Abstract
Objective To establish the occurrence and intensity of podocyturia and its relation to grade of disease activity, as defined by clinical and laboratory criteria. Methods Prospective, cross-sectional study involving 50 patients with lupus nephritis and 29 controls, which had podocyturia levels determined from random urine samples using an immunofluorescence technique. Disease activity was graded by BILAG (renal criteria) and an additional system used in the service (S2). Results Fifty patients with lupus nephritis (WHO classes III, IV and V), with a median age of 37 years, were evaluated. Of these, 86.5% were female, and 52% were BILAG A. Podocyturia quantification in the lupus nephritis and control groups differed significantly (p = 0.009). This score was higher in relation to classes III, IV and V. The correlation with C3 consumption was stronger (p = 0.011) than with C4. The highest levels were found in the most active groups (A and B of BILAG and S2). Lower podocyturia correlated with a lower dose of prednisone. There was no association with the intensity of proteinuria, hematuria or pyuria, serum creatinine levels, among others. Conclusions Podocyturia assessment, which was performed by immunofluorescence in this study, can be used as an indicator of disease activity with the advantage of being a urinary biomarker. The levels proved to be higher in patients with lupus nephritis than in the controls and were particularly higher in class IV.
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease with frequent renal involvement at diagnosis, particularly characterized by the development of glomerulonephritis, which increases as the disease progresses. One of the greatest difficulties in monitoring SLE and lupus nephritis is that its course is punctuated by episodes of reactivation, which is why various biomarkers are being explored. Podocyturia assessment has recently emerged as an interesting alternative biomarker.Citation1–3 Podocyturia has been identified before the onset of proteinuria in diseases such as diabetes, focal segmental glomerulosclerosis, IgA nephropathy and lupus nephritis.Citation4–7 Under certain conditions, apoptosis of the podocytes has been observed along with consequent detachment into the urinary space, thus leading to podocyturia and podocytopenia. Moreover, the intensity of this phenomenon has been related to the prognosis of kidney disease.Citation5 One of the most common techniques to evaluate podocyturia is indirect immunofluorescence using specific antibodies directed against podocyte antigens. Currently, methodology, the most suitable antibodies for podocyturia assessment, and its applicability as a diagnostic test in the evaluation of renal injury are not yet sufficiently defined. The aim of this study was to investigate the frequency and intensity of podocyturia in lupus nephritis and its relation to the grade of disease activity, as defined by clinical and laboratory criteria.
Materials and methods
This study was approved by the Ethics Committee of the Federal University of São Paulo (UNIFESP) and informed consent was given by the participants.
Patients
Fifty patients were evaluated, all of whom were monitored at the Glomerulopathies Outpatient Unit of UNIFESP, with established SLE diagnosis based on the American College of Rheumatology criteria.Citation8 The categorization of lupus nephritis into classes was defined by renal biopsy according to the World Health Organization (WHO) classification. The histological indices of activity and chronicity described in renal biopsy were also used.Citation9
Grading of disease activity was performed using BILAG scores for renal involvement and a second reinforcing system (S2) of clinical and laboratory criteria observed by the physician at each visit. In S2, the signs and symptoms of the patient and the required immunosuppressive treatment were used as clinical criteria. Routine tests used in monitoring patients with SLE within our service corresponded to the laboratory criteria, as described by Solorzano et al.Citation10 The disease was thus graded as active, moderately active, slightly active or inactive. The need for renal replacement therapy during the study was also evaluated.
The control group consisted of 29 healthy subjects without urinary abnormalities who underwent clinical evaluation and a urine dipstick test at the collection time for podocyturia measurement.
Podocyturia assessment
Midstream urine specimens were collected in a sterile bottle and kept refrigerated at 4–8 °C until processing time. An aliquot was used for urinary protein and creatinine measurements. Approximately 30 mL of urine was transferred to a tube and subjected to centrifugation at 2000 rpm for 5 min. The supernatant was discarded and the sediment resuspended in 50% ethanol. After washing with HDF solution, cytocentrifugation was performed on filter paper attached to a slide.
The prepared slides were fixed with formaldehyde and sucrose in PBS. After washing, they were treated with Triton X-100 (Sigma-Aldrich, St. Louis, MO), and incubated with blocking solution. After a further washing step, the slides were incubated with a primary antibody specific for podocytes, an anti-podocin rabbit antibody (Sigma-Aldrich, St. Louis, MO) at 1:250 dilution, and then with the secondary fluorescein-linked goat anti-rabbit IgG antibody (FITC, Sigma-Aldrich, St. Louis, MO) at 1:320 dilution.
Podocytes were measured by counting the number of podocytes in 30 random fields, corrected by urinary creatinine concentration in the same sample. Only one trained observer evaluated podocyte count in urine and the person was blinded to patient characteristics.
Laboratory tests
Creatinine, albumin, anti-DNA, C3 and C4, CH50 levels were determined in serum. Glomerular filtration rate (GFR) was estimated by the MDRD equation. Additionally, the 24-h proteinuria, urinalysis and protein/creatinine (P/C) ratio in a random urine sample were evaluated.
Creatinine and proteinuria measurements were performed using a commercial kit on an Olympus AU 400 analyzer instrument (Mishima Olympus Co. Ltd., Shizuoka, Japan). In urinalysis, the total counts of erythrocytes and leukocytes were used for comparison (average per field after quantification in 10 fields).
All tests available at the time of the study and necessary to fulfill BILAG and S2 criteria were considered in the overall evaluation of disease activity. Descriptive and inferential statistical analyses were performed the latter including Kruskal–Wallis, Mann–Whitney, Pearson’s Chi-square and the Spearman correlation coefficient. A level of significance α = 5% was used for inferential analysis.
Results
The clinical and laboratory characteristics of the 50 patients and 29 healthy controls are shown in . It is noteworthy that, using BILAG, 52% of patients were classified as A (active), 20% as D (inactive), and the remainder as moderately or mildly active (B and C, respectively), as shown in .
Table 1. Clinical and laboratory characteristics of healthy subjects and patients with lupus nephritis.
Table 2. Distribution of podocyturia levels (podocytes/mg creatinine) in patients with lupus nephritis according to activity grading criteria and evaluated histological characteristics.
All patients had undergone prior renal biopsy at different lag times (indication of the biopsies was not related to this study). Regarding division by histological classification of lupus nephritis (WHO) in those biopsies, 65% had class IV, 29% class V and 5% class III.
Patients with lupus nephritis exhibited median levels of podocyturia (number of podocytes/mg urinary creatinine) that were statistically higher than the podocyturia levels in the controls (p = 0.009).
Podocyturia levels tended to be related to histological class (p = 0.058). and show that patients from class III had lower podocyturia levels than those patients from classes IV and V and that the highest levels corresponded to class IV. When grouping classes III and IV and comparing them with class V, no significant difference in podocyturia levels was observed.
Figure 1. Podocyturia levels (podocytes/mg creatinine) in patients with lupus nephritis according to BILAG and S2 criteria and histological grade.
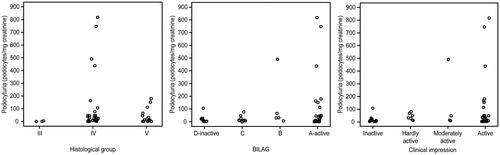
Regarding BILAG and S2 activity classification criteria, the moderately active and active groups showed the highest podocyturia counts. However, again, no statistically significant relationships were observed between podocyturia and activity grade according to either criteria (p = 0.377 and p = 0.140, respectively), or with age (p = 0.336), GFR estimated by MDRD (p = 0.283), 24-h proteinuria (p = 0.449), P/C ratio (p = 0.346), histological activity (p = 0.243), chronicity indices (p = 0.812) or need of renal replacement therapy (p = 0.130).
There was also no statistically significant difference when grouping cases with a higher grade of activity (BILAG A and B; active and moderately active S2) and less active cases (BILAG C and D; S2 mildly active or inactive). However, the highest urine podocyte levels were seen in the groups with the highest activity ().
Individuals with reduced levels of C3 and C4 complement showed higher podocyturia counts than individuals with normal C3 and C4 levels (p = 0.011).
Anti-DNA was positive in only 5 of the cases, and this was the reason why comparisons were not performed between this marker and podocyturia.
In the multivariate analysis (ANOVA) involving C3 (p = 0.014), C4 (p = 0.700), BILAG index (p = 0.250), S2 activity index (p = 0.936), histological class of lupus nephritis (p = 0.586), GFR (p = 0.574), use of renoprotective medications (p = 0.254) and use of sodium mycophenolate (p = 0.129), only C3 levels were statistically related to the podocyturia levels.
Regarding drug treatment, different ranges of corticosteroid doses, oral versus intravenous administration, and whether there was use of other immunosuppressive medications were evaluated. Patients who were taking prednisone at a dosage of 5 mg/day had lower podocyturia levels than patients who were on higher doses (p = 0.083). It is important to point out that all patients were on prednisone, and 5 mg was the lowest dose. The use of mycophenolate sodium and renoprotective drugs (angiotensin-converting enzyme inhibitors and/or angiotensin receptor blockers) was not associated with podocyturia levels.
Discussion
The presence of podocyturia, representing podocyte injury, has been described in various glomerulopathies, including IgA nephropathy, focal segmental glomerulosclerosis, membranous nephropathy and diabetic nephropathy, among others.Citation4,Citation11–15 Nevertheless, studies regarding the meaning and applicability of podocyturia as a biomarker for clinical purposes and its relationship to the pathogenesis of diseases are still in the early stages. Little is known about the profile of podocyturia in lupus nephritis, and the possibility that podocyturia may prove to be an early indicator of activity in this condition is of particular interest. This indicator might precede, for instance, proteinuria increases, as described in preeclampsia.Citation16,Citation17
In podocyturia measurement, antibodies against various proteins are currently being used.Citation2,Citation3,Citation18,Citation19 We tested some of these antibodies and chose the anti-podocin antibodies, which allowed better reproducibility in a previous study conducted by our group than antibodies against nephrin and synaptopodin.Citation20 While the referred study was predominantly methodological, the present one has its focus on clinical application of the laboratory methods tested and previously approved. Here, in a new group of patients, we have also emphasized the use of a widely known system of classification of lupus activity (BILAG) to facilitate understanding and comparison.
It is noteworthy that, in clinical practice, it is difficult in most cases of lupus nephritis to evaluate disease activity using only the established criteria.Citation21,Citation22 Doubt is particularly common because criteria such as hematuria and proteinuria may not always reflect the real presence and intensity of glomerular damage or even translate into renal disease activity. Here an additional urinary biomarker would be useful.
In the present study the highest urinary podocyte levels were found in patients with lupus nephritis clinically classified as active or moderately active. When the parameters used to characterize activity were considered separately, we evaluated complement serum levels, which are widely accepted and even assist in making treatment decisions, as indicative of the “immune” activity of SLE.Citation23 In this study, a significant association was observed between C3 consumption and higher podocytes counts in urine, as well as a greater tendency to higher podocyturia with low levels of C4.
One of the difficulties in studying lupus nephritis is the existence of a considerable variety of histological presentations. A total of 65% of our study population fell within class IV, which was expected, as it is usually more frequent in lupus patients undergoing renal biopsy.Citation24 In searching for possible associations between histological classes and podocyturia, the highest levels were found in patients with class IV. Due to the small number of patients with class III lupus nephritis (5.8%) we combined classes III and IV, who share the condition of proliferative glomerulonephritis (focal and diffuse, respectively), for additional analysis. No statistically significant association was observed with regard to podocyturia counts between these morphologically proliferative subgroups (III and IV) and the nonproliferative subgroup (V). However, the size of the subgroups as well as the different periods of time since renal biopsy may have influenced our results and prevented any differences between them from being ascertained. Small population size is one of the limitations of the present study, and eventual associations could not have been identified due to the sample size. It is known that in other glomerulopathies, excluding lupus nephritis, certain authors have observed higher urine podocyte counts in proliferative glomerulonephritis.Citation25
No relationship between levels of GFR and podocyturia was observed. About 70% of patients had estimated GFR equal to or greater than 60 mL/min, and podocyturia was not significantly different between them and those with reduced estimated GFR. A recently published study by our group likewise did not observe such association.Citation20
Although a statistical relationship between proteinuria and podocyturia has not been demonstrated, we cannot rule out a possible association between these parameters, as described by certain authors.Citation4,Citation7 It is possible that other factors, such as size sample, affected the result. Alternatively, higher levels of proteinuria or podocyturia have been described at different times in the same patient. Follow-up studies indicate that the start and end of podocyturia and proteinuria changes are different, with the former being detected earlier.Citation3
There are reports suggesting that the previous use of various therapies, particularly immunosuppressants, statins and antiplatelet drugs, may interfere with the excretion of podocytes in the urine.Citation26,Citation27 Most of our patients had used medications from the former two groups.
Our lupus nephritis sample revealed that patients who were taking prednisone at a dosage of 5 mg/day tended to have lower podocyturia than patients who required higher doses.Citation25,Citation27 In general, this low dosage is used as a maintenance treatment and reflects in our patients’ renal activity control after initial treatment. On the other hand higher doses are administered in more serious and active stages of the disease. Certain authors have observed the disappearance of podocyturia after 3 months of treatment with intravenous and oral corticosteroids,Citation27 thus reinforcing our findings; in addition, othersCitation25 have demonstrated that immunosuppressive therapy reduced podocyturia. However, the same was not observed by Wang et al.Citation1 in relation to the use of corticosteroids in combination with cyclophosphamide and/or mycophenolate; in that study, patients with complete or partial remission did not differ from nonresponsive cases in terms of urinary podocyte markers.
In relation to therapeutic measures, we evaluated if blockade of the renin–angiotensin system interferes with podocyturia levels, but no significant difference was found between the podocyturia of patients using these medications and of patients who had not. As the patients in our study were taking various drugs, further studies are needed to evaluate this aspect more clearly.
In conclusion, our study suggests that podocyturia measurement would possibly be useful as an indicator of disease activity in patients with lupus nephritis. One must consider that podocyturia is simple to carry out for being a urinary evaluation and, as has happened with other biomarkers, this test may be able to provide an early warning sign regarding episodes of activity. The results of this study should be regarded as a contribution to the diagnosis of active lupus nephritis, which need to be confirmed in follow-up studies.
Disclosure statement
All the authors declare no conflict of interests.
Funding information
J.B.M. and G.M.K. received research grants from the Brazilian Council for Scientific and Technological Development (CNPq) during this study.
References
- Wang G, Lai FM, Tam LS, et al. Messenger RNA expression of podocyte-associated molecules in urinary sediment of patients with lupus nephritis. J Rheumatol. 2007;34:2358–2364.
- Petermann A, Floege J. Podocyte damage resulting in podocyturia: A potential diagnostic marker to assess glomerular disease activity. Nephron Clin Pract. 2007;106:c61–c66.
- Yu D, Petermann A, Kunter U, Rong S, Shankland SJ, Floege J. Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J Am Soc Nephrol. 2005;16:1733–1741.
- Hara M, Yanagihara T, Kihara I. Cumulative excretion of urinary podocytes reflects disease progression in IgA nephropathy and Schonlein-Henoch purpura nephritis. Clin J Am Soc Nephrol. 2007;2:231–238.
- Skoberne A, Konieczny A, Schiffer M. Glomerular epithelial cells in the urine: What has to be done to make them worthwhile? Am J Physiol Renal Physiol. 2009;296:F230–F241.
- Konieczny A, Ryba M, Wartacz J, Czyzewska-Buczynska A, Hruby Z, Witkiewicz W. Podocytes in urine, a novel biomarker of preeclampsia? Adv Clin Exp Med. 2013;22:145–149.
- Vogelmann SU, Nelson WJ, Myers BD, Lemley KV. Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol. 2003;285:F40–F48.
- Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277.
- Austin HA III, Muenz LR, Joyce KM, et al. Prognostic factors in lupus nephritis. Contribution of renal histologic data. Am J Med. 1983;75:382–391.
- Solorzano G. Marcadores de atividade lúpica. 1 ed. São Paulo: Sarvier; 2008:116–120.
- Petermann AT, Krofft R, Blonski M, et al. Podocytes that detach in experimental membranous nephropathy are viable. Kidney Int. 2003;64:1222–1231.
- Petermann AT, Pippin J, Krofft R, et al. Viable podocytes detach in experimental diabetic nephropathy: Potential mechanism underlying glomerulosclerosis. Nephron Exp Nephrol. 2004;98:e114–e123.
- Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: Podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005;54:1626–1634.
- Lemley KV, Lafayette RA, Safai M, et al. Podocytopenia and disease severity in IgA nephropathy. Kidney Int. 2002;61:1475–1485.
- Achenbach J, Mengel M, Tossidou I, et al. Parietal epithelia cells in the urine as a marker of disease activity in glomerular diseases. Nephrol Dial Transplant. 2008;23:3138–3145.
- Garovic VD, Wagner SJ, Turner ST, et al. Urinary podocyte excretion as a marker for preeclampsia. Am J Obstet Gynecol. 2007;196:320 e1–327.
- White WM, Garrett AT, Craici IM, et al. Persistent urinary podocyte loss following preeclampsia may reflect subclinical renal injury. PloS One. 2014;9:e92693
- Jefferson JA, Alpers CE, Shankland SJ. Podocyte biology for the bedside. Am J Kidney Dis. 2011;58:835–845.
- Aita K, Etoh M, Hamada H, et al. Acute and transient podocyte loss and proteinuria in preeclampsia. Nephron Clin Pract. 2009;112:c65–c70.
- Sabino AR, Teixeira Vde P, Nishida SK, Sass N, Mansur JB, Kirsztajn GM. Detection of podocyturia in patients with lupus nephritis. J Bras Nefrol. 2013;35:252–258.
- Yee CS, Isenberg DA, Prabu A, et al. BILAG-2004 index captures systemic lupus erythematosus disease activity better than SLEDAI-2000. Ann Rheum Dis. 2008;67:873–876.
- Reyes-Thomas J, Blanco I, Putterman C. Urinary biomarkers in lupus nephritis. Clin Rev Allergy Immunol. 2011;40:138–150.
- Dall’Era M, Stone D, Levesque V, Cisternas M, Wofsy D. Identification of biomarkers that predict response to treatment of lupus nephritis with mycophenolate mofetil or pulse cyclophosphamide. Arthritis Care Res (Hoboken). 2011;63:351–357.
- Mok CC, Kwok RC, Yip PS. Effect of renal disease on the standardized mortality ratio and life expectancy of patients with systemic lupus erythematosus. Arthritis Rheum. 2013;65:2154–2160.
- Rodrigues PG, Bringhenti RN, do Nascimento JF, et al. Expression patterns of podocyte-associated mRNAs in patients with proliferative or non-proliferative glomerulopathies. Int J Clin Exp Pathol. 2014;7:2185–2198.
- Nakamura T, Ushiyama C, Hirokawa K, et al. Effect of cerivastatin on proteinuria and urinary podocytes in patients with chronic glomerulonephritis. Nephrol Dial Transplant. 2002;17:798–802.
- Nakamura T, Ushiyama C, Shimada N, et al. Effect of the antiplatelet drug dilazep dihydrochloride on urinary podocytes in patients in the early stage of diabetic nephropathy. Diabetes Care. 2000;23:1168–1171.
