Abstract
The objective of the present study was to determine whether preischemic administration of syringic acid (SA) would attenuate renal ischemia-reperfusion injury (IRI). Rats were divided into three groups: Sham group; IR group; and IR + SA group. The effects of SA were examined using biochemical parameters including serum ischemia-modified albumin (IMA), total antioxidant status (TAS), total oxidant status (TOS), oxidative stress index (OSI), tissue superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT) and malondialdehyde (MDA). The apoptosis status and histopathological changes were evaluated. After calculating the score for each histopathological change, the total score was obtained by summing all the scores. In the SA group, MDA, IMA, TOS, and OSI decreased significantly compared to the IR group. After SA administration, the increase in GPx activity was found to be significant. Apoptosis decreased significantly in the SA group compared with the IR group. The total score significantly decreased after administration of SA. Taken together, our findings suggest that SA preconditioning is effective in reducing tissue damage induced in kidney IRI. Renal histology also showed convincing evidence regarding the protective nature of SA.
Introduction
Structural and functional damage as a result of transient interruption and delayed restoration of blood flow to the kidneys is called renal ischemia-reperfusion injury (IRI). Renal IRI is a common cause of acute kidney injury which often occurs due to hypovolemic conditions, septic shock, cardiovascular surgery, kidney transplantation and partial nephrectomy. The reasons for the IRI are numerous. Hypotension, hypoperfusion, hypoxia, oxidative stress and renal vasoconstriction contribute to its pathogenesis. Eventually, it becomes a complicated inflammatory condition, including ATP depletion, reactive oxygen species (ROS) accumulation, proinflammatory cytokine production and apoptotic pathway activation. In spite of advances in supportive precautions and preventive strategies, acute kidney injury is associated with high morbidity and mortality. Presently there is no specific medication in clinical use for acute kidney injury arising from IRI.Citation1–4
Polyphenols are among the most common antioxidants in the human diet. These are categorized into four different classes as phenolic acids, flavonoids, lignins, and stilbenes.Citation5 Syringic acid (SA), naturally found in many plants such as olive, grape, pomegranate, green tea and mushroom, is classified within the phenolic acid group.Citation6,Citation7 Along with anti-inflammatory, chemo protective and antimicrobial properties, SA is a powerful antioxidant. It has been shown that SA is a strong inhibitor of low density lipoprotein oxidation, supporting scavenging of free radicals and reducing production of malondialdehyde (MDA).Citation5,Citation8–11
The objective of the present study was to investigate the preventive effect of the active ingredient SA on renal injury due to ischemia reperfusion (IR).
Methods
Experimental animals and study groups
SA was obtained from Sigma-Aldrich’s Turkish distributor, İnterlab (Sigma-Aldrich Lot: S6881). Twenty-four male Wistar-Albino rats aged 8–12 weeks, weighing 275 ± 25 g, were procured from Çanakkale Onsekiz Mart University Experimental Research Application and Research Center. All animals were fed ad libitum with 7–8 mm pellet rat food and tap water. To provide 12 h darkness, 12 h light environment, photoperiodic white fluorescent light was used and the temperature and humidity were held at 21 ± 2 °C and 55–60% humidity. Food and water were withheld from all animals for 12 h prior to the surgical procedures. The methods used for animal experiments were in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals protocols. Permission was granted by Canakkale Onsekiz Mart University Animal Experiments Local Ethics Committee (No: 2014/11–13). The rats were randomly divided into three groups:
Group 1 (Sham operation group, n=8) (Sham) Three intraperitoneal doses (at 0, 12, 24 h) of 1 mL/kg 10% ethanol were administered and the group was Sham operated without renal pedicle clamping 30 min after the last dose.
Group 2 (ischemia-reperfusion group, n=8) (IR) Three intraperitoneal doses of 1 mL/kg 10% ethanol were administered, half an hour after the last dose bilateral renal pedicles were occluded for 30 min, and the rats were sacrificed 60 min after removal of the clamps.
Group 3 (ischemia-reperfusion + syringic acid, n=8) (SA) Three intraperitoneal doses of 10 mg/kg, 10% SA (1 mL/kg solution prepared with 10% ethanol)Citation12 were given before surgery (at 0, 12, 24 h). Half an hour after the last dose, bilateral renal pedicles were occluded for 30 min, and the rats were sacrificed 60 min after removal of the clamps.
At the end of the experiment all animals were deeply anesthetized with ketamine (50–60 mg/kg), the left renal tissue was removed for biochemical analyses and histopathological evaluation. Blood samples were collected from each rat and the rats were killed thereafter.
Surgical procedure
All groups were anesthetized with 5 mg/kg xylazine (Bayer, Istanbul, Turkey) and 50 mg/kg ketamine hydrochloride (Parke Davis, Istanbul, Turkey) with spontaneous respiration at room temperature placed on a sterile disposable towel over a warming pad. A midline incision was made and the renal pedicles were bluntly dissected. Bilateral renal pedicles were occluded for 30 min using an atraumatic mini-clamp and subsequently allowed to reperfuse for 60 min following removal of the clamp.Citation7,Citation13 The animals received 50 mL/kg of warm saline instilled into the abdominal cavity during the entire procedure. Successful ischemia or reperfusion was evaluated by monitoring the transformation in tissue color from red to navy blue or from navy blue to red, respectively. The incision was closed with 3-0 silk and rats were returned to cages to recover. Animals were sacrificed following 60 min of reperfusion. Subsequently, blood samples were collected by heart puncture for biochemical analysis and the left kidney was harvested and subjected to further histopathological and immunohistochemical investigation.
Laboratory analysis
Rat blood and tissue samples were obtained at the end of the procedures in each group of animals. Blood samples were collected from all subjects into tubes with no anticoagulants. The blood samples were centrifuged at 4000 rpm for 10 min for analyses. The resultant serum samples were aliquoted into polypropylene tubes and stored at −80 °C until biochemical analysis. The tissues were prepared at +4 °C to measure their MDA, superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) activities. After washing with 0.9% NaCl, kidney tissue samples were weighed and cut into small pieces, and homogenized in ice-cold tris–HCl buffer (50 mM, pH 7.4) containing 0.50 mL L-1 Triton X-100 solution. Tissues from all experimental groups were homogenized using IKA Ultra-Turrax t 25 Basic (Staufen, Germany). Assays were performed on the supernatant of the homogenate prepared by centrifugation at 4000 rpm for 5 min at +4 °C.
Blood and tissue biochemical markers were determined by spectrophotometric measurements. The protein contents of the tissues were determined according to the method of Lowry et al.Citation14
Serum total antioxidant status (TAS, Product Code: RL0017) and total oxidant status (TOS, Product Code: RL0024) activities were determined with spectrophotometric kits (Rel Assay Diagnostics, Gaziantep, Turkey) as previously described. The ratio of TOS to TAS was used to calculate the oxidative stress index (OSI), an indicator of the degree of oxidative stress. For calculation, the unit of TAS, mmol Trolox equivalent/L, was converted to μmol Trolox equivalent/L, and OSI was calculated as follows: OSI (arbitrary unit) = [(TOS, μmol H2O2 equivalent/L)/(TAS, μmol Trolox equivalent/L)].Citation15 Serum ischemia-modified albumin (IMA) activity was measured by using the colorimetric method discovered by Bar-Or et al.Citation16 The results are reported as absorbance units (ABSUs).
Tissue MDA activities were measured as described by Wasowicz et al.Citation17 The results are expressed as nmol/g protein. The principle of the total (Cu–Zn and Mn) SOD (EC 1.1.15.1.1) enzyme activity method is based on the inhibition of nitroblue tetrazolium (NBT) reduction by O2 generated by the xanthine/XO system.Citation18 Tissue SOD activity was also expressed as units per milligram protein (U mg prot-1). Tissue GPx (EC 1.6.4.2) activity was measured by using the method of Paglia and Valentine.Citation19 Results were expressed as units per milligram protein. Tissue CAT (EC 1.11.1.6) activity was measured according to the method of Aebi.Citation20 Activities were expressed as k (rate constant) per g protein.
Histopathological investigation
The renal tissue samples were immersed in 4% buffered formaldehyde, dehydrated, embedded in paraffin and then cut into 4 μm slices. Tissue samples were examined under a light microscope (Olympus BX51) and photographed with a camera system (Olympus DP72) connected to the microscope. Evaluation of histopathological changes included the dilatation of Bowman’s space (DBS), tubular dilatation (TD), tubular cell sloughing (TCS), tubular cell degeneration – necrosis (TCDN), hyaline casts (HC) and interstitial lymphocytic infiltration (ILI). Tissue damage was quantified in a blinded manner and scored according to the percentage of damaged tubules in the sample: 0 = normal kidney (no damage); 1 = minimal damage (<25% damage); 2 = mild damage (25–50% damage); 3 = moderate damage (50–75% damage); and 4 = severe damage (>75% damage). After calculating the score for each of the histopathological changes, the total score was obtained by summing all the scores. Morphologic assessments were examined by an experienced renal pathologist who was unaware of the groups and treatments.
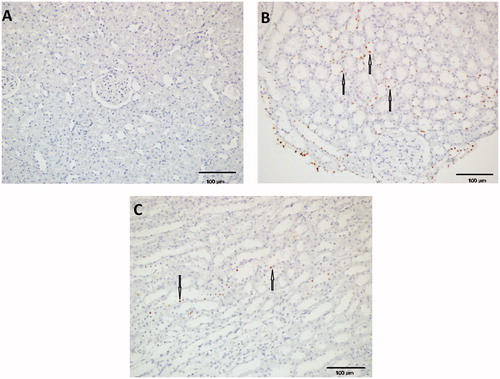
The terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) method, which detects fragmentation of DNA in the nucleus during apoptotic cell death in situ, was employed using an apoptosis detection kit (ApopTag, MILLIPORE, USA). The percentage of positive cells with TUNEL staining in five 400× sights served as apoptosis index.
Statistical evaluation
Statistical analyses were carried out using the statistical software SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). In normally distributed groups, the results are presented as mean ± SD, otherwise they are given as median (min–max). The significance of the differences between groups was determined using the Student unpaired t-test for normal distribution, and using the Mann–Whitney U test for abnormal distribution. Histopathological findings were evaluated with the Kruskal–Wallis test. Pearson correlation coefficient and Spearman correlation coefficient were used to test the strength of any associations between different variables. p-Values less than 0.05 were accepted as the level of significance.
Results
Biochemical findings
Blood biochemical results for the experimental groups are given in . Plasma IMA increased significantly in the IR group compared with the Sham group. In the SA group, it decreased significantly compared to the IR group (p=0.002). TAS values decreased in the IR group. Although this value increased in the SA group compared to the IR group, the increase was not significant. OSI and TOS values increased significantly in the IR group. In the group treated with SA, these values decreased significantly compared to the IR group (respectively p=0.021; p=0.010).
Table 1. Serum IMA, TAS, TOS and OSI activities.
Tissue biochemical results for the groups are given in . SOD, GPx and CAT values decreased in the IR group. Although this value increased in the SA group compared to the IR group, the increase was significant only for GPx (p=0.033). Tissue MDA increased significantly in the IR group compared with the Sham group (p<0.001). In the SA group, MDA decreased significantly compared to the IR group (p=0.001).
Table 2. Tissue SOD, GPx, CAT and MDA activities.
Histopathologic findings
Apoptosis and histopathological analysis results for the groups are given in . A higher apoptosis degree, as well as tissue architecture disarrangement, was found in the IR group when compared to SA and Sham groups. Tunel assay showed an increase in the number of positive cells in the IR group compared with Sham group (p=0.001). Apoptosis decreased significantly in the SA group compared with the IR group (p=0.011) ().
Figure 1. Hematoxylin–eosin (H&E ×200) kidney stained sections showed normal structure of the renal cortex and medulla of Sham operated group (A). Thin arrows show tubules and thick arrows show glomerulus. This was markedly affected in the renal ischemia-reperfusion group (B) with severe tubular dilatation (thin arrow), moderate to severe tubular cell degeneration and necrosis (TCDN; red (small) arrows), and severe dilatation of Bowman’s space (DBS; thick arrows). In addition to this, the number of generated hyaline cast was high (C). The normal structure was somewhat regained in the syringic acid pretreated group (D): mild DBS (thick arrows), mild to moderate TD (thin arrows) and minimal TCDN (red (grey) arrows).
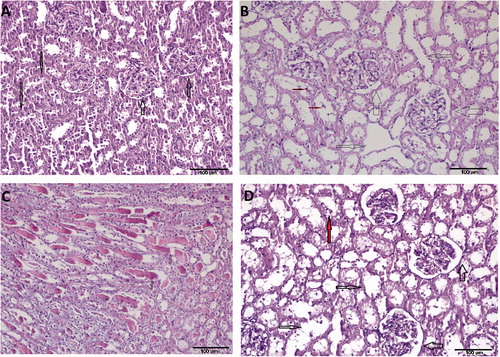
Table 3. Histopathological analysis and apoptosis score results.
DBS, TD, TCS, TCDN, HC and ILI found in the IR group represent IR-induced morphological kidney damage markers. SA preconditioning caused tubular morphology recovery, and as a result, this group had better preserved renal morphology (). Although levels of DBS, TD, HC, ILI and total score were significantly increased in the IR group compared to the Sham group; the increase in TCS and TCDN scores were found to be insignificant. Compared to the IR group, there was a significant decrease in levels of DBS, TD, HC and total score in the SA group. The decrease in levels of TCS and TCDN scores and ILI was not significant. After calculating the score for each of the histopathological changes, the total score was obtained by summing all the scores from morphological kidney damage markers. The total score was increased with IR damage when compared with the Sham group (9.75 ± 0.84, 2.13 ± 0.29; p=0.001). But the total score fell significantly after administration of SA (4.13 ± 0.71; p=0.001 compared with IR group).
Discussion
According to our literature survey, this is the first biochemical and histopathological research into the possibility of a protective effect of SA against renal injury induced by IR in rats. This study demonstrated that IR-induced oxidative stress reduced with SA preconditioning as per biochemical results and histopathological analysis. In the IR group, the biochemical parameters indicating oxidative stress of serum IMA, TOS, OSI and tissue MDA activities were higher compared to the Sham group. It was demonstrated that SA reduces these oxidative stress parameters. Although serum TAS values were lower in the IR group and higher in the SA pretreated group, no significant changes in TAS values were detected between all the studied groups. However, after SA administration, the increase in tissue GPx activity was found to be significant. Also the total histopathological scores of the IR group were significantly higher than the Sham group and this was lower in the SA group.
As is well known, morphological changes indicating ischemic necrosis of renal tubular cells are checked after minimum 30 min of ischemia and at least an hour of reperfusion.Citation13,Citation21,Citation22 Tissue damage in IR is well documented and defined, and includes the early involvement of activated neutrophils with subsequent diapedesis and leukocyte adhesion in the chain of inflammatory events that culminate in tissue disarrangement, fibrosis, necrosis and loss of function.Citation3,Citation13 ROS created during the ischemic process trigger apoptotic and necrotic cell death through several pathways.Citation3,Citation13,Citation22 Various causal factors like ROS, cytokines, and chemokine synthesis contribute to the pathogenesis of kidney injury.Citation4
To reduce kidney damage caused by renal IR, experimental studies of many possible protective agents have been completed. Polyphenols behave as antioxidants by scavenging ROS, which produce oxidative stress and can adversely affect many cellular processesCitation23. In our study, the phenolic compound syringic acid, one of the major benzoic acid derivatives in edible plants and fruits, which has multipharmacological properties such as suppressing fibrosis and strong antioxidant, antiendotoxin, antiproliferative, and anti-cancer effects was used.Citation6,Citation24
Studies in recent years have defined IMA as a new marker for diseases related to inflammation.Citation25 IMA activities are found to be higher in many inflammatory and oxidative stress-associated diseases.Citation26 Several reports proved that IMA increases within a few minutes after ischemia, remains high during 6–12 h and returns to normal within 24 h.Citation27 In our study, serum IMA values were significantly higher in the IR group than those in the Sham group. In the SA treatment group, however, these parameters decreased compared to the IR group.
Clinical studies emphasize that ROS play a major role in the pathogenesis of IR-induced kidney damage.Citation7 It was found that ROS linked to high activities of MDA reacts with nucleic acids, proteins, and lipids, leads to massive protein oxidation and degradation, and lipid peroxidation in biological membranes.Citation28 In recent years, serum lipid peroxidation activities have been analyzed by evaluating the TOS.Citation29 The protecting enzymes, SOD, CAT and GPx, react against the devastating actions of ROS and these molecules comprise the TAS. The advantage of TAS measurement is that it measures the antioxidant capacity of all antioxidants in a biological sample.Citation30 More importantly, the ratio of the TOS to TAS is accepted as the OSI, which is an indicator of oxidative stress.Citation10
Kim et al. found that a caffeic acid–syringic acid hybrid compound showed a strong neuroprotective effect in transient cerebral ischemia.Citation29 Although Kumar et al.Citation24 showed that SA administration preserves the functional capacity of the kidney from adverse effects in hypertensive rats, there was no study showing the effect of SA on renal IR found in our review of the literature.
Itoh et al. reported that SA significantly suppressed the increase in inflammatory cytokines TNF-α, IFN-γ and IL-6; and the suppressive effect of SA on Concanavalin A-induced liver injury might be due to scavenging of ROS generated by activated NADPH oxidase in the lymphocytes.Citation23 They stated that SA could scavenge ROS to suppress hepatocyte death. Consistent with this study, we found that the fibrosis and oxidative stress marker IMA and the valuable oxidative stress parameters MDA, TOS and OSI decreased significantly in the SA group.
Kumar et al. demonstrated that SA significantly inhibited lipid membrane damage as evidenced by the decreased activities of lipid peroxidation products in rats.Citation24 In our study, TOS activity showing serum lipid peroxidation degree reduced significantly with SA therapy. They also illustrated that administration of SA improved the activities of SOD, CAT and GPx. In our study, serum TAS activity was insignificantly lower in the IR group compared to the Sham group and insignificantly increased with SA therapy. However, it was found that tissue GPx activities were significantly increased with SA therapy.
Renal histology also showed convincing evidence regarding the protective nature of SA against kidney injury induced during renal ischemia reperfusion. Apoptosis decreased significantly in the SA group compared with the IR group. The total score indicating the sum of the levels of DBS, TD, TCS, TCDN, HC and ILI was high in the IR group. It was observed that the total score significantly decreased with administration of SA. Briefly, improvements were detected in the SA group in the form of remarkable regression of histopathological changes caused by IR injury. Also, glomeruli and tubules were observed to be apparently healthy.
The present study suggests that treatment with SA preconditioning is effective in reducing tissue damage induced in kidney IRI and also that the protective effect of SA is mediated via reducing MDA, TOS and OSI, increasing GPx activity and preventing apoptosis. Consequently, SA might be helpful in protecting the kidneys from IR induced damage in humans, probably through its antiapoptotic and antioxidant properties. More studies should be conducted to provide a better understanding of the potential benefits and fully assess the action of SA on IR damage to the kidney.
The limitations of the current study are that we did not evaluate the effect of SA in a dose dependent manner. However, the dosage was determined as 10 mg/kg body weight based on previously studies. The other limitation is that there was not the group of Sham + SA.
Disclosure statement
None of the contributing authors have any conflict of interest, including specific financial interests or relationships and affiliations relevant to the subject matter or materials discussed in the manuscript.
References
- Shokeir AA, Barakat N, Hussein AM, et al. Activation of Nrf2 by ischemic preconditioning and sulforaphane in renal ischemia/reperfusion injury: A comparative experimental study. Physiol Res. 2015;64:313--323.
- Lempiainen J, Finckenberg P, Mervaala EE, et al. Dexmedetomidine preconditioning ameliorates kidney ischemia-reperfusion injury. Pharmacol Res Perspect. 2014;2:45--59.
- Wang L, Chen H, Liu XH, et al. Ozone oxidative preconditioning inhibits renal fibrosis induced by ischemia and reperfusion injury in rats. Exp Ther Med. 2014;8:1764–1768.
- Malek M, Nematbakhsh M. The preventive effects of diminazene aceturate in renal ischemia/reperfusion injury in male and female rats. Adv Prev Med. 2014;2014:740647. doi: 10.1155/2014/740647.
- Kakkar S, Bais S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014;2014:952943. doi: 10.1155/2014/952943.
- Itoh A, Isoda K, Kondoh M, et al. Hepatoprotective effect of syringic acid and vanillic acid on CCl4-induced liver injury. Biol Pharm Bull. 2010;33:983–987.
- Sancaktutar AA, Bodakci MN, Hatipoglu NK, Soylemez H, Basarili K, Turkcu G. The protective effects of pomegranate extracts against renal ischemia-reperfusion injury in male rats. Urol Ann. 2014;6:46–50.
- Birosova L, Mikulasova M, Vaverkova S. Antimutagenic effect of phenolic acids. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2005;149:489–491.
- Aziz NH, Farag SE, Mousa LA, Abo-Zaid MA. Comparative antibacterial and antifungal effects of some phenolic compounds. Microbios. 1998;93:43–54.
- Keith ES, Powers JJ. Effect of phenolic acids and esters on respiration and reproduction of bacteria in urine. Appl Microbiol. 1965;13:308–313.
- Morton LW, Croft KD, Puddey IB, Byrne L. Phenolic acids protect low density lipoproteins from peroxynitrite-mediated modification in vitro. Redox Rep. 2000;5:124–125.
- Tokmak M, Yuksel Y, Sehitoglu MH, et al. The neuroprotective effect of syringic acid on spinal cord ischemia/reperfusion injury in rats. Inflammation. 2015;38:1969–1978.
- Soares BL, de Freitas MA, Montero EF, Pitta GB, Miranda F. Jr. Alprostadil attenuates inflammatory aspects and leucocytes adhesion on renal ischemia and reperfusion injury in rats. Acta Cir Bras. 2014;2:55–60.
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275.
- Kosecik M, Erel O, Sevinc E, Selek S. Increased oxidative stress in children exposed to passive smoking. Int J Cardiol. 2005;100:61–64.
- Bar-Or D, Lau E, Winkler JV. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia—A preliminary report. J Emerg Med. 2000;19:311–315.
- Wasowicz W, Neve J, Peretz A. Optimized steps in fluorometric determination of thiobarbituric acid-reactive substances in serum: Importance of extraction pH and influence of sample preservation and storage. Clin Chem. 1993;39:2522–2526.
- Durak I, Yurtarslanl Z, Canbolat O, Akyol O. A methodological approach to superoxide dismutase (SOD) activity assay based on inhibition of nitroblue tetrazolium (NBT) reduction. Clin Chim Acta. 1993;214:103–104.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169.
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126.
- Pararajasingam R, Weight SC, Bell PR, Nicholson ML, Sayers RD. Prevention of renal impairment following aortic cross-clamping by manipulation of the endogenous renal nitric oxide response. Eur J Vasc Endovasc Surg. 2000;19:396–399.
- Ushigome H, Sano H, Okamoto M, et al. The role of tissue factor in renal ischemic reperfusion injury of the rat. J Surg Res. 2002;102:102–109.
- Itoh A, Isoda K, Kondoh M, et al. Hepatoprotective effect of syringic acid and vanillic acid on concanavalin a-induced liver injury. Biol Pharm Bull. 2009;32:1215–1219.
- Kumar S, Prahalathan P, Raja B. Syringic acid ameliorates (L)-NAME-induced hypertension by reducing oxidative stress. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:1175–1184.
- Ellidag HY, Eren E, Aydin O, et al. Ischemia modified albumin levels and oxidative stress in patients with bladder cancer. Asian Pac J Cancer Prev. 2013;14:2759–2763.
- Roy D, Quiles J, Gaze DC, Collinson P, Kaski JC, Baxter GF. Role of reactive oxygen species on the formation of the novel diagnostic marker ischaemia modified albumin. modified. Heart. 2006;92:113–114.
- Anwaruddin S, Januzzi JL, Jr., Baggish AL, Lewandrowski EL, Lewandrowski KB. Ischemia-modified albumin improves the usefulness of standard cardiac biomarkers for the diagnosis of myocardial ischemia in the emergency department setting. Am J Clin Pathol. 2005;123:140–145.
- Chatterjee PK, Cuzzocrea S, Brown PA, et al. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000;58:658–673.
- Kim IH, Yan BC, Park JH, et al. Neuroprotection of a novel synthetic caffeic acid-syringic acid hybrid compound against experimentally induced transient cerebral ischemic damage. Planta Med. 2013;79:313–321.
- Kusano C, Ferrari B. Total antioxidant capacity: A biomarker in biomedical and nutritional studies. J Cell Mol Biol. 2008;7:1–15.