Abstract
Purpose The objective of this study is to examine the incidence, clinical characteristics, and outcome (90-day mortality) of critically ill Chinese patients with septic AKI. Methods Patients admitted to the ICU of a regional hospital from 1 January 2011 to 31 December 2013 were included, excluding those on chronic renal replacement therapy. AKI was defined using KDIGO criteria. Patients were followed till 90 days from ICU admission or death, whichever occurred earlier. Demographics, diagnosis, clinical characteristics, and outcome were analyzed. Results In total, 3687 patients were included and 54.7% patients developed AKI. Sepsis was the most common cause of AKI (49.2%). Compared to those without AKI, AKI patients had higher disease severity, more physiological and biochemical disturbance, and carried significant co-morbidities. Ninety-day mortality increased with severity of AKI (16.7, 27.5, and 48.3% for KDIGO stage 1, 2, and 3 AKI, p < 0.001). Full renal recovery was achieved in 71.6% of AKI patients. Compared with non-septic AKI, septic AKI was associated with higher disease severity and required more aggressive support. Non-recovery of renal function occurred in 2.5% of patients with septic AKI, compared with 6.4% in non-septic AKI (p < 0.001). Cox regression analysis showed that age, emergency ICU admission, post-operative cases, admission diagnosis, etiology of AKI, disease severity score, mechanical ventilation, vasopressor support, and blood parameters (like albumin, potassium and pH) independently predicted 90-day mortality. Conclusions AKI, especially septic AKI is common in critically ill Chinese patients and is associated with poor patient outcome. Etiology of AKI has a significant impact on 90-day mortality and may affect renal outcome.
Introduction
Acute kidney injury (AKI) is a common clinical problem in critically ill patients and is associated with increased mortality and morbidity.Citation1 The lack of consensus on the definition of AKI before 2004 has resulted in a broad range of estimated incidence in the intensive care unit (ICU). The Acute Dialysis Quality Initiative (ADQI) published the Risk, Injury, Failure, Loss, and End-Stage (RIFLE) criteria in 2004 which defined AKI in terms of changes in serum Cr from baseline and urine output.Citation2 Subsequently, the Acute Kidney Injury Network (AKIN) proposed a more sensitive guideline for the diagnosis of AKI.Citation3 The latest Kidney Disease Improving Global Outcomes (KDIGO) definition is a combination of AKIN and RIFLE.Citation4 Study by Luo et al.Citation5 showed that KDIGO identified more patients with AKI than RIFLE or AKIN classification system.
The etiology of AKI is often multifactorial. However, sepsis has consistently been found to be a leading contributing factor to AKI in critically ill.Citation6 Patients with septic AKI are generally sicker, with a higher disease severity couple with greater abnormalities in acute physiology when compared to patients with non-septic AKI.Citation7 Moreover, septic AKI is independently associated with higher mortality and longer hospital length of stay. Most outcome data on septic AKI come from Caucasians. Data among Chinese population are relatively sparse. Our objective is to examine the incidence, clinical characteristics and outcome (90-day mortality) of critically ill Chinese patients with AKI, especially in those with sepsis.
Subjects and methods
This study was approved by the Hong Kong East Cluster Ethics Committee. Written informed consent was waived. It was a retrospective, single-centered, cohort study conducted at the ICU of Pamela Youde Nethersole Eastern Hospital, a 2300-bed acute care tertiary hospital. The ICU is a 22-bed closed mixed medical-surgical unit with an average admission of 1300 patients per year. Patients electively or emergently admitted from 1 January 2011 to 31 December 2013 were recruited with the exclusion of those receiving chronic renal replacement therapy including hemodialysis, hemofiltration, or peritoneal dialysis. Only the first ICU admission episode was analyzed for recurrent ICU admission during the same hospitalization episode. Admissions involving the same patient in different hospitalization episodes were treated independently. Medical records were reviewed. Patients were followed till 90 days from ICU admission or death, whichever occurred earlier. Demographics, diagnosis, clinical characteristics, severity of kidney injury, and outcome were recorded.
Definitions
Septic AKI was defined as the development and progression of sepsis associated AKI in severe sepsis without other apparent cause.Citation7–9 Severe sepsis was defined according to the American College of Chest Physician (ACCP)/Society of Critical Care (SCCM) criteria,Citation10 i.e. Systemic Inflammatory Response Syndrome in the presence of suspected or known infection which led to organ dysfunction. Cardiogenic AKI was defined as AKI due to type I cardiorenal syndrome.Citation11 Hypovolemia associated AKI was defined as AKI due to volume depletion (clinical dehydration ± hypotension) in the absence of other apparent cause of AKI. Those defined as “others” included post-renal causes, contrast induced AKI, glomerulonephritis, and other. For patients with more than one potential causes of AKI, the predominant cause was decided based on clinical judgment.
We used KDIGO criteria to diagnose and grade the severity of AKI ().Citation4 Baseline renal function was defined as the lowest known SCr value in the preceding 3 months before current ICU admission. It was estimated by the Modification of Diet in Renal Disease (MDRD) equation for those without the preceding value (assuming a lower limit of normal baseline glomerular filtration rate (GFR) of 75 mL/min).Citation2,Citation12 Chronic kidney disease (CKD) was defined as CKD stage 3 or above.
Table 1. KDIGO criteria to diagnose and grade the severity of AKI.
Primary outcome was 90-day mortality. Secondary outcomes were ICU and hospital mortality, ICU and hospital length of stay. Renal replacement therapy was defined as continuous or intermittent renal replacement therapy, which was started based on fluid, electrolyte, and acid–base status and also the clinical progress. Renal recovery at 90 days from ICU admission was evaluated. It was defined as full recovery if serum creatinine ≤125% baseline, partial recovery if serum creatinine >125% and ≤300% baseline, and non-recovery if serum creatinine >300% baseline or required dialysis support.Citation2,Citation13
Statistical analysis
Comparisons were performed between those (1) with and without AKI, (2) with septic AKI and without AKI, (3) septic and non-septic AKI. Results were expressed as mean ± standard deviation (SD) or as number of cases and percentages as appropriate. Categorical variables were compared using Pearson chi-square tests or Fisher’s exact test as appropriate. Student t-test or Mann–Whitney U test was used to compare continuous variables. Cox regression analysis using forward stepwise (Likelihood ratio) strategy was used to identify factors associated with 90-day mortality in patients. Trend analysis was performed using Chi squared test for trend in proportions. On conversion of continuous variables to categorical variables, the appropriate cut-off values were determined by the receiver operating characteristic method. All the analyses were performed using the Statistical Package for Social Sciences for Windows, version 20 (SPSS Inc., Chicago, IL) and R statistical program version 3.2 (R Foundation, http://www.r-project.org/).
Results
All patients
A total of 3809 patients were admitted into our ICU during the study period from 1 January 2011 to 31 December 2013. One hundred and twenty two patients (3.2%) were excluded because they were receiving chronic renal replacement therapy before ICU admission. A total of 3687 patients were further analyzed with baseline characteristics shown in . Their mean age was 64 ± 17.2 years old, 58.4% were male and the mean Acute Physiology and Chronic Health Evaluation (APACHE) IV score was 69.2 ± 35.4 corresponding to 25 ± 27% predicted risk of hospital death. The most common diagnostic categories for ICU admission were neurological (21.5%), followed by gastrointestinal (20.8%), and sepsis (17.8%). Baseline SCr were available in 3217 (87.3%) patients and the rest were estimated using MDRD equation. The mean baseline SCr was 94.6 ± 53.5 μmol/L. One fourth (25.9%) of all patients had CKD. The ICU, hospital and 90-day mortality were 10.3, 16.8, and 19.8%, respectively. The most common causes of death included pneumonia (28.0%), cardiovascular complications (17.2%), cerebral vascular complications (14.2%), sepsis other than pneumonia (16.1%), malignancy (7.1%), trauma (6.6%), and others such as ischemic bowel disease, gastrointestinal hemorrhage, and suicide (10.8%).
Table 2. Characteristics and laboratory results of patients with and without acute kidney injury.
Comparison between those with and without AKI
Of 3687 patients, 2018 (54.7%) patients developed AKI during ICU stay. Sepsis was the most common cause of AKI (49.2%) followed by hypovolemia (32.3%), cardiogenic (14.1%), and others (4.4%). Among those with AKI, 666 (33.0%) had KDIGO stage 1 AKI, 691 (34.2%) had stage 2 AKI, and 661 (32.8%) had stage 3 AKI. Using SCr criteria or urine output criteria alone for diagnosing AKI would have underdiagnosed 707 (35.0%) patients and 501 (24.8%) patients, respectively. The proportion of patients who required MDRD SCr estimation were higher in those with AKI compared to those without AKI (14.5% versus 10.7%, p = 0.001). The percentage of patients suffered from AKI was similar across the study period (55.3% in 2011, 53.6% in 2012, and 55.5% in 2013, p = 0.872).
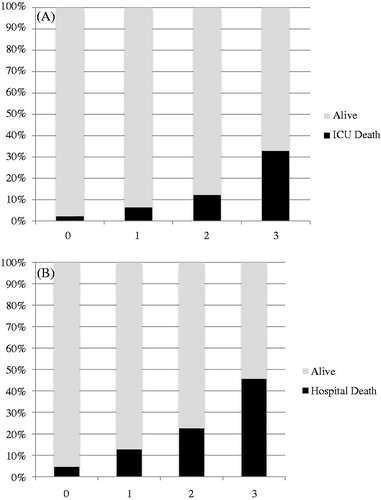
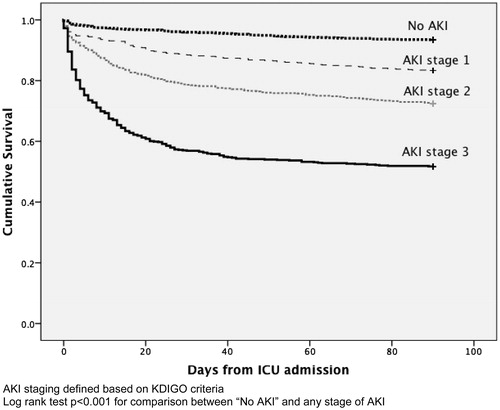
Acute kidney injury (AKI) patients were older, more likely to be male, heavier and have more significant comorbidities (including hypertension, diabetes mellitus, CKD, and underlying malignancy) (). They were more likely to be emergency admission (89.1% versus 73.6%) and came from medical specialty (44.1% versus 30.9%). Sepsis (24.4%) was the most common diagnostic category. Their APACHE scores were higher indicating greater disease severity. AKI patients were more likely to be on mechanical ventilation (62.5% versus 34.0%) and required the use of vasopressor (47.5% versus 15.0%). Renal replacement therapy (RRT) was initiated in 360 patients (17.8%), while 83% received continuous venovenous hemofiltration (CVVH) and 17% received sustained low-efficiency hemodialysis or hemodiafiltration (SLED or SLED-f). AKI patients also had lower albumin, hemoglobin, platelet count, pH and GFR, and higher white cell count (WCC) on first 24 h of ICU admission when compared to those without AKI (). The ICU (17% versus 2.2%) and hospital mortality (26.8% versus 4.6%) were higher for patients with AKI when compared to those without AKI. Mortality increased with severity of AKI (). The Kaplan–Meier survival plot for 90-day mortality differed significantly between those with and without AKI (). The 90-day renal recovery was good for those with AKI. Full renal recovery was achieved in 71.6% patients and only 4.5% patients were classified as non-recovery. The mean ICU and hospital length of stay (LOS) for those with and without AKI differed by 2.8 and 7.3 days, respectively. Logistic regression analysis using Forward LR methods (Hosmer and Lemeshow test χ2 = 4.916, df = 8, p = 0.767) showed that male gender, emergency ICU admission, the presence of diabetes or CKD as comorbidity, ideal bodyweight >60 kg, hemoglobin <10 g/dL, albumin <27.5 g/L, APACHE IV score >65, and requirement of mechanical ventilation or vasopressor were independent risk factors for predicting occurrence of AKI ().
Table 3. Logistic regression analysis for independent predictors of AKI.
90-Day mortality
Cox regression analysis showed that age, emergency ICU admission, post-operative cases, admission diagnosis (neurological, trauma, malignancy, and genitourinary cases), type of AKI, APACHE IV score, requirement of mechanical ventilation and vasopressor support, and blood parameters (albumin, potassium, and pH) independently predicted 90-day mortality ().
Table 4. Cox regression analysis for independent predictors of 90-day mortality.
Comparison between septic AKI and without AKI
Among all patients, 1635 patients (44.3%) were documented to have sepsis at the time of ICU admission (1224 patients) or after ICU admission but during ICU stay (411 patients). The causes of sepsis included pneumonia (57%), gastrointestinal and hepato-biliary sepsis (18%), and others such as urosepsis and musculoskeletal infection (24%). Surgical wound infection accounted for 0.3% of septic cases. The isolated micro-organisms included Escherichia coli (10%), Pseudomonas (9.7%), Methicillin-resistant Staphylococcus aureus (MRSA) (8%), Klebsiella species (7.8%), Coagulase negative staphylococcus (7.7%), Candida albicans (6.4%), Stenotrophomonas maltophilia (5.1%), Alpha hemolytic streptococci (4.9%), Staphylococcus aureus (3.3%), Acinetobacter (3.2%), Enterococcus (3%), Enterobacter (2.8%), and Klebsiella pneumoniae (2%). Among those septic patients, 1171 of them (71.6%) developed AKI and septic AKI was regarded as the predominant cause in 992 patients (84.7%). Patients were classified as KDIGO stage 1, 2, and 3 AKI in 28.1, 32.6, and 39.3%, respectively. Compared with those without AKI, septic AKI patients were older, more likely to be male, had slightly higher body weight and have more significant comorbidities (including hypertension, diabetes, CKD, and underlying malignancy) (). All of them were emergency ICU admission and predominantly from medical specialty (57.9%). Sepsis (47.2%) was the most common diagnostic category. They had higher APACHE scores indicating greater disease severity, corresponding to ∼40% predicted hospital mortality rate. AKI patients were more likely to be on mechanical ventilation (64.6% vs. 34.0%) and required use of vasopressor (57.2% vs. 15.0%). Up to 23.3% of patients with septic AKI required RRT. They also had lower albumin, hemoglobin, platelet count, pH, GFR, and higher WCC on first 24 h of ICU admission compared with those without AKI (). The ICU (17.7% vs. 2.2%), hospital (28.0% vs. 4.6%), and 90-day mortality (32.4% vs. 6.5%) were significantly higher in patients with septic AKI. They also had longer ICU and hospital LOS.
Comparison between septic and non-septic AKI
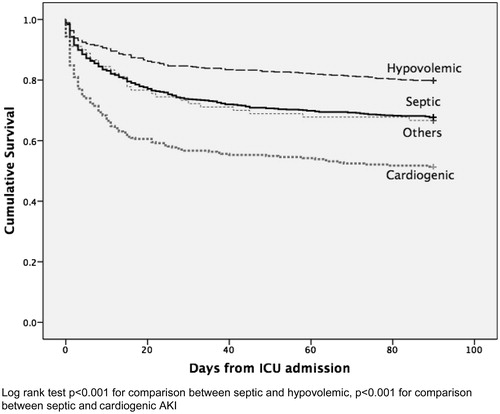
Comparing to patients with non-septic AKI, patients with septic AKI were older, more likely to be emergency admission, and were admitted from medical specialty (). The presence of significant comorbidities was similar between two groups except malignancy, which was more common among those with septic AKI. Septic AKI patients had higher APACHE scores, with 10% difference of the predicted risk of hospital death when compared with non-septic AKI. Vasopressor (57.2% vs. 38.2%) and RRT (23.3% vs. 12.6%) was more commonly adopted in septic AKI patients. Among those who received RRT, CVVH was more commonly performed in septic AKI patients than in non-septic AKI patients (85% vs. 80%, p < 0.001). Compared with non-septic AKI, patients with septic AKI had lower albumin, higher starting SCr, bilirubin, and WCC within the first 24 h of ICU admission. Despite higher APACHE score predicted risk of death, ICU, hospital, and 90-day mortality were similar between both septic and non-septic AKI patients. The unadjusted Kaplan Meier survival plot for 90-day mortality showed significant mortality difference between different causes of AKI (). Full renal recovery at 90 days from ICU admission could be achieved in 70% of patients with septic AKI, while 27.5% had partial recovery. Non-recovery of renal function only occurred in 2.5% of patients with septic AKI compared with 6.4% for those patients with non-septic AKI (p < 0.001). The mean ICU and hospital LOS for those with septic and non-septic AKI differed by 2.2 and 4.4 days, respectively.
Table 5. Septic AKI versus non-septic AKI.
Discussion
This study evaluated the incidence, clinical characteristics, and outcome of critically ill patients with septic AKI among local population. We found that among 3687 recruited patients, 54.7% (2018 patients) had AKI and half of them (992 patients, 49.2% among those with AKI, 26.9% among all recruited critically ill patients) suffered from septic AKI. Among those with documented sepsis on ICU admission or during ICU stay, 71.6% developed AKI and septic AKI being the predominant cause (84.7%). Due to the varied clinical background prior to ICU admission, we do not have accurate data on urine output prior to ICU admission in these patients. Nevertheless, based on serum creatinine data alone, 395 patients (10.7%) had AKI at the time of ICU admission. We believe that this is an under-estimate of the true prevalence. As the deterioration of renal function was often a continuous process with many patients showing further decline of renal function and urine output after ICU admission, the eventual overall incidence noted at and during ICU stay is the more clinically meaningful and reliable parameter than the percentage prior to ICU admission.
Globally, there are a numbers of studies that evaluated the epidemiology of septic AKI () but the majority of them examined Caucasians. Data among Chinese population are relatively sparse. Most of the published studies were conducted in critical care setting. The largest one is by Bagshaw et al. which involved 57 Australian ICUs with >120,000 critically ill patients.Citation7 AKI was classified according to the RIFLE system with a modification of the urine output criteria. He reported a septic AKI incidence of 11.7%. Wang et al. reported the largest multi-center study among Chinese populations, which involved 30 ICUs in Beijing with >3100 critically ill patients.Citation14 AKI was defined and classified according to the KDIGO guidelines.Citation4 Sepsis was present in 29.5% patients and 361 patients (39.4% among those with sepsis and 11.6% among all recruited patients) developed septic AKI, which is similar to that reported by Bagshaw et al.Citation7 The reported incidence of septic AKI from other studies ranged from 1.6% to 16.8%, although study by Gurjar et al. reported an exceptionally high level of 31% (). Obviously, difference of AKI diagnostic criteria among studies affects the reported incidence. Majority of studies used RIFLE system for diagnosing AKI. However, modifications of RIFLE criteria are commonly performedCitation7,Citation15 which may have contributed to a misclassification of some patients and may influence the overall incidence of septic AKI. In this study, we adopted KDIGO criteria. KDIGO identified more AKI due to the addition of the criterion of an absolute increase in creatinine of ≥26.5 μmol/L over 48 h to the RIFLE definition and extending the time-limit from 48 h to 7 days for percentage increase of creatinine of ≥50% in the AKIN definition.Citation16 A comparison of the performance of the three AKI classification systems in 1900 post-cardiac surgery patients showed that AKI incidence (25.9%) and staging were identical when using KDIGO and AKIN, while RIFLE identified fewest (24.9%) patients with AKI.Citation17 Similar findings were also reported in a study with 3100 critically ill patients.Citation5 KDIGO identified more patients with AKI than RIFLE (51% vs. 46.9%; p = 0.001) or AKIN (51% vs. 38.4%; p < 0.001). Therefore, based on currently available evidence, KDIGO is at least as good as, and in some situations even better than the AKIN and RIFLE. Difference in the population studied also contributed to variation of septic AKI incidence. Different ICU using different admission criteria and case-mix are highly variable. Moreover, disease severity is assessed using different tools which make it difficult to compare across different studies.
Table 6. Summary of major studies describing the incidence of septic AKI.
We found that septic AKI patients differ from patients with non-septic AKI. Septic AKI patients were older and had greater biochemical disturbance. They had higher disease severity that required more invasive and intensive organ support. However, the ICU, hospital, and 90-day mortality were similar between septic and non-septic AKI patients, which were unexpected. Study by Bagshaw et al. showed that septic AKI was associated with greater risks for both ICU and hospital death.Citation7 We could have a better understanding on this issue by examining the etiologies of non-septic AKI. In fact, the 90-day mortality rate for patients with septic AKI was 32%, which was higher than those with hypovolemia induced AKI (19%, log rank test p < 0.001) but lower than those with cardiogenic AKI (46%, p < 0.001) (). This finding carries significant prognostic implication and stresses the importance to identify the etiology of AKI. Currently, the differentiation between different causes of AKI is largely based on clinical judgment. Utilization of multiple functional and injury biomarkers specific for different pathways may be able to earlier diagnose, as well as to identify the underlying etiology of AKI.Citation18
Despite more patients with septic AKI required RRT when compared with non-septic AKI, they had better renal recovery. This finding is actually similar to that reported by Cruz et al. and Bagshaw et al.,Citation8,Citation19 signifying a difference in the pathophysiologyCitation20–22 between septic and non-septic AKI. Instead, Singer et al proposed the cell cycle arrest hypothesis and mentioned that multi-organ failure induced by critical illness is primary a functional, rather than structural abnormality.Citation23 His hypothesis is supported by recent clinical studies, which demonstrated that two G1 cell cycle arrest markers, namely, insulin-like growth factor-binding protein 7 (IGFBP7) and tissue inhibitor of metalloproteinase 2 (TIMP-2), predicted AKI in critically ill, and cardiac surgical patients.Citation24–26 This adaptive response may be controlled by mitochondria, with the aim to limit further cellular damage and provide cells with the opportunity to recover function.Citation27 By shutting down some non-essential cellular functions, remaining energy can be directed to other functions that are required for cell survival.Citation27
Our study has several limitations. First, this is a single center study. Patients outcome are affected by our clinical practice which may differ from other centers. Second, the retrospective nature of this study may potentially make it susceptible to several forms of bias. Change of management strategies over time may alter patients’ outcome. Third, we did not include some potentially important variables in data collection, such as mode and intensity of RRT, timing for initiation of RRT, fluid status and appropriateness of antibiotic for septic patients. Fourth, some of patients' baseline creatinine concentrations were not available (12.7%); therefore, we had to perform back-calculation. This may have led to misclassification of some patients. Finally, the duration of follow-up is short and the long-term outcome of those AKI patients is unclear.
Conclusion
We showed that incidence of AKI is high in critically ill patients, with sepsis being the commonest etiology. Patients who developed AKI were sicker, had more physiological and biochemical disturbance, and carried more significant co-morbidities. ICU, hospital, and 90-day mortality increased with severity of AKI. Compared with non-septic AKI, septic AKI patients had more severe disease and required more aggressive support in ICU. However, despite the fact that more patients with septic AKI required RRT when compared with non-septic AKI patients, they had better renal recovery. This could be explained by the difference in underlying pathophysiology between different types of AKI. Etiology of AKI has significant impact on 90-day mortality. Future researches should investigate the usefulness of biomarkers to differentiate different causes of AKI and delineate the appropriate therapeutic strategies.
Acknowledgements
We would like to thank all nursing staff of our unit for their cooperation and support.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lameire NH, Bagga A, Cruz D, et al. Acute kidney injury: An increasing global concern. Lancet. 2013;382:170–179.
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute dialysis quality initiative w. acute renal failure – Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212.
- Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31.
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184.
- Luo X, Jiang L, Du B, et al. A comparison of different diagnostic criteria of acute kidney injury in critically ill patients. Crit Care. 2014;18:R144.
- Zarjou A, Agarwal A. Sepsis and acute kidney injury. J Am Soc Nephrol. 2011;22:999–1006.
- Bagshaw SM, George C, Bellomo R, Committee ADM. Early acute kidney injury and sepsis: A multicentre evaluation. Crit Care. 2008;12:R47.
- Bagshaw SM, Uchino S, Bellomo R, et al. Septic acute kidney injury in critically ill patients: Clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2:431–439.
- Poukkanen M, Vaara ST, Pettila V, et al. Acute kidney injury in patients with severe sepsis in Finnish Intensive Care Units. Acta Anaesthesiol Scand. 2013;57:863–872.
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637.
- House AA, Anand I, Bellomo R, et al. Definition and classification of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transpl. 2010;25:1416–1420.
- Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34:1913–1917.
- Pannu N, James M, Hemmelgarn B, Klarenbach S. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol. 2013;8:194–202.
- Wang X, Jiang L, Wen Y, et al. Risk factors for mortality in patients with septic acute kidney injury in intensive care units in Beijing, China: A multicenter prospective observational study. BioMed Res Int. 2014;2014:172620.
- Bagshaw SM, Lapinsky S, Dial S, et al. Acute kidney injury in septic shock: Clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. 2009;35:871–881.
- Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9:12–20.
- Bastin AJ, Ostermann M, Slack AJ, Diller GP, Finney SJ, Evans TW. Acute kidney injury after cardiac surgery according to risk/injury/failure/loss/end-stage, Acute Kidney Injury Network, and Kidney Disease: Improving Global Outcomes classifications. J Crit Care. 2013;28:389–396.
- Endre ZH, Kellum JA, Di Somma S, et al. Differential diagnosis of AKI in clinical practice by functional and damage biomarkers: Workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol. 2013;182:30–44.
- Cruz MG, Dantas JG, Levi TM, et al. Septic versus non-septic acute kidney injury in critically ill patients: Characteristics and clinical outcomes. Rev Bras Ter Intensiva. 2014;26:384–391.
- Takasu O, Gaut JP, Watanabe E, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med. 2013;187:509–517.
- Langenberg C, Gobe G, Hood S, May CN, Bellomo R. Renal histopathology during experimental septic acute kidney injury and recovery. Crit Care Med. 2014;42:e58–e67.
- Lerolle N, Nochy D, Guerot E, et al. Histopathology of septic shock induced acute kidney injury: Apoptosis and leukocytic infiltration. Intensive Care Med. 2010;36:471–478.
- Singer M, De Santis V, Vitale D, Jeffcoate W. Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet. 2004;364:545–548.
- Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25.
- Meersch M, Schmidt C, Van Aken H, et al. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One. 2014;9:e93460.
- Meersch M, Schmidt C, Van Aken H, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury after pediatric cardiac surgery. PLoS One. 2014;9:e110865.
- Gomez H, Ince C, De Backer D, et al. A unified theory of sepsis-induced acute kidney injury: Inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41:3–11.
- Hoste EA, Lameire NH, Vanholder RC, Benoit DD, Decruyenaere JM, Colardyn FA. Acute renal failure in patients with sepsis in a surgical ICU: predictive factors, incidence, comorbidity, and outcome. JASN. 2003;14:1022–1030.
- Oppert M, Engel C, Brunkhorst FM, et al. Acute renal failure in patients with severe sepsis and septic shock––a significant independent risk factor for mortality: results from the German Prevalence Study. Nephrology, Dialysis, Transplantation. 2008;23:904–909.
- Lopes JA, Jorge S, Resina C, et al. Acute kidney injury in patients with sepsis: a contemporary analysis. Intl J Infect Dis.: IJID. 2009;13:176–181.
- Yegenaga I, Tuglular S, Ari E, et al. Evaluation of sepsis/systemic inflammatory response syndrome, acute kidney injury, and RIFLE criteria in two tertiary hospital intensive care units in Turkey. Nephron. Clin Practice. 2010;115:c276–282.
- Plataki M, Kashani K, Cabello-Garza J, et al. Predictors of acute kidney injury in septic shock patients: an observational cohort study. Clin J Am Soc Nephrol.: CJASN. 2011;6:1744–1751.
- Payen D, Lukaszewicz AC, Legrand M, et al. A multicentre study of acute kidney injury in severe sepsis and septic shock: association with inflammatory phenotype and HLA genotype. PloS One. 2012;7:e35838.
- Kim WY, Huh JW, Lim CM, Koh Y, Hong SB. Analysis of progression in risk, injury, failure, loss, and end-stage renal disease classification on outcome in patients with severe sepsis and septic shock. J Crit Care. 2012;27:104 e101–107.
- Kim WY, Huh JW, Lim CM, Koh Y, Hong SB. A comparison of acute kidney injury classifications in patients with severe sepsis and septic shock. Am J Med Sci. 2012;344:350–356.
- Gurjar M, Baronia AK, Azim A, et al. Septic acute kidney injury in critically ill Indian patients. Ind J Crit Care Med. 2013;17:49–52.
- Suh SH, Kim CS, Choi JS, Bae EH, Ma SK, Kim SW. Acute kidney injury in patients with sepsis and septic shock: risk factors and clinical outcomes. Yonsei Med J. 2013;54:965–972.
- Medeiros P, Nga HS, Menezes P, Bridi R, Balbi A, Ponce D. Acute kidney injury in septic patients admitted to emergency clinical room: risk factors and outcome. Clin Exp Nephrol. 2015;19:859–866.