Abstract
Purpose Renal ischemia/reperfusion (I/R) injury is a common clinical problem associated with significant mortality and morbidity. One newly described strategy to reduce this damage is remote perconditioning (RPEC), in which short-time ischemia of a limb during renal ischemia reduces the I/R-induced kidney injury. This study aimed to assess whether RPEC confer protection through changes in pro-inflammatory mediators. Methods Rats were subjected to right nephrectomy and randomized into: sham (no intervention), I/R (subjected to 45-min left renal ischemia) and RPEC group (subjected to four cycles of 5-min I/R of the femoral artery administered during renal ischemia). After 24-h, blood, urine, and kidney samples were collected. Biochemical indicators of renal dysfunction were measured in the cases of Neutrophil gelatinase-associated lipocalin (NGAL), and N-acetyl-B-diglucosaminidase (NAG) activity. Inflammatory cytokines [interleukin (IL)-6 and tumor necrosis factor-alpha, TNF-α] expression in the renal tissues as well as Periodic acid-Schiff stained histological sections were evaluated. Results I/R resulted in renal dysfunction, as evidenced by higher renal NGAL expression and urinary NAG activities. This was accompanied by increased TNF-α and IL-6 expressions as well as histological changes in this group. However, RPEC improved renal histology and function compared with the I/R group. Furthermore, the RPEC group showed decreases in TNF-α and IL-6 expression. Conclusions These results suggest that RPEC reduces the dysfunction and injury associated with I/R of the kidney. This technique reduced the pro-inflammatory cytokine in the kidney. RPEC could be a promising strategy against I/R-induced acute kidney injury partly by down-regulation of inflammatory mediators.
Introduction
In clinical settings, renal ischemia/reperfusion (I/R) injury is observed either by direct renal ischemia during kidney surgery and transplantation, or indirectly in shock states followed by resuscitation (e.g., hemorrhage and sepsis). I/R injury (IRI) is one of the main causes of acute kidney injury (AKI) which is a common condition that develops in some of hospitalized patients. The occurrence of AKI is associated with mortality, and also with high financial costs. There is much evidence that destructive events during I/R injury (IRI) are triggered or mediated by a complex response to I/R, including formation of reactive oxygen species (ROS), cytokines, and chemokines. IRI is associated with oxidant-induced damage secondary to increased generation of ROS and a decrease in endogenous anti-oxidant availability at the cellular level. In addition, IRI is associated with a distinct inflammatory response characterized by an increase in inflammatory cytokine production and neutrophil accumulation in the ischemic tissue. Both oxidants and pro-inflammatory mediators contribute to tubular damages and post-ischemic renal injury.Citation1,Citation2
In recent years, many studies have focused on innate tissue protective strategies that attenuate the destructive effects of IRI. For example, remote ischemic conditioning, which was first described in 1993 by Przyklenk et al.Citation3 in the heart, decreased IRI when applied before ischemia. Remote ischemic conditioning involves short-time ischemic stimulus of an organ or tissue that affords protection to a remote organ or tissue subjected to a long-time ischemic insult. Short time I/R of a remote organ can be applied before the onset of a prolonged ischemia which is termed remote ischemic pre-conditioning (RIPC). The inherent tissue protection of RIPC has been applied to clinical situations where the onset of ischemia is predictable, including cardiac surgery and transplantation. In the kidney, RIPC has been reported to attenuate post-ischemic injury.Citation4 Although RIPC reduces post-ischemic injury in many organs, its application requires foreknowledge of the ischemic event, which is difficult to anticipate in most circumstances, with the exception of surgery and transplantation.
Recently, studies have attempted to circumvent this issue by modification of the reperfusion phase. Ischemic post-conditioning (PoC), defined as a series of brief alternating periods of arterial reperfusion and re-occlusions applied at the onset of reperfusion, has emerged as a clinically promising innate protective maneuver. We have recently demonstrated the protective effect of remote PoC in a renal IRI model.Citation5 However, post-conditioning has also limited clinical applicability and is mostly applicable to patients undergoing manual reperfusion.
Some detrimental effects of AKI begin during ischemia and before reperfusion which may result in the activation of programmed cell death during the reperfusion phase. Thus, it may be more protective to induce the ischemic conditioning strategies during ischemia, especially during prolonged ischemic periods. Furthermore, because the timing of ischemia is usually unpredictable, clinically relevant renoprotective strategies must be effective when delivered after the onset of ischemia, targeting the reperfusion-induced injury. Regarding this, Schmidt et al.Citation6 described the concept of remote perconditioning (RPEC). They demonstrated that, transient episodes of lower limb I/R during myocardial ischemia reduce myocardial IRI. We have recently reported that RPEC stimulus, induced by transient ischemia of the limb, can provide potent renoprotection when delivered during renal ischemia and before reperfusion in an animal model.Citation7 In this study, we investigated the effectiveness of RPEC on glomerular and tubular protection through changes in pro-inflammatory mediator expression of the kidneys.
Materials and methods
Animal maintenance and preparation
All the processes of the study were approved by the Animal Ethics Committee of Tehran University of Medical Sciences in accordance with the Animals (Scientific Procedures) Act 1986. Male Sprague–Dawley rats, weighing 220–270 g, were kept in a temperature-controlled environment, had a 12-h light/dark cycle, and were freely allowed standard chow pellets and drinking water. Randomly selected rats were anesthetized by an intraperitoneal injection of sodium pentobarbital (60 mg/kg). Additional doses (12 mg/kg) were administered as needed. Core body temperature was maintained at 37 °C using a heating pad. Systolic blood pressure and heart rate were monitored by a tail-cuff connected to a pulse transducer device. The transducer was connected to a PowerLab/4SP data-acquisition system. Animals with blood pressure below 60 mmHg were excluded.
Remote perconditioning protocol
Preparation of left femoral artery was performed before the beginning of renal ischemia as the following. A small skin incision was made in the left thigh and the femoral neurovascular bundle was exposed. The femoral artery was then separated from the femoral vein and nerve at the proximal position near the groin. Clamping and declamping of femoral artery with an atraumatic microvessel clamp was performed for induction of RPEC. The protocol of RPEC was four cycles of 5-min of ischemia and 5-min of reperfusion just at the beginning of the renal ischemia.
Experimental protocol
Three groups with a minimum of six animals in each were used. The total anesthesia time was equal in all groups. All animals underwent right nephrectomy through a flank incision. In addition, each group was subjected to a specific procedure as follows:
Sham-operated group (sham): Only underwent laparotomy, mobilization of the kidney, and mobilization of the right femoral vascular bundle;
Ischemia/reperfusion group (I/R): The left renal pedicle was rendered ischemic for 45 min followed by 24 h reperfusion, using a bulldog clamp;
Remote perconditioning group (RPEC): Four cycles of 5 min ischemia followed by 5 min reperfusion of lower limb (achieved by clamping and declamping of the left femoral artery) were applied starting at the beginning of renal ischemia.
At the end of the surgery, the wounds were sutured, animals were placed in metabolic cages and urine was collected over a period of 24 h. After 24 h reperfusion, the rats were killed and the serum was extracted from inferior vena cava. The animal’s kidneys were harvested before being washed and dissected in cold phosphate-buffered saline. Part of the kidney was fixed in 10% formalin for histological evaluation, and the rest was snap-frozen in liquid nitrogen and stored at −70 °C until further use. For evaluation of gene expression, a part of kidney was immersed in RNA later before being snap-frozen.
Assessment of post-ischemic renal impairment
Urine samples were collected at the end of the reperfusion period and total volume recorded. Activity of urinary N-acetyl-β-d-glucosaminidase (NAG), a specific indicator of tubular damage was measured based on the enzymatic hydrolysis of p-nitrophenyl-n-acetyl-glucosaminide at pH 4.4 and the subsequent detection of liberated p-nitrophenol at 405 nm by spectrophotometry.Citation8
Renal tissue Neutrophil gelatinase-associated lipocalin (NGAL) expression as one of the most dramatically up-regulated genes in the kidney after ischemia and a sensitive biomarker of acute renal damage was evaluated by quantitative real-time RT-PCR which is described at the following.
Concentrations of blood urea nitrogen (BUN) and creatinine in the serum were measured by colorimetric methods, using Hitachi 704 autoanalyzer (Hitachi, Tokyo, Japan).
Quantitative real-time RT-PCR analysis of mRNA expression
For analysis of gene expression, total RNA was extracted from snap-frozen renal tissues using RNeasy Mini kit (Qiagen, Valencia, CA), according to the manufacturer protocol. RNA quality was tested using NanoDrop (Thermo Scientific). After that, the first strand cDNA synthesis was carried out using RevertAid M-MuLV enzyme in the reaction mixture according to the instructions of the manufacturer (Vivantis, Surrey, UK). Real-time PCR with SYBR Green fluorescence detection was performed using an ABI Prism 7000 ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Carlsbad, CA) with specific primers of IL-6 and NGAL (for sequences of primer pairs, see ). For normalizing the difference in the amount of used cDNAs, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) a housekeeping gene was used as the internal standard. All data were normalized against the GAPDH mRNA levels and expressed as fold increases relative to control ± SEM.
Table 1. Sequences of primers for NGAL and IL-6.
Western blot analysis of TNF-α
TNF-α in the renal tissue were detected by Western blot analysis. Protein was extracted from the tissue by homogenizing samples in ice-cold lysis buffer [50 mmol/L Tris-HCl, 150 mmol/L NaCl, 0.1% Triton X-100, 0.25% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1 mmol/L EDTA and 1% protease inhibitor cocktail], followed by two cycles of centrifugation at 15 700 × g for 15 min at 4 °C. Equal amounts of proteins were loaded onto 12.5% SDS-PAGE and transferred to nitrocellulose membrane. Protein concentrations of lysate were measured by the Bradford assay. Equal amount of proteins from each sample were loaded onto 12% SDS-PAGE and then blotted onto polyvinylidene difluoride membranes. Membranes were blocked by incubation with 2% skim milk prepared in TBST (Tris-buffered saline and 0.1% Tween 20) for 75 min at room temperature and then incubated with TNF-α antibody (Cell Signaling, Danvers, MA) overnight at 4 °C. After they were washed three times in TBST, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Cell Signaling, Danvers, MA) for 60 min at room temperature and finally visualized using the enhanced chemiluminescence detection system (GEHealthcare, Amersham, UK). To confirm that loading was equal, the membrane was stripping and reprobing with β-actin rabbit monoclonal antibody (Cell Signaling). Finally, the quantification of the TNF-α protein bands intensity was performed using Image J software (National Institute of Health, Bethesda, MD) and was normalized to the corresponding band density of the β-actin bands.
Histological assessment
Kidneys were processed for evaluation with light microscopy, according to standard procedures. Tissues were collected and fixed in buffered 10% formalin, processed for paraffin sectioning, sectioned at 4 μm, and stained with periodic acid-Schiff reagent (PAS). Histological evaluation included loss of brush borders, tubular dilatations, cellular degenerations and formation of luminal debris.
Statistical analysis
All data were expressed as the mean ± SEM. Statistical analyses were performed with SPSS software version 11.5 (SPSS Inc., Chicago, IL) and Graph Pad Prism version 5.01 (Graph Pad, La Jolla, CA). Data were then analyzed by one-way analysis of variance and individual group means compared using Tukey’s multiple group comparison test. p Values of <0.05 were considered statistically significant.
Results
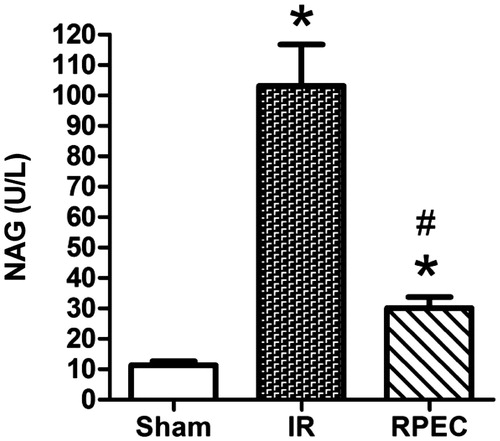
Remote perconditioning decreases the urinary NAG activity
Forty-five minutes of renal ischemia and 24 h reperfusion resulted in a significant increase in NAG activities compared with that in the sham group (103.2 ± 13.6 vs. 11.4 ± 1.3 U/L, respectively; p < 0.05). However, RPEC significantly decreased NAG (30.1 ± 3.6; p < 0.05) compared with the I/R group ().
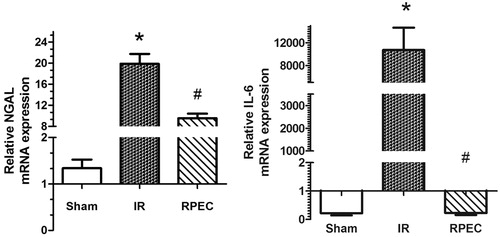
Remote perconditioning attenuates NGAL and Il-6 gene expression in the kidney
Significant up-regulation of NGAL and IL-6 gene expressions were seen in the I/R animals compared with sham group. These values were significantly attenuated by RPEC ().
Remote perconditioning decreases the BUN and creatinine levels
Forty-five minutes of renal ischemia and 24 h reperfusion resulted in a significant increase in BUN (108.2 ± 6.8 vs. 17.5 ± 1.9 mg/dL; p < 0.05) and creatinine (3.54 ± 0.48 vs. 0.62 ± 0.21 mg/dL; p < 0.05) levels compared with that in the sham group. However, RPEC significantly decreased BUN (52.2 ± 6.2 mg/dL; p < 0.05) and creatinine (1.51 ± 0.36 mg/dL; p < 0.05) compared with the I/R group. These data confirm the results of our first report of RPEC which was published in the Journal of Transplantation in 2011.Citation5
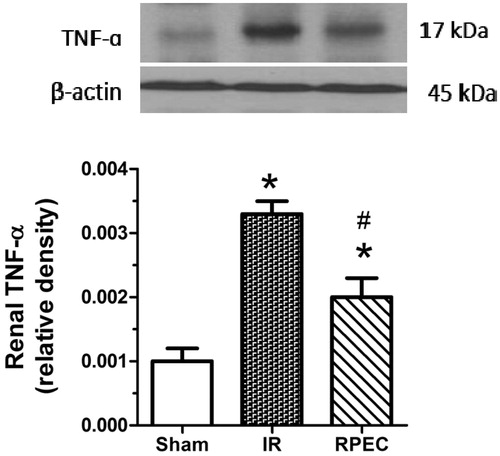
Remote perconditioning attenuates TNF-α protein expression in the kidney
Significant up-regulation of TNF-α protein expression was seen in the I/R compared with sham group. However, RPEC significantly attenuated TNF-α expression compared with the I/R group ().
Figure 3. Effects of RPEC on renal TNF-α protein expression. I/R, ischemia/reperfusion group; RPEC, remote perconditioning group; Top panels show representative western blot data; quantitative results were obtained from densitometric analysis of scanned X-ray films. Data were normalized against β-actin as a loading control and are shown as relative density. Each column and bar represents the mean ± SEM (n = 3–5 in each group). *p < 0.05 compared with the sham group; #p < 0.05 compared with the I/R.
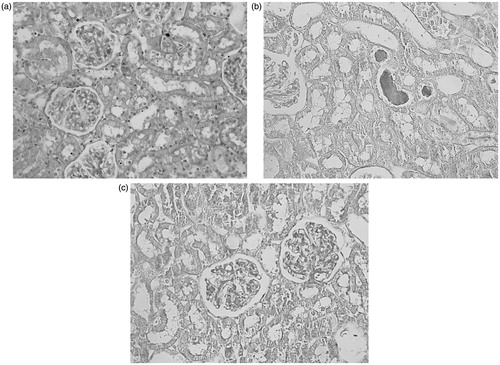
Remote perconditioning improves morphological damages
Sections from the sham group exhibited only minimal or no changes. Brush borders are healthy and observable. However, in the ischemia/reperfusion group brush borders are completely lost. Cellular destruction and severe tubular dilatation due to cellular damages are seen. Cast formation and obstruction of the more tubules are present. In the RPEC group, some of the brush borders are preserved. Flattening of the tubules and destructive changes in the cells are less comparing to the IR group ().
Discussion
Remote ischemic perconditioning is the newest ischemic conditioning method described to mitigate ischemia and reperfusion injury in many organs, such as heart, brain and liver.Citation9,Citation10 In this study, remote perconditioning treatment suppressed renal pro-inflammatory mediator expression demonstrated by decreased TNF-α and IL-6 expressions. It was associated by favorable histological outcome. Furthermore, RPEC attenuated the ischemia/reperfusion-induced renal dysfunction indicated by lessen renal expression level of NGAL as well as decreased urinary NAG activity.
In this study, for post-ischemic renal function assessment, we first measured NAG activity as a urinary biomarker of kidney injury. This marker was significantly decreased in the RPEC animals compared with the I/R group. Also, we assessed the plasma levels of BUN and creatinine as the traditional renal functional indices which was significantly lower in RPEC-treated animals compared with I/R group. However, since in some cases, these markers might not be sensitive enough to detect early kidney injury. We also detected NGAL expression in the renal tissues, a biomarker of early ischemic acute kidney injury. It is reported that NGAL levels in patients with acute kidney injury is associated with the severity of their prognosis which make it a useful biomarker for acute renal damages.Citation11 NGAL gene expressions were significantly induced in the I/R group. It was accompanied with morphological damages in the renal tissue. However, RPEC significantly improved renal function and histological damages. The present findings are consistent with those of other studies, which revealed that ischemic conditioning inhibits the effects I/R in functional and morphological impairment of ischemic injured kidney.Citation12
There are experimental and clinical studies demonstrating the potent necrosis size-reducing effect of RPER in the myocardium or liver.Citation9,Citation10 Recently Kiss et al. published interesting results regarding lower limbs RPER in a rat model of myocardial infarction. They reported significant necrosis reduction with the application of perconditioning compared with non-treated animals.Citation9 In our study, we attempted to evaluate the effects of RPER on histopathologic alterations of renal tissue after reperfusion. It was found that RPEC attenuated the morphological changes compared with the sections from I/R group which exhibited complete loss of brush borders, cellular destruction, and severe tubular dilatation due to cellular damages. Considering the results detailed previously, this study revealed remarkably attenuate target tissue injury on application of RPER. The data obtained from our study are in line with and complement previous findings of other authors investigating the tissue injury reducing effects of remote per-conditioning.Citation9,Citation10
Inflammatory response is one of the important mechanisms for renal IRI.Citation2 The results of our study showed that renal levels of pro-inflammatory cytokines were significantly up-regulated after 24 h reperfusion in the I/R group compared with the sham-operated animals. This is in agreement with previous works which have demonstrated that I/R can cause nuclear translocation of the nuclear factor-κB (NF-κB) complex from the cytosol in a very early stage of reperfusion. This further caused the elevation of serum pro-inflammatory cytokines, such as TNF-α and IL-6. TNF-α released during renal IRI process can promote a cascade reaction of pro-inflammatory cytokines, which further increases the formation of other pro-inflammatory cytokines, such as IL-1 and IL-6.Citation13 Not only these cytokines cause apoptosis and attract neutrophils to the inflammation site, but also they can be direct stimuli of the production of reactive oxygen species (ROS) and their metabolites. In addition, up-regulation of TNF-α in the ischemic tissue is able to induce mitochondria damage indicated by mitochondria swelling, disruption of crista and reduced density, which would finally induce irreversible renal IRI.Citation14
Remote ischemic perconditioning is a novel concept of protection against IRI in which controlled episodes of ischemia of the limb confers a powerful systemic protection against prolonged ischemia in a distant organ. The most fascinating question, which remains as yet unanswered, is how these brief controlled episodes of ischemia of the limb confer a protection in case of the kidneys.
The results of this study revealed that RPEC produced an anti-inflammatory effect indicated by decreased level of expression of pro-inflammatory mediators. It is in line with other studies showing that other methods of remote ischemic conditioning provoke a systemic protective response involving modulation of immune cells either at post-translational level or through transcriptional regulation.Citation15 Microarray analysis of blood samples obtained from healthy human volunteer subjected to intermittent short-time forearm ischemia revealed suppression of pro-inflammatory genes encoding proteins involve in leukocyte chemotaxis, adhesion, migration, and exocytosis, as well as innate immunity responses, cytokine synthesis, and apoptosis. On the other hand, anti-inflammatory genes were up-regulated.Citation16 In the other study, ischemic pre-conditioning of liver has also been shown to attenuate up-regulation in P-selectin expression and neutrophil infiltration in multiple remote organs through inhibition of systemic TNFα production.Citation17 Further studies showed up-regulation of genes associated with cytoprotection and redox regulation by remote ischemic pre-conditioning of the limb.Citation16 In 2011, Wei et al.Citation18 proposed that remote post-conditioning changes the inflammatory reactions by reduction of polymorphonuclear leukocyte infiltration and monocyte chemoattractant protein-1 expression in the heart ischemic tissue. The result of our study in line with findings of others can be suggestive of functional changes in leukocytes. Then, after reperfusion the conditioned leukocytes perfuse into the ischemic regions which have modulated pattern of gene expression and function.
TNF-α has different actions in different tissues, due to the multiple biological activities. Due to these different actions, TNF-α is sometimes described as representing a double edged sword in IRI, with different effects, depending on time and conditions.Citation19 This study shows that compared with the I/R group, renal levels of TNF-α at 24 h reperfusion was significantly lower in the RPEC group. Jia et al. reported the improvement of kidney allograft function by ischemic pre-conditioning which was associated by decreased TNF-α expression.Citation20 In another study, ischemic conditioning caused a marked decrease in the TNF-α concentration in hepatic homogenates after 1 h of reperfusion which persisted during further phases of reperfusion (6 and 72 h).Citation21 In 2013, Czigany et al. reported the protective effects of RPEC on liver morphology, redox homeostasis, and microcirculation. Also, they proposed the changes of TNF-α expression as a result of RPEC in the liver.Citation10 The protective effect of limb remote conditioning in lung injury after pulmonary resection was associated by decreased serum level of TNF-α and IL-6 in a randomized control trial study by Li et al.Citation22 Taken together, these studies support a possible role for TNF-α in short-time ischemic conditioning treatment.
IL-6 which is released by macrophages in the site of ischemia increases renal inflammation by recruiting more neutrophils into the injured kidney, stimulation of neutrophil release from the bone marrow,Citation23 prevention of neutrophil apoptosis and activation of neutrophils to produce toxic enzymes.Citation24 In our study, increased IL-6 expression in the renal tissues, 24 h after reperfusion is consistent with the previous reports. However, the protective effects of RPEC were associated by down-regulation of IL-6 expression in the kidney. This is in line with the study by Zhou et al. demonstrating that limb ischemic pre-conditioning reduces heart and lung damages after an open heart operation in infants which was associated with decreased plasma level of IL-6.Citation25 Recently, in an animal study of Pan et al. ischemic pre-conditioning reduced brain injury and the systemic inflammatory response, reported by decreased IL-6 levels.Citation26 Altogether, it could be concluded that reduced IL-6 levels may be an important consequence of ischemic conditioning in the ischemic kidney.
Several molecular pathways are implicated in remote ischemic condition models which predominantly implicates systemic multifactorial anti-inflammatory, neuronal, and humoral signaling pathways.Citation27 This involves remote ischemic conditioned-facilitated suppression of the inflammatory response subsequent to I/R and promotion of an anti-apoptotic and anti-inflammatory gene transcription profile in the target ischemic tissue. One important feature of RPEC is that in case of unpredicted ischemic insult, application of ischemic conditioning during the ischemia in the remote organ may release some protective signaling molecules or modulate the pattern of gene expression in the local leukocytes. Therefore, it can be assumed that, at the starting time of reperfusion many candidate protective molecules such as adenosine, nitric oxide or bradykinin, as well as conditioned leucocytes will enter to the insolent region. Therefore, they may attenuate the fore coming reperfusion-induced kidney injury.
Conclusion
Our findings suggest that the protective effect of remote perconditioning is closely related to the decreased expression level of pro-inflammatory mediators, IL-6, and TNF-α in renal tissues. This clinically applicable method may be used in the prevention of renal injury caused by the ischemia/reperfusion which occurs inevitably in surgical procedures when the kidneys remain without blood supply for some times.
Acknowledgements
The authors thank Professor Abolhassan Ahmadiani and Dr Leila Dargahi (Neuroscience Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran) for their help with the western blot assay.
Disclosure statement
All authors declare that they have no conflict of interest.
Funding information
This study was funded by Tehran University of Medical Sciences [grant number 15828] to Mehri Kadkhodaee.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
References
- Glodowski SD, Wagener G. New insights into the mechanisms of acute kidney injury in the intensive care unit. J Clin Anesth. 2014;27:175–180.
- Jiang G, Wang M, Wang L, et al. protective effect of nesfatin-1 against renal ischemia-reperfusion injury in rats. Ren Fail. 2015;24:1–8.
- Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic 'preconditioning' protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–899.
- Yamanaka T, Kawai Y, Miyoshi T, et al. Remote ischemic preconditioning reduces contrast-induced acute kidney injury in patients with ST-elevation myocardial infarction: A randomized controlled trial. Int J Cardiol. 2014;178:136–141.
- Kadkhodaee M, Seifi B, Najafi A, Sedaghat Z. First report of the protective effects of remote per- and postconditioning on ischemia/reperfusion-induced renal injury. Transplantation. 2011;92:e55.
- Schmidt MR, Smerup M, Konstantinov IE, et al. Intermittent peripheral tissue ischemia during coronary ischemia reduces myocardial infarction through a KATP-dependent mechanism: First demonstration of remote ischemic perconditioning. Am J Physiol Heart Circ Physiol. 2007;292:1883–1890.
- Sedaghat Z, Kadkhodaee M, Seifi B, et al. Remote per-conditioning reduces oxidative stress, downregulates cyclo-oxygenase-2 expression and attenuates ischemia-reperfusion-induced acute kidney injury. Clin Exp Pharmacol Physiol. 2013;40:97–103.
- Horak E, Hopfer SM, Sunderman FW. Jr., Spectrophotometric assay for urinary N-acetyl-beta-D-glucosaminidase activity. Clin Chem. 1981;27:1180–1185.
- Kiss A, Tratsiakovich Y, Gonon AT, et al. The role of arginase and rho kinase in cardioprotection from remote ischemic perconditioning in non-diabetic and diabetic rat in vivo. PLoS One. 2014;9:e104731.
- Czigány Z, Turóczi Z, Ónody P, et al. Remote ischemic perconditioning protects the liver from ischemia-reperfusion injury. J Surg Res. 2013;185:605–613.
- Tasanarong A, Hutayanon P, Piyayotai D. Urinary Neutrophil Gelatinase-Associated Lipocalin predicts the severity of contrast-induced acute kidney injury in chronic kidney disease patients undergoing elective coronary procedures. BMC Nephrol. 2013;14:270.
- Chen H, Xing B, Wang L, Weng X, Chen Z, Liu X. Aged kidneys are refractory to ischemic postconditioning in a rat model. Ren Fail. 2014;36:1575–1580.
- Lambertsen KL, Biber K, Finsen BJ. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012;32:1677–1698.
- Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66:486–491.
- Saxena P, Shaw OM, Misso NL, et al. Remote ischemic preconditioning stimulus decreases the expression of kinin receptors in human neutrophils. J Surg Res. 2011;171:311–316.
- Konstantinov IE, Arab S, Li J, et al. The remote ischemic preconditioning stimulus modifies gene expression in mouse myocardium. J Thorac Cardiovasc Surg. 2005;130:1326–1332.
- Peralta C, Fernández L, Panés J, et al. Preconditioning protects against systemic disorders associated with hepatic ischemia-reperfusion through blockade of tumor necrosis factor-induced P-selectin up-regulation in the rat. Hepatology. 2001;33:100–113.
- Wei M, Xin P, Li S, et al. Repeated remote ischemic postconditioning protects against adverse left ventricular remodeling and improves survival in a rat model of myocardial infarction. Circ Res. 2011;108:1220–1225.
- Aggarwal BB. Signalling pathways of the TNF superfamily: A double-edged sword. Nat Rev Immunol. 2003;3:745–756.
- Jia RP, Xie JJ, Luo FY, Zhu JG. Ischemic preconditioning improves rat kidney allograft function after ischemia/reperfusion injury: The role of tumor necrosis factor-alpha. Transplant Proc. 2008;40:3316–3320.
- Helewski K, Kowalczyk-Ziomek G, Czecior E, et al. Protective effect of intermittent clamping of the portal triad in the rat liver on liver ischemia-reperfusion injury. Hepat Mon. 2011;11:445–451.
- Li C, Xu M, Wu Y, et al. Limb remote ischemic preconditioning attenuates lung injury after pulmonary resection under propofol-remifentanil anesthesia: A randomized controlled study. Anesthesiology. 2014;121:249–259.
- Suwa T, Hogg JC, English D, Van Eeden SF. Interleukin-6 induces demargination of intravascular neutrophils and shortens their transit in marrow. Am J Physiol Heart Circ Physiol. 2000;279:H2954–H2960.
- McLoughlin RM, Witowski J, Robson RL, et al. Interplay between IFN-gamma and IL-6 signaling governs neutrophil trafficking and apoptosis during acute inflammation. J Clin Invest. 2003;112:598–607.
- Zhou W, Zeng D, Chen R, et al. Limb ischemic preconditioning reduces heart and lung injury after an open heart operation in infants. Pediatr Cardiol. 2010;31:22–29.
- Pan LN, Zhu W, Li Y, et al. Astrocytic Toll-like receptor 3 is associated with ischemic preconditioning-induced protection against brain ischemia in rodents. PLoS One. 2014;9:e99526.
- Gassanov N, Nia AM, Caglayan E, Er F. Remote ischemic preconditioning and renoprotection: From myth to a novel therapeutic option? J Am Soc Nephrol. 2014;25:216–224.