architect c16000 analyzer was used to measure uCr and uMA, with uMA expressed in mg/L and uMA/Cr expressed in mg/g.
Serum samples
Blood samples immediately were centrifuged for 10 min at 2000 × g. Aliquots of serum were stored at −80 °C for assaying. Serum levels of HSP70 were assessed by ELISA technique. Serum HSP70 levels were analyzed using Human HSP70 ELISA Kit (Cat no.: 20959) purchased from Bio Medical Assay (BMASSAY, Beijing, China) following the manufacturer’s instructions. Their levels were expressed as ng/mL. The detection and quantification limits were set at <0.05 ng/mL. The ELISA kit shows no cross reactivity with any of the cytokines. The intra-assay CV of HSP70 was 6.1% and the inter-assay CV was 7.4%.
Approval for this study was obtained from the Ethical Committee of the Istanbul University Istanbul Medical Faculty.
Statistical analysis
Statistical calculations were performed with NCSS 2007 program for Windows. Besides standard descriptive statistical calculations (mean, standard deviation, median, and IQR), Wilcoxon signed-rank test was employed in the assessment of 2012 and 2013 values, Mann–Whitney U test was used in the comparison of two groups, and chi-square test was performed during the evaluation qualitative data. The results were evaluated within a 95% confidence interval. Statistical significance level was established at p < 0.05.
To calculate the sensitivity, specificity, positive predictive value, negative predictive value, and positive likelihood ratio for the HSP27/Cr, HSP40/Cr, HSP60/Cr, HSP70/Cr, HSP90/Cr measurements at varying cutoff values, a conventional receiver operating characteristic (ROC) curve was generated. ROC analysis was used to calculate the AUC for HSP27/Cr, HSP40/Cr, HSP60/Cr, HSP70/Cr, HSP90/Cr and to find the best HSP27/Cr, HSP40/Cr, HSP60/Cr, HSP70/Cr, HSP90/Cr cutoff values for identifying diabetic kidney injury.
Results
Urine excretion of HSPs in patients and control groups
When we compare patients with controls according to the results obtained in 2012, mean uHSP27/Cr, uHSP40/Cr, uHSP60/Cr, uHSP70/Cr, uHSP90/Cr of patient group were significantly higher than in controls (p < 0.05) ().
Table 2. Urine heat shock protein/creatinine of patients and control groups in 2012 and 2013.
We investigated the relationship between uHSPs and prognostic factors of T1DM such as age of onset, presence of puberty at the time of the diagnosis, and HbA1c level. None of these markers was correlated to the age of onset of T1DM (p > 0.05) (). Ten patients were found to have signs of puberty at the time of the diagnosis. Urine HSPs/Cr of these patients were not significantly different than in the prepubertal patients (p > 0.05). There were no correlations between HbA1c and uHSPs/Cr (p > 0.05) (). It could not be evaluated if there was a relationship between uHSPs and obesity because obesity was established in only 3 patients in our group.
Table 3. Correlations of urine heat shock protein/creatinine with age of onset diabetes and HbA1c.
We also evaluated whether uHSPs/Cr changed with time measuring uHSPs at two consecutive years. In 2013, all of the uHSPs/Cr ratios in patient group were significantly higher than in controls except uHSP60/Cr. Comparisons of uHSPs/Cr between the patients and controls in 2013 are given in .
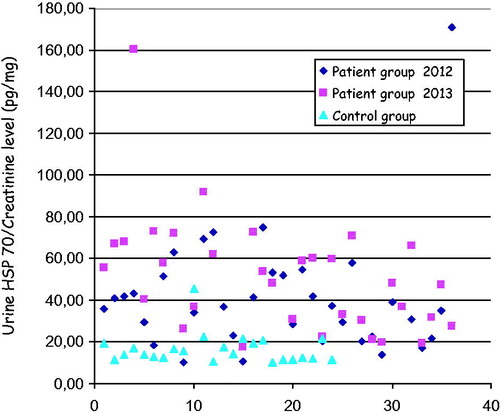
Comparing uHSPs/Cr in 2012, uHSP70/Cr significantly increased in 2013 (p = 0.001). Whereas uHSP60/Cr significantly decreased (p = 0.043) and uHSP27/Cr, uHSP40/Cr, uHSP90/Cr remained stable (p > 0.05). The distribution of uHSP70/Cr in patients and control was shown in .
Comparison of AUC values of uHSPs/cr
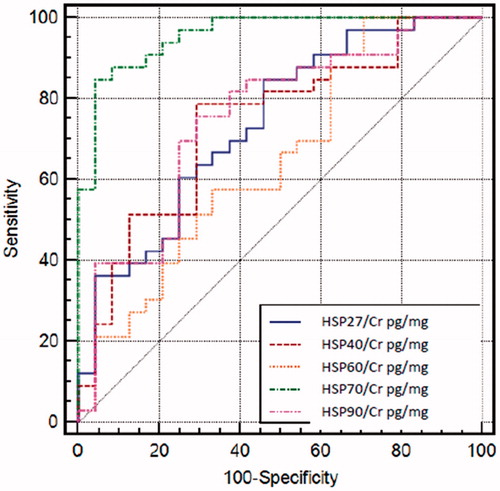
Comparing all uHSPs to each other, uHSP70/Cr seems to be the best indicator for diabetic kidney injury with AUC value of 0.957. Comparison of the AUC values of uHSPs/Cr was shown in . ROC analysis revealed that the optimal cutoff value to predict diabetic kidney injury was 22.59 pg/mgCr for uHSP70/Cr. At this cutoff point, sensitivity was 84.85%, specificity was 95.83%, positive predictive value was 96.6% and negative predictive value was 82.1%. Above this cutoff value, the possibility of diabetic kidney injury increased 20-fold (positive likelihood ratio of 20.36). The cutoff values of all uHSPs/Cr, sensitivity and specificity of them were given in .
Table 4. The cutoff values, sensitivity, and specificity of urine heat shock protein/creatinine ratios.
Fractional excretion rate of HSP70
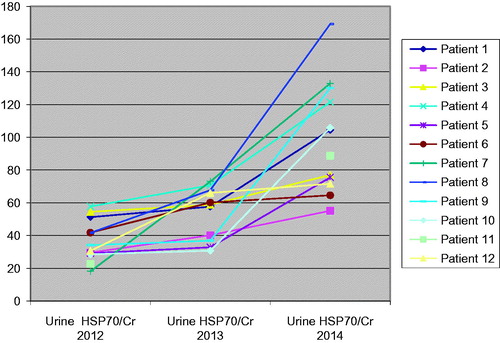
In 2014, the mean uHSP70/Cr was 133.40 pg/mg and was higher than the values of 2012 and 2013 (p = 0.0001) (). The mean value of urine HSP70 was 6.5 times higher than the mean serum HSP70 level (117.30 ± 53.84 pg/mL vs. 22.10 ± 11.40 pg/mL) and fractional excretion rate of HSP70 was 4.12 ± 4.06.
Discussion
Although DN is accepted as a glomerular disease, current evidences suggest that the renal tubular interstitial fibrosis is an important part of diabetic kidney injury and the rate of deterioration of renal function correlates with the degree of tubulointerstitial fibrosis.Citation20 Nonspecific renal interstitial inflammation affects renal survival in diabetic patients.Citation20 Moreover, tubular basement membrane width is known to be a constant part of early phases of DN.Citation20 Previous studies showed some renal tubular biomarkers to be increased before microalbuminuria in diabetic children. Zachwieja et al.Citation21 notified that urine neutrophil gelatinase-associated lipocalin (NGAL) levels were higher in normoalbuminuric children with T1DM than in controls. Also Soltysiak et al.Citation22 found higher urine NGAL and lower urine cathepsin-L levels in normoalbuminuric diabetic children than in control group and the authors concluded that the changes in these parameters indicate early signs of diabetic kidney injury, especially tubular injury.Citation21,Citation22 Similarly, HSP70 may indicate early diabetic kidney injury because it has been shown in experimental models of acute kidney injury and gentamicin-induced renal tubular injury in Human Kidney tubular cell line (HK-2) that uHSP70 is a useful marker for early detection of proximal tubular cell injury.Citation23,Citation24
DN is a consequence of exposure to consistent high plasma glucose concentration and various other metabolic factors such as advanced glycosylated end products (AGEs), oxidative stress, growth factors, and cytokines.Citation1,Citation2 AGEs bind their specific receptors on cell surface.Citation2,Citation25 This interaction results in activation of Janus kinase 2-signal transducers and activators of transcription (JAK2/STAT) pathway and induction of cellular oxidative stress.Citation2,Citation25 Moreover, AGEs stimulate proliferation of interstitial fibroblast cells in rat kidney via activation of the same pathway.Citation25,Citation26 HSP70 expression was enhanced by JAK/STAT pathway activation.Citation27 Chen et al.Citation25 demonstrated that AGEs also stimulate HSP70 expression in kidney interstitial fibroblasts in JAK2-dependent manner. Furthermore, exposure to high glucose concentration induces HSP70 gene expression in renal proximal tubular cells suggesting that increase of HSP70 expression maybe related to several factors in DN.Citation28
Calabrese et al.Citation29 evaluated systemic oxidative stress markers such as AGEs, protein oxidation and lipid oxidation products in plasma, lymphocytes, and urine of patients with type 2 DM as well as HSP32, HSP60, HSP70 levels in only lymphocytes. They found that levels of oxidative stress markers and all these cellular HSPs increased in lymphocytes of the patients with DN in comparison with control group.Citation29 Barutta et al.Citation5 evaluated HSP27 (human)/25 (rodents), HSP60, HSP70 (human)/72 (rodents), HSP90 in experimental model of DN. The authors conclude that HSP27/25, HSP60, and HSP70/72 overexpressed in the outer medulla and phosphorylated HSP27 was increased in podocytes of diabetic animals.Citation5 Increased levels of HSPs may be an indicator of DN according to these studies.
We evaluated uHSP27/Cr, uHSP40/Cr, uHSP60/Cr, uHSP70/Cr, uHSP90/Cr in order to determine whether these biomarkers could indicate diabetic kidney injury in children with T1DM. All of these parameters were found to be significantly higher in diabetic children than in controls indicating the presence of diabetic kidney injury in our patient group who did not have uremia and proteinuria. Comparing all uHSPs with each other, uHSP70/Cr appears to be the best indicator for diabetic kidney injury because of the highest AUC, sensitivity, specificity values and, as an additional feature, the possibility of being indicator of progression in diabetic kidney injury. The possibility of diabetic kidney injury increases 20-fold, when uHSP70/Cr is higher than determined cutoff value. We consider that uHSPs70/Cr may be elevated as a protective mechanism against high glucose exposure, AGEs and oxidative stress in diabetes because all these factors contribute to develop DN as well as to increase HSPs in experimental studies mentioned above. Since majority of our patient group has no microalbuminuria, it may be considered that uHSPs/Cr elevation represents very early phases of diabetic kidney injury. Hyperfiltration may be the only evidence that can be observed in the early stages of DN.Citation18 The majority of our patients have elevated eGFR suggesting that they are in the first stage of DN.
At this point, a question may arise “which factor caused augmentation of uHSP70 in our patients, the nephropathy or diabetes itself?” First of all, it is well known that the DN begins before microalbuminuria emerges. Moreover, fractional excretion rate of HSP70 was >1 in our patients suggesting that the urinary HSP70 might originate from the kidney cells. Lebherz-Eichinger et al.Citation30 found urine HSP70 levels to be elevated in the patients with chronic kidney disease stage 4 and 5 comparing the controls and fractional excretion rate of HSP70 to be >1, similar to our study. The authors advocate that HSP70 in urine derived from renal cells according to their findings. In addition, there was no relationship between uHSP70/Cr and HbA1c in our study as well as the study of Calabrese et al.Citation29 In another study, Molinas et al.Citation31 evaluated HSP70 levels in urine, kidney and liver samples of the rats receiving acetaminophen which led to hepatic and renal toxicity. The authors found that HSP70 levels increased in renal cortical tissue and in urine simultaneously, although HSP70 levels in liver tissue did not increase despite the elevation of plasma ALT levels. This study showed that the source of urine HSP70 was kidney cells. Taking into account these points, we consider that nephropathy was responsible for high uHSP70 levels, although our study cannot prove it directly.
The factors contributing to progression of DN are glycemic control, blood pressure control, obesity, presence of puberty at the time of diagnosis of T1DM.Citation1,Citation2 None of the uHSPs/Cr values were found to be related to these prognostic factors in our study.
The limitations of our study are that the number of patients in subgroups is not sufficient for statistical analysis, that very few patients have microalbuminuria in our study group and that follow-up duration is only one year. Despite these limitations, this study has important results. First, this study demonstrated that urine excretion of HSPs increased in diabetic children without uremia suggesting that diabetic kidney injury begins before the onset of microalbuminuria. Second, uHSP70/Cr may be a useful biomarker to detect early phases of diabetic kidney injury in children. Third, uHSP70/Cr may indicate progression of diabetic kidney injury with time. Manifest nephropathy may be prevented with close follow-up of these patients. Further investigations are needed to answer the questions about whether administration of renoprotective agents in patients with high urine HSP70/Cr precludes kidney injury.
Conclusions
Diabetic kidney injury begins before the onset of microalbuminuria. Therefore, new biomarkers are needed to identify diabetic kidney injury at earlier phases. These new biomarkers may allow to diagnose the injury earlier and to find alternative therapeutic options. Urine excretion of HSPs increases in diabetic children without uremia and microalbuminuria. Urine HSP70/Cr may be a useful biomarker to detect early phases of diabetic kidney injury in children. Urine HSP70/Cr may indicate progression of diabetic kidney injury with time.
Disclosure statement
The authors declare that they have no conflict of interest.
Funding information
This work has been supported by a grant from Istanbul University Scientific Research Project Foundation.
References
- Caramori ML, Mauer M. Pathogenesis and pathophysiology of diabetic nephropathy. In: Greenberg A, ed. Primer on Kidney Diseases. Philadelphia, PA: Saunders, 2009:214–223.
- Marcovecchio ML, Chiarelli F. Diabetic nephropathy. In: Avner DE, Harmon EV, Niaudet P, Yoshikawa N, eds. Pediatric Nephrology. Berlin: Springer, 2009:1199–1217.
- Beck FX, Neuhofer W, Müller E. Molecular chaperones in the kidney: Distribution, putative roles and regulation. Am J Physiol Renal Physiol. 2000;279:203–215.
- Musial K, Zwolinska D. Heat shock proteins in chronic kidney disease. Pediatr Nephrol. 2011;26:1031–1037.
- Barutta F, Pinach S, Giunti S, et al. Heat shock protein expression in diabetic nephropathy. Am J Phhysiol Renal Physiol. 2008;295:1817–1824.
- Buraczynska M, Swatowski A, Buraczynska K, Dragan M, Ksiazek A. Heat-shock protein gene polymorphisms and the risk of nephropathy in patients with type 2 diabetes. Clin Sci. 2009;116:81–86.
- Mueller T, Bidmon B, Pichler P, et al. Urinary heat shock protein-72 excretion in clinical and experimental renal ischemia. Pediatr Nephrol. 2003;18:97–99.
- O’Neill S, Harrison EM, Ross JA, Wigmore SJ, Hughes J. Heat-shock proteins and acute ischemic kidney injury. Nephron Exp Nephrol. 2014;126:167–174.
- Vidyasagar A, Wilson NA, Djamali A. Heat shock protein 27 (HSP27): Biomarker of disease and therapeutic target. Fibrogenesis Tissue Repair. 2012;5:7.
- Razzaque MS, Ahsan N, Taguchi T. Heat shock protein 47 in renal scarring. Nephron. 2000;86:339–341.
- Kai M, Nakatsura T, Egami H, Senju S, Nishimura Y, Ogawa M. Heat shock protein 105 is overexpressed in a variety of human tumors. Oncol Rep. 2003;10: 1777–1782.
- Takashima M, Kuramitsu Y, Yokoyama Y, et al. Proteomic profiling of heat shock protein 70 family members as biomarkers for hepatitis C virus-related hepatocellular carcinoma. Proteomics. 2003;3:2487–2493.
- Ohashi S, Abe H, Takahashi T, et al. Advanced glycation end products increase collagen-specific chaperone protein in mouse diabetic nephropathy. J Biol Chem. 2004;279:19816–19823.
- Sanchez-Niño MD, Sanz AB, Sanchez-Lopez E, et al. HSP27/HSPB1 as an adaptive podocyte antiapoptotic protein activated by high glucose and angiotensin II. Lab Invest. 2012;92:32–45.
- Bundak R, Furman A, Gunoz H, Darendeliler F, Bas F, Neyzi O. Body mass index references for Turkish children. Acta Paediatr. 2006;95:194–198.
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576.
- Schwartz GJ, Feld LG, Langford DJ. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263.
- Ficociello LH, Perkins BA, Roshan B, et al. Renal hyperfiltration and the development of microalbuminuria in type 1 diabetes. Diabetes Care. 2009;32:889–893.
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553.
- Phillips AO, Steadman R. Diabetic nephropathy: The central role of renal proximal tubular cells in tubulointerstitial injury. Histol Histopathol. 2002;17:247–252.
- Zachwieja J, Soltysiak J, Fichna P, et al. Normal-range albuminuria does not exclude nephropathy in diabetic children. Pediatr Nephrol. 2010;25:1445–1451.
- Soltysiak J, Skowrońska B, Fichna P, et al. Neutrophil gelatinase-associated lipocalin and cathepsin L as early predictors of kidney dysfunction in children with type 1 diabetes. Endokrynol Pol. 2014;65:479–484.
- Barrera-Chimal J, Pérez-Villalva R, Cortés-González C, et al. Hsp72 is an early and sensitive biomarker to detect acute kidney injury. EMBO Mol Med. 2011;3:5–20.
- Wang Z, Liu L, Mei Q, Liu L, Ran Y, Zhang R. Increased expression of heat shock protein 72 protects renal proximal tubular cells from gentamicin-induced injury. J Korean Med Sci. 2006;21:904–910.
- Chen SC, Guh JY, Chen HC, Yang YL, Huang JS, Chuang LY. Advanced glycation end-product-induced mitogenesis is dependent on Janus kinase 2-induced heat shock protein 70 in normal rat kidney interstitial fibroblast cells. Transl Res. 2007;149:274–281.
- Guh JY, Huang JS, Chen HC, Hung WC, Lai YH, Chuang LY. Advanced glycation end product-induced proliferation in NRK-49F cells is dependent on the JAK2/STAT5 pathway and cyclin D1. Am J Kidney Dis. 2001;38:1096–1104.
- Madamanchi NR, Li S, Patterson C, Runge MS. Reactive oxygen species regulate heat-shock protein 70 via the JAK/STAT pathway. Arterioscler Thromb Vasc Biol. 2001;21:321–326.
- Qi W, Chen X, Gilbert RE, et al. High glucose-induced thioredoxin-interacting protein in renal proximal tubule cells is independent of transforming growth factor-beta1. Am J Pathol. 2007;171:744–754.
- Calabrese V, Mancuso C, Sapienza M, et al. Oxidative stress and cellular stress response in diabetic nephropathy. Cell Stress Chaperones. 2007;12:299–306.
- Lebherz-Eichinger D, Ankersmit HJ, Hacker S, et al. HSP27 and HSP70 serum and urine levels in patients suffering from chronic kidney disease. Clin Chim Acta. 2012;413:282–286.
- Molinas SM, Rosso M, Wayllace NZ, et al. Heat shock protein 70 induction and its urinary excretion in a model of acetaminophen nephrotoxicity. Pediatr Nephrol. 2010;25:1245–1253.