Abstract
Polymorphisms in the vitamin D receptor (VDR) gene have recently been reported to be associated with urinary calculi in pediatric and adult cases, but no studies have looked at the youngest period of life. The purpose of this study was to investigate the role of VDR gene polymorphisms in infantile urolithiasis in a Turkish population. We compared a study group of 104 infants (55 girls and 49 boys, mean age 6.94 ± 3.81 months) with a control group of 96 infants (51 girls and 45 boys, mean age 7.51 ± 3.23) to evaluate their demographics and metabolic risk factors. PCR-based restriction analysis of the polymorphisms on the VDR gene (BsmI and TaqI) showed statistically significant differences between study and control groups (p = 0.001 and 0.043, respectively). In addition, the prevalence of the BsmI genotype was significantly different between the hypercalciuric and normocalciuric stone formers (p = 0.007). Allelic frequencies were similar between the urolithiasis and control groups (p > 0.05). The B allele of BsmI and the A allele of ApaI were more prevalent in the hypercalciuric stone formers than in the normocalciuric stone formers (p = 0.018 vs.0.036, respectively). These results suggest that the BsmI and TaqI VDR genotypes could be candidate genes leading to infantile urolithiasis.
Introduction
The prevalence of urolithiasis is rising, both in countries in which stone disease is endemic, such as Turkey, and also in non-endemic ones,Citation1–5 either because of increasing awareness or because of the greater use of radiologic diagnostic techniques. Factors leading to urolithiasis include metabolic abnormalities, genetics, nutrition, environment, and specific medicines.Citation6,Citation7 Hypercalciuria is the most common metabolic abnormality.Citation2,Citation8 Infantile urolithiasis represents 9–23% of all pediatric cases; approximately, 60% of infants have family history of stone disease.Citation1,Citation2,Citation8
While knowledge is limited on how genetic factors mediate susceptibility to urolithiasis, one important factor is the function of the vitamin D receptor (VDR). Vitamin D plays a critical role in intestinal calcium absorption and renal calcium reabsorption,Citation9 and the VDR, a member of the steroid receptor family, mediates effects of the active metabolite 1,25-dihydroxyvitamin D3.Citation10 More than 60 polymorphisms have been reported in the VDR gene,Citation11 primarily related to such diseases as cancer, renal diseases, and diabetes.Citation12 An increase in the number of VDR has been observed in hypercalciuric rats, and a susceptibility locus has been defined in hypercalciuric patients.Citation13,Citation14 These findings suggest that allelic variation of the VDR gene could be responsible for the genetic susceptibility associated with urolithiasis. Other studies have evaluated the association of the VDR gene with urolithiasis, hypercalciuria, and hypocitraturia,Citation9,Citation12,Citation15 but not for infantile urolithiasis.
The aim of this study is to evaluate urolithiasis in the first year of life, its risk factors, demographic factors, and relationship with VDR gene polymorphisms. Since genotype and allele frequencies are known to vary in different ethnic populations, we focused our investigation on BsmI, ApaI, and TaqI in urolithiasis of infants in Turkey.
Materials and methods
The study group comprised 104 infants (55 girls and 49 boys, mean age 6.94 ± 3.81 mo) who, in our pediatric nephrology clinic, had been diagnosed by ultrasound (US) as having urolithiasis. Microlithiasis was defined as calculi < 3 mm in US examination. Small and newly developed stones, however, may not produce posterior shadowing, especially if less than 3 mm. Infants were fed before US, and those with fever or an acute illness, such as diarrhea were excluded. Their mean birth weight was 2971.5 ± 682 g. Ten of the 104 were born prematurely, but they were not excluded from the study group because prematurity is a known risk factor for stone formation, shown in a recent study to constitute 12% of cases.Citation2 The control group included 96 age- and gender-matched healthy infants (51 girls and 45 boys, mean age 7.51 ± 3.23 mo) proven to be stone-free by USG. Patients with abnormal renal function and renal tubular acidosis were recruited from the control and study groups.
Each subject’s medical history included age, gender, weight, height, birth history, length of breastfeeding in months, time of the start of supplementary feeding, average daily dosage of vitamin D supplementation, previous diagnosis of urinary tract infection, and symptoms, such as restlessness, crying, vomiting, and hematuria. Serum samples were obtained for routine tests. For metabolic investigation, urine was analyzed for calcium, uric acid, oxalate, and citrate; blood was analyzed for vitamin D gene polymorphism, venous blood gas, calcium, phosphorus, 25 hydroxy vitamin D level, alkaline phosphatase, potassium, uric acid, creatinine, and sodium. Measurements for citrate and oxalate were made in the hospital’s clinical laboratory using standard enzymatic and photometric methods (autoanalyzer, Cobbas 6000, Roche, Ben S.r.l. Via Toselli®, 4 20, 127 Milano, Italy and Trinity Biotech Oxalate®, Bray, Ireland, respectively).
A ratio of spot values to creatinine was used to rule out the influence of urinary dilution or concentration. Normal spot urine calcium/creatinine ratios were defined as < 0.8 mg/mg creatinine for infants 0–6 mo and < 0.6 mg/mg for infants 7–12 mo. Normal spot urine oxalate/creatinine levels were accepted as < 0.28–0.26 mg/mg creatinine for infants <6 mo, < 0.11–0.14 mg/mg creatinine for infants 7–24 mo, and spot urine uric acid <2.2 for infants <1 year. For hypocitraturia, a spot urine citrate/creatinine ratio of 0.42 mg/mg creatinine was used.Citation1
Blood samples added to test tubes containing EDTA were used to isolate the genomic DNA from peripheral blood leukocytes by means of a DNA isolation kit (Invitrogen, Carlsbad, CA). All purified DNA samples were stored at +4 °C until PCR application. DNA could be obtained from 78 infants in the study group and 60 infants in the control group.
Vitamin D receptor gene polymorphisms (the BsmI and ApaI sites in intron 8 and the TaqI site in exon 9) were analyzed using the previously described PCR-restriction fragment length polymorphism (PCR-RFLP) assay, but with minor modifications.Citation16 We used 25 μL total PCR mixtures containing 10.0 pmol of each primer, specifically, 1.0 mM dNTP (deoxynucleotide triphosphates), 25 mM MgCl2, 2.5U Taq DNA polymerase in the supplied reaction buffer [Taq Buffer with (NH4)2SO4], and 100–200 ng DNA. PCR was performed with the forward and reverse primers (), with the initial denaturization at 94 °C for 5 min, following 33 cycles of 94 °C for 30 s, 65 °C for 30 s, 72 °C for 30 s, and 72 °C for 5 min. PCR products become visible on 2% agarose gel with ethidium bromide staining, digested by restriction enzymes and then genotyped. Three genotypes for each polymorphism were determined by their distinct banding patterns (). The BsmI band patterns (#FD0964, Thermo Scientific, Waltham, MA) were 825 bp for the BB (AA) genotype; 825, 650, and 175 bp for the Bb (AG) genotype; and 650, 175 bp for the bb (GG) genotype. The ApaI band patterns (#FD1,414, Thermo Scientific) were 740 bp for the AA (TT) genotype; 740, 520, and 220 bp for the Aa (TG) genotype; and 520, 220 bp for the aa (GG) genotype. The TaqI band patterns (#FD0,674, Thermo Scientific) were 495, 245 bp for the TT (TT) genotype; 495, 290, 245, and 205 bp for the Tt (TC) genotype; and 290, 245, 205 bp for the tt (CC) genotype.Citation16
Figure 1. Genotyping for the BsmI, ApaI, and TaqI polymorphims in the VDR gene. (A) Representative gel showing the genotype for BsmI. The first lane of gel contains a 50 bp DNA ladder. (B) Representative gel showing the genotype for ApaI. The first lane of gel contains a 100 bp DNA ladder. (C) Representative gel showing the genotype for TaqI. The first lane of gel contains a 100 bp DNA ladder.
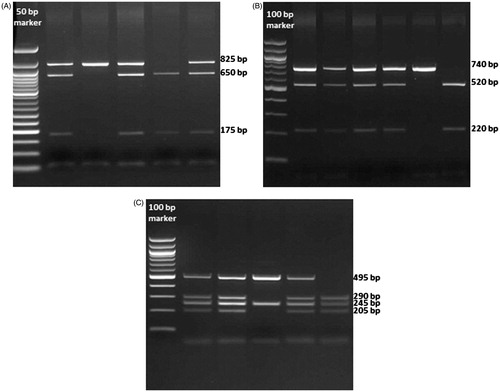
Table 1. Primer sequences in VDR gene polymorphisms.
The genotype analysis was performed using Pearson’s chi-square test. Statistical analyses were performed with SPSS version 17.0 (Chicago, IL). Data are presented as the mean ± standard deviation. Differences in variable means between the stone-forming group and the control group were compared by Student’s t-test. A p-value < 0.05 was considered statistically significant.
The study was approved by our local ethics committee. Written inform consent was obtained from one or both parents of each infant.
Results
Gender distribution and the comparisons of mean ages, weight, height, and birth weight revealed no statistically significant differences between the two groups (p > 0.05) (). Family history of stone disease was detected in 55% of the infants with urolithiasis. In the study group, 43% of infants were exclusively breastfed, 1% was on formula, and 55% were mixed fed; control group percentages were 58, 13, and 27%, respectively. The study group’s daily vitamin D supplementation was slightly higher than that of the controls (499.48 ± 126.58 vs.459.45 ± 124.96, units/day; p = 0.042).
Table 2. Demographic and anthropometric features of the study and control groups.
The clinical characteristics of infants with urolithiasis are shown in . Mean stone diameter was 3.36 (1–14 mm); 30 infants had stones with a diameter > 3 mm. Locations of the stones were 15% on right kidneys, 55% on left kidneys, and 30% on both kidneys.
Table 3. The clinical characteristics of the infants with urolithiasis.
Infants with urolithiasis most commonly presented with restlessness (53.9%). Vomiting was present in 23 patients (22.5%), low appetite in 25 (24.5%), and hematuria in 10 (9.8%). Hydronephrosis were detected in 3, pelvicaliectasia in 2, and renal ectopia in 1 infant. UTI was present in 44 infants.
The study and control groups did not statistically differ in levels of serum calcium (ca), phosphate, 25 hydroxyvitamin D (25OHD), alkaline phosphatase (ALP), uric acid, creatinine, and serum electrolytes (). The urinary calcium/creatinine ratio was significantly higher in stone formers compared with the stone-free control group (p = 0.021), but their urinary excretions of oxalate, citrate, and uric acid did not statistically differ (). Among the stone-formers, hypercalciuria was detected in 21, hypocitraturia in 10, hyperuricosuria in 7, and hyperoxaluria in 2 patients. Three infants had more than one metabolic abnormality: one had both hypercalciuria and hyperuricosuria, one had both hyperuricosiuria and hypocitratruia, and one had hypercalciuria along with hypocitraturia. Also in the study group, 3 patients had elevated serum uric acid levels, 8 had hypercalcemia (Ca > 11 mg/dL), and one had an elevated vitamin D level. Serum phosphorus levels were normal in all the study group infants.
Table 4. Serum and urine metabolic markers of the stone-forming children and controls.
We further analyzed the study group and divided into two groups according to calculi dimensions. A comparison of the demographic and clinical features of infants with microlithiasis (≤3 mm) and stones (>3 mm) disclosed no statistically significant differences for gender, associated symptoms, or metabolic features.
Genotype and allele frequencies for BsmI
As evident in , children with urolithiasis had a different VDR-BsmI genotype distribution from the control group (p = 0.001); therefore, we further analyzed subdivisions of the two groups and found that the bb genotype was greater than the BB + Bb genotype (p < 0.001). Allelic frequencies were not significantly different between infants with urolithiasis and the controls (p = 0.065). The hypercalciuric infants with urolithiasis have a different distribution of the BsmI genotype than the infants with normocalciuric urolithiasis () (p = 0.007). Both the frequency of BB + Bb and the expression of the B allele were greater in the hypercalciuric stone formers than in the normocalciuric stone formers (p = 0.015 and p = 0.018, respectively).
Table 5. Comparision of the VDR BsmI, ApaI, and TaqI genotype distributions between the stone forming children and the controls.
Table 6. Comparison of the VDR BsmI, ApaI, and, TaqI genotype distributions between the hypercalciuric and normocalciuric stone formers.
Genotype and allele frequencies for ApaI
discloses no significant differences between the percent prevalence of the VDR-ApaI genotypes and allele frequencies for the infants of each group (p = 0.222), nor for allelic frequencies (p = 0.318). Furthermore, hypercalciuric infants with urolithiasis had distribution of the ApaI genotype similar to that of normocalciuric infants () (p = 0.077), but the expression of the A allele is significantly greater in hypercalciuric stone formers (p = 0.036).
Genotype and allele frequencies for TaqI
As seen in , the VDR-TaqI genotype differs significantly between the study and control groups (p = 0.043); therefore, we performed further analyses by subdividing the two groups. The TT + tt genotype was greater than the Tt genotype in the study group (p = 0.023), but no significant difference arose in allelic frequencies between the infants with urolithiasis and the controls (p = 0.889). In the hypercalciuric infants with urolithiasis, the distribution of the TaqI genotype and allelic distribution was similar to the infants with normocalciuric urolithiasis, as evident in (p = 0.102 vs.0.052, respectively).
A comparison of infants according to stone size (i.e. <3 mm and ≥3 mm) showed similar polymorphism distributions (p > 0.05).
Discussion
Genetic factors are presumed to be an important risk for the development of urolithiasis, as demonstrated by the evidence of positive family history. Susceptibility genes for stone disease are the VDR gene, calcitonin receptor gene, interleukin 1 gene cluster, urokinase gene, arginine p21, VEGF, AR&ER receptor, and e-cadherin gene.Citation17 The most popular is the VDR gene, which is 5.6 kb in size and is located on chromosome 12q12–14. VDR is related to genomic and non-genomic effects of 1.25(OH)D on renal tubular cells and modulates citrate metabolism and transport of calcium and phosphate.Citation18 Allelic variations are hypothesized to affect translational efficiency or stability in messenger RNA that may result in changes in VDR expression and further changes in target genes.Citation19 For this reason, several polymorphisms of the VDR gene were evaluated, especially four single nucleotide polymorphisms (SNP): ApaI, BsmI, TaqI, and Fox I. The SNPs evaluated in this study are ApaI, BsmI, and TaqI. ApaI and BsmI are located between the 8 and 9 exons, in the 3' region of the VDR gene; TaqI is located on the 3' noncoding sequence in exon 9. These polymorphisms of the VDR gene cause no amino acid change in the protein, but they have strong linkage disequilibrium. Linkage disequilibrium is the non-random association of alleles at linked loci and is required to assess genes predisposition to complex diseases, such as urolithiasis.Citation17,Citation20,Citation21
Many studies have shown that VDR gene polymorphism has an undeniable relationship with urolithiasis and hypercalciuria. Hypercalciuria may result from increased calcium absorption and decreased renal calcium or phosphorus reabsorption and/or enhanced bone demineralization. The VDR overexpression in the intestinal system of genetic hypercalciuric rats and duodenum and kidney cortex of genetic hypercalciuric stone forming rats was demonstrated in experimental data.Citation13,Citation22 Also a link between idiopathic calcium stone formation and the microsatellite marker D12S339 (near the VDR locus) has been proved in humans.Citation14 In one meta-analysis, which included Asians and Caucasians, ApaI and TaqI were found to be related to uroltihiasis susceptibility, and hypercalciuric patients who harbored the b allele had increased risk of urolithiasis.Citation20 The other meta-analysis found a significant association of TaqI polymorphisms with urolithiasis risk.Citation23 Much research has been performed with pediatric or adult populations, but the power of our study is in selecting the first year of life, a spatial age group which has unique characteristics.
The present study revealed a significant difference in the distribution of BsmI and TaqI VDR genotypes between the stone formers and the controls (p = 0.001 and p = 0.043, respectively). Also, the distribution of the BsmI genotype was significantly different in hypercalciuric stone formers when compared with normocalciuric stone formers (p = 0.007). Allelic frequencies were similar between the urolithiasis and control groups (p > 0.05). The B allele of BsmI and the A allele of ApaI were found more prevalent in stone formers with hypercalciuria than in normocalciuric stone formers (p = 0.018 vs.0.036, respectively).
We found that the bb genotype is related to urolithiasis susceptibility in the infancy period, but interestingly, the expression of the bb allele was significantly higher in normocalciuric stone formers compared with hypercalciuric stone formers. We thought that having a bb allele could be an independent risk factor for stone formation in normocalciuric infants, but in other studies from Turkey, Gunes et al.Citation21 and Özkaya et al.Citation24 found no significant association between BsmI VDR polymorphism and stone formation, nor did Relan et al.Citation9
In this study, TaqI distribution differed significantly between the study and control groups, but ApaI did not. In contrast, Ozkaya et al.Citation24 and Seyhan et al.Citation25 found that the frequency of the ApaI AA genotype was significantly higher in the children with calcium nephrolithiasis than in their controls. Ozkaya presented results showing that the distribution of TaqI genotypes of patients with urolithiasis was similar to that of the control group, but Seyhan showed that the TaqI tt genotype was significantly greater in recurrent calcium stone formers.Citation24,Citation25 Gunes found no significant association of TaqI and ApaI with urolithiasis.Citation21 Nishijima et al.Citation26 reported results somewhat similar to ours, finding that TaqI TT and tt genotypes are significantly higher in stone formers, and that the ApaI genotype is similar between the controls and stone formers.
The diversity of these results may be due to racial genotype variability, complexity of urolithiasis etiology, sample size, differences in population characteristics and relation with environment, and gene–gene and gene–environment interactions. As mentioned above, polymorphisms on the VDR gene does not cause a particular amino acid sequence. Ebihara et al.Citation27 performed a study on a novel rat population which retains the intron 8 of VDR during alternative splicing, and showed that this event resulted in negative modulation in the vitamin D signaling pathway through a set of target genes. That the same type of change could occur in the human VDR gene is highly probable, and the relationship between VDR gene polymorphisms and phenotypes could be explained by the differences in the intron.
This study also investigated the daily vitamin D consumption and metabolic risk factors for urolithiasis. Turkey’s health policy is daily administration of 400 units of vitamin D during the infancy period. Dosage of vitamin D was slightly higher in the study group, but their serum 25OHD levels were similar to that of the controls, perhaps because of the 10 premature infants among the 104 in the study group. In the study group, eight infants had hypercalcemia, but vitamin D level was elevated only in one of these, showing that vitamin D sensitivity differs in infants, and genetic differences on the receptor may play an important role in stone formation.
There are some limitations of this study. One is the limited number of participants. The other is the absence of calculus analysis. Also the study population included premature infants. This study was conducted in Turkish infants, so the results should be interpreted carefully for the other ethnicity as this study assessed the genetic predisposition to urolithiasis.
In contrast to adults, metabolic abnormalities are more associated with urolithiasis in childhood: more than 40% of all children with urolithiasis have identifiable metabolic abnormalities.Citation6 This study found that 38.5% of the infants with urolithiasis have some metabolic abnormality, as follows: 20% had hypercalciuria, 9.6% had hypocitraturia, 6.7% had hyperuricosuria, and 1.9% had hyperoxaluria. By way of comparison, in infantile cases, hypercalciuria was detected in 48% in Baştuğ’s study,Citation1 25% in Güven’s study,Citation2 and 30.8% in Alpay’s study.Citation8
In conclusion, BsmI and TaqI polymorphisms seem to be candidate genetic markers in cases of infantile urolithiasis. Identification of susceptible genes may enable determination of high-risk patients and their families, which may then allow for early diagnosis and treatment. This is the first study concerning VDR gene polymorphisms in the first year of life. Further studies should be done in order to gain a better understanding of the dynamics of infantile urolithiasis and the relationship of VDR polymorphisms with stone formation.
Acknowledgements
The authors appreciate the contributions and editorial assistance made by S. Delacroix, a native speaker of English.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Baştuğ F, Gündüz Z, Tülpar S, Poyrazoğlu H, Düşünsel R. Urolithiasis in infants: Evaluation of risk factors. World J Urol. 2013;31:1117–1122.
- Guven AG, Koyun M, Baysal YE, et al. Urolithiasis in the first year of life. Pediatr Nephrol. 2010;25:129–134.
- Milošević D, Batinić D, Turudić D, Batinić D, Topalović-Grković M, Gradiški IP. Demographic characteristics and metabolic risk factors in Croatian children with urolithiasis. Eur J Pediatr. 2014;173:353–359.
- VanDervoort K, Wiesen J, Frank R, et al. Urolithiasis in pediatric patients: A single center study of incidence, clinical presentation and outcome. J Urol. 2007;177:2300–2305.
- Edvardsson V, Elidottir H, Indridason OS, Palsson R. High incidence of kidney stones in Icelandic children. Pediatr Nephrol. 2005;20:940–944.
- Cameron MA, Sakhaee K, Moe OW. Nephrolithiasis in children. Pediatr Nephrol. 2005;20:1587–1592.
- Gürgöze MK, Sarı MY. Results of medical treatment and metabolic risk factors in children with urolithiasis. Pediatr Nephrol. 2011;26:933–937.
- Alpay H, Gokce I, Özen A, Bıyıklı N. Urinary stone disease in the first year of life: Is it dangerous? Pediatr Surg Int. 2013;29:311–316.
- Relan V, Klullar M, Singh SK, Sharma SK. Association of vitamin D receptor genotypes with calcium excretion in nephrolithiatic subjects in northern India. Urol Res. 2004;32:236–240.
- Bid HK, Chaudhary H, Mittal RD. Association of vitamin D and calcitonin receptor gene polymorphism in paediatric nephrolithiasis. Pediatr Nephrol. 2005;20:773–776.
- Basiri A, Shakhssalim N, Houshmand M, et al. Coding region analysis of vitamin D receptor gene and its association with active calcium stone disease. Urol Res. 2012;40:35–40.
- Valdivielso JM, Fernandez E. Vitamin D receptor polymorphisms and diseases. Clin Chim Acta Chim. 2006;371:1–12.
- Li XQ, Tembe V, Horwitz GM, Bushinsky DA, Favus MJ. Increased intestinal vitamin D receptor in genetic hypercalciuric rats. A cause of intestinal calcium hyperabsorption. J Clin Invest. 1993;91:661–667.
- Scott P, Ouimet D, Valiquette L, et al. Suggestive evidence for a susceptibility gene near the vitamin D receptor locus in idiopathic calcium stone formation. J Am Soc Nephrol. 1999;10:1007–1013.
- Zhu C, Ye Z, Chen Z, Xia D, Hu J. Association between vitamin D receptor gene polymorphisms and idiopathic hypocitraturia in the Chinese population. Urol Int. 2010;85:100–105.
- Israni N, Goswami R, Kumar A, Rani R. Interaction of vitamin D receptor with HLA DRB1 0301 in type 1 diabetes patients from North India. PLoS One. 2009;4:e8023
- Mittal RD, Bid HK, Manchandra PK, Kapoor R. Predisposition of genetic polymorphism with the risk of urolithiasis. Indian J Clin Biochem. 2008;23:106–116.
- Mossetti G, Rendina D, Viceconti R, et al. The relationship of 3’ vitamin receptor haplotypes to urinary supersaturation of calcium oxalate salts and to age at onset and familial prevalence of nephrolithiasis. Nephrol Dial Transplant. 2004;19:2259–2265.
- Mossetti G, Vuotta P, Rendina D, et al. Association between vitamin D receptor gene polymorphisms and tubular citrate handling in calcium nephrolithiasis. J Intern Med 2003;253:194–200.
- Zhang P, Nie W, Jiang H. Effects of vitamin D receptor polymorphisms on urolithiasis risk: A meta-analysis. BMC Med Genet. 2013;14:104.
- Gunes S, Bilen CY, Kara N, Asci R, Bagcı H, Yılmaz AF. Vitamin D receptor gene polymorphisms in patients with urolithiasis. Urol Res. 2006;34:47–52.
- Yao J, Kathpalia P, Bushinsky DA, et al. Hyperresponsiveness of vitamin D receptor gene expression to 1,25-dihydroxyvitamin D3: a new characteristic of genetic hypercalciuric stone-forming rats. L Clin Invest. 1998;101:2221–2232.
- Liu W, Chen M, Ma H, Tong S, Lei Y, Qi L. Vitamin D receptor gene (VDR) polymorphisms and the urolithiasis risk: An updated meta-analysis based on 20 case-control studies. Urolithiasis. 42:45–52.
- Özkaya O, Söylemezoğlu O, Mısırlıoğlu M, Gönen S, Buyan N, Hasanoğlu E. Polymorphisms in the vitamin D receptor gene and the risk of calcium nephrolithiasis in children. Eur Urol. 2003;44:150–154.
- Seyhan S, Yavascaoglu I, Kılıcarslan H, Doğan HS, Kordan Y. Association of vitamin D receptor gene Taq I polymorphism with recurrent urolithiasis in children. Int J Urol. 2007;14:1060–1062.
- Nishijima S, Sugaya K, Naito A, Morocumi M, Hatano T, Ogawa Y. Association of vitamin D receptor gene polymorphism with urolithiasis. J Urol. 2002;167:2188–2191.
- Ebihara K, Masuhiro Y, Kitomoto T, et al. Intron retention generates a novel isoform of the murine vitamin D receptor that acts in a dominant negative way on the vitamin D signaling pathway. Mol Cell Biol. 1996;16:3393–3400.
