Abstract
Objective: It is known that connective tissue growth factor (CTGF) and β-catenin are involved in DN; however, the underlying molecular mechanisms remain unknown. Here we hypothesized that podocytes undergo epithelial–mesenchymal transition (EMT) in high-glucose condition and CTGF mediates high-glucose induced EMT by activating β-catenin in podocytes.
Methods: The differentiated podocytes were cultured and divided into three groups: the normal glucose group (5 mmol/L glucose), the high-glucose group (30 mmol/L glucose), and the osmotic control group (5 mmol/L glucose supplemented with 25 mmol/L mannitol). The morphology of cultured podocytes was observed under phase contrast microscopy. To study the relevant markers of EMT, as well as CTGF and β-catenin, the mRNA and protein expressions were analyzed by real-time PCR and western blotting, respectively. In addition, the effects of inhibition CTGF by anti-CTGF antibody on high-glucose-induced EMT and β-catenin expression in podocytes were studied.
Results: High glucose not only induced phenotypic transition of podocytes but also increased the expression of CTGF and β-catenin. Under high-glucose condition, podocytes underwent EMT, which were demonstrated by downregulation of nephrin and upregulation of desmin. Moreover, high-glucose-induced EMT and β-catenin overexpression in podocytes were attenuated by anti-CTGF antibody.
Conclusion: CTGF and β-catenin are involved in the EMT of podocytes in diabetes. CTGF mediates high-glucose induced EMT through activation of β-catenin in podocytes. CTGF inhibition may protect podocytes from EMT in diabetes.
Introduction
With the changes of lifestyle and enhancement of material life level, nowadays, the prevalence of diabetes is surging. It is estimated that the number of patients suffer from diabetes will reach 439 million by the year 2030 from 285 million in 2010.Citation1 As a common chronic complication of diabetes, diabetic nephropathy (DN) is becoming the leading cause of end-stage renal disease worldwide. It is well recognized that podocytes play a critical role in the progression of DN.Citation2–6 Furthermore, during DN, podocytes undergo epithelial–mesenchymal transition (EMT).Citation7–10
Recent studies including our previous study have found that CTGF is an important media of EMT, which is involved in the development of DN.Citation11,Citation12 However, the mechanisms underlying its pathogenesis are not completely clarified.
Other studies have found that the Wnt/β-catenin signal activation was involved in the podocyte injury and the development of proteinuria and inhibition of β-catenin attenuated EMT in podocytes and delayed kidney fibrosis.Citation13–15
In diabetes, the role of CTGF on β-catenin expression and the effect of CTGF inhibition on EMT in podocytes are unknown. In this study, we hypothesized that podocytes underwent EMT in high-glucose condition and CTGF mediated high-glucose induced EMT by activating β-catenin in podocytes.
Materials and methods
Podocyte cultures
Conditionally immortalized mouse podocyte cell line was obtained from Pro. Mundel (Albert Einstein College of Medicine, Bronx, NY) and were cultured as described previously.Citation16 Briefly, to stimulate podocytes proliferation, they were cultured at 33 °C in RPMI 1640 medium (Gibco, Waltham, MA) supplemented with 10% fetal bovine serum (FBS, Gibco, Waltham, MA), 10 U/mL mouse recombinant IFN-γ (Sigma, St. Louis, MO), and 100 U/mL of penicillin/streptomycin in type collagen-coated flasks. To induce differentiation, the podocytes were grown under non-permissive conditions at 37 °C in the absence of IFN-γ for 10 d.
Experimental design
The differentiated podocytes were cultured and divided into three groups as follows: the normal glucose group (5 mmol/L glucose, 5 mM GS), the high-glucose group (30 mmol/L glucose, 30 mM GS), and the osmotic control group (5 mmol/L glucose supplemented with 25 mmol/L mannitol, 5 mM GS + 25 Man). To study the effect of CTGF on podocytes, some of them were preincubated with anti-CTGF antibody (FG-3019, 10 μg/mL, FibroGen, San Francisco, CA) for 2 h and then incubated with high glucose for another 24 h, as described in previous study.Citation17 Then podocytes were gathered for subsequent study. The morphology of podocytes in different culture conditions, relevant markers of EMT, as well as CTGF and β-catenin expression was studied.
Morphological observation
After removing the old culture medium and washing two times with PBS, fresh medium were added to the podocytes, their appearances in different conditions were observed using inverted phase contrast microscopy (Olympus, Tokyo, Japan).
RNA extraction and real-time RT-PCR
Total RNA was isolated from cultured podocytes using the RNAiso plus reagent, and then the first strand of cDNA was synthesized using the reverse transcription system kit according to the manufacturer’s instructions (Takara, Tokyo, Japan). Reverse transcription was performed at 37 °C for 15 m, followed by an inactivation reaction at 85 °C for 5 s. Then real-time RT-PCR was performed using an ABI PRISM 7300 real-time PCR System (Applied Biosystems, Waltham, MA). The cycling condition was performed using a two-step process: 95 °C for 30 s, 40 cycles at 95 °C for 5 s, and 60 °C for 31 s. PCR without the cDNA template was used as a negative control. All measurements were performed in triplicate and the results were analyzed using the ΔΔCt technique, as previously described.Citation18 The relative expression of mRNA was calculated by using the formula: target gene expression of sample/target gene expression of control. Objective gene sequences of primers and the amplified product length are shown in .
Table 1. Primers used for real-time RT-PCR.
Western blot analysis
At the end of the experiment, podocytes were washed with PBS and lysed in RIPA buffer to extract protein. Total protein concentration was measured using a BCA protein assay kit (Sigma, St. Louis, MO). Equal amounts of proteins were separated by electrophoresis on 10% SDS-PAGE and transferred to PVDF membranes (Millipore, Darmstadt, Germany), which were then washed and blocked with 5% nonfat milk in Tris-buffered saline with 0.5% Tween 20 overnight at 4 °C. Next, they were incubated with primary antibodies of goat anti-CTGF (1:200), mouse anti-β-catenin (1:200), rabbit anti-desmin (1:100) and goat anti-nephrin (1:100, all from Santa Cruz Technology, Santa Cruz, CA) for 1 h at room temperature. After washing twice in PBS, the membranes were incubated with the HRP-conjugated secondary antibodies of donkey anti-goat, goat anti-mouse or goat anti-rabbit (each 1:1000, Santa Cruz, Technology, Santa Cruz, CA) for another 1 h at room temperature, respectively. Finally, antibody labeling was visualized by ECL advanced system (GE Healthcare, Buckinghamshire, UK).
Statistical analysis
Experimental values are presented as mean ± SD, which were performed using SPSS 15.0 statistical software (SPSS Inc., Chicago, IL). Comparisons between the groups were made by a paired Student’s t test or one-way ANOVA followed by post hoc tests for either selected or multiple comparisons. p values of <0.05 were considered to be significant.
Results
High glucose induced the phenotypic alternations of podocytes
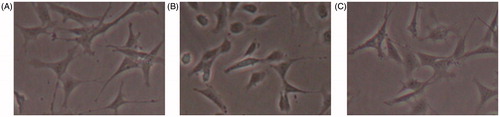
In normal conditions, podocytes displayed the characteristic of epithelial cells, showed by a typical dendritic appearance, which was consistent with previous studies. In response to high glucose, the phenotypes of podocytes change from dendritic to cobblestone. Whereas no significant change was seen in the osmotic controls ().
Figure 1. The morphology of podocytes observed by phase contrast microscopy in different conditions. (A) Podocytes showed a spreading arborized morphology with processes in normal glucose conditions. (B) Under high-glucose conditions, podocytes displayed a cobblestone or elongating morphology. (C) Under osmotic control conditions, the appearances of podocytes are almost similar with that in normal glucose conditions. Original magnification, 200×.
High glucose induced upregulation of CTGF and nuclear β-catenin in podocytes
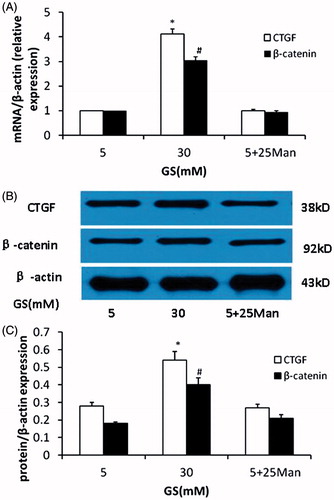
Besides inducing the phenotypic altercations of podocytes, as shown in , high glucose induced upregulation of CTGF and nuclear β-catenin in podocytes compared with normal glucose (mRNA and protein expression, p < 0.05, respectively). However, the expression of CTGF and nuclear β-catenin showed no significant changes in the osmotic controls compared to that of controls (p > 0.05).
Figure 2. Effects of high glucose on CTGF and β-catenin expression in podocyte. (A) High glucose induced overexpression of CTGF and β-catenin mRNA in podocytes compared to normal glucose. (B and C) Western blot analyses showed the upregulation of CTGF and activation of nuclear β-catenin in podocytes in high-glucose condition. The osmotic pressure have no effects on CTGF and β-catenin expression (GS, glucose; Man, mannitol; mM:mmol/L, *p < 0.05 vs. 5 mM GS, #p < 0.05 vs. 5 mM GS, n = 3).
Blockade of CTGF attenuated high glucose induced epithelial–mesenchymal transition in podocytes
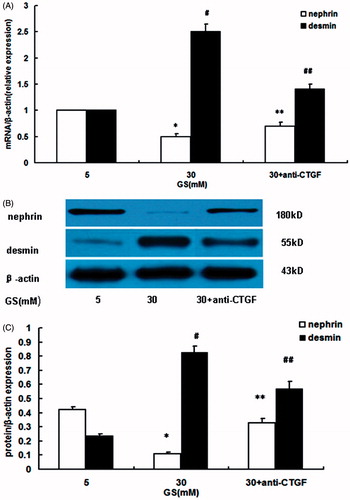
As showed in , compared with the normal glucose group, high glucose induced EMT in podocytes, which was demonstrated by downregulation of nephrin and upregulation of desmin (p < 0.05, respectively). Blockade of CTGF by anti-CTGF antibody (FG-3019) ameliorated EMT in podocytes under high-glucose conditions (p < 0.05 vs. high-glucose, respectively).
Figure 3. Effects of CTGF inhibition by anti-CTGF antibody on high glucose-induced EMT in podocytes. (A) Nephrin and desmin mRNA expression. (B and C) Nephrin and desmin protein expression. Compared with normal glucose conditions, high glucose downregulated nephrin mRNA and protein expression, but upregulated desmin mRNA and protein expression. Treatment with anti-CTGF antibody reversed EMT, showing by preventing nephrin downregulation and desmin overexpression (GS, glucose; Man, mannitol; mM:mmol/L,*p < 0.05 vs. 5 mM GS, #p < 0.05 vs. 5 mM GS, **p < 0.05 vs. 30 mM GS, ##p < 0.05 vs. 30 mM GS, n = 3).
Blockade of CTGF prevented upregulation of nuclear β-catenin in podocytes induced by high glucose
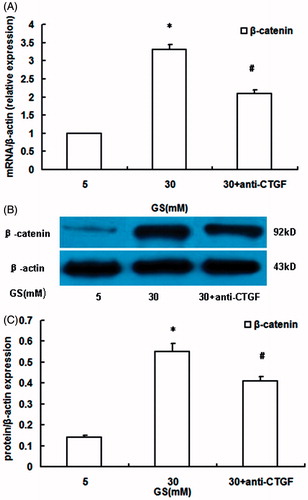
To determine whether high glucose-induced nuclear β-catenin overexpression was mediated by CTGF, podocytes were preincubated with anti-CTGF antibody (10 μg/mL) for 2 h and then were incubated under high-glucose conditions for 24 h. The results showed that treatment with anti-CTGF antibody partially attenuated the upregulation of β-catenin mRNA () and protein expression () induced by high glucose, demonstrating that high glucose induced nuclear β-catenin expression in podocytes was mediated at least in part by CTGF.
Figure 4. Effects of CTGF inhibition by anti-CTGF antibody on high glucose-induced activation of nuclear β-catenin in podocytes. (A) β-catenin mRNA expression. (B and C) nuclear β-catenin protein expression. High glucose induced β-catenin overexpression in podocytes than that in normal glucose conditions, which was prevented by anti-CTGF antibody (GS, glucose; Man, mannitol; mM:mmol/L, *p < 0.05 vs. 5 mM GS, #p < 0.05 vs. 30 mM GS, n = 3).
Discussion
It is well accepted that podocytes injury plays an important role in the progression of DN.Citation4,Citation6,Citation19 There are different important styles of podocytes injury in DN, such as apoptosis, detachment, and EMT. EMT in podocytes is contributed to DN, which was widely confirmed by our previous and other studies.Citation7,Citation9,Citation20 During the course of EMT, podocytes loss some normal epithelial markers but acquire mesenchymal markers.Citation7,Citation9,Citation12 In this study, the epithelial marker of nephrin and the mesenchymal marker of desmin were investigated; the results indicated that podocytes underwent EMT under high-glucose conditions, which were consistent with previous studies, demonstrated by downregulation of nephrin and upregulation of desmin. Again, high glucose induced CTGF and nuclear β-catenin expression in podocytes, inhibition of CTGF by anti-CTGF antibody prevented the expression of nuclear β-catenin and ameliorated EMT of podocytes.
CTGF was originally identified as a protein secreted by human umbilical endothelial cells,Citation21 moreover, it was involved in cell proliferation, migration and adhesion, and tissue repair.Citation22,Citation23 Its overproduction was proposed to play a major role in many fibrotic diseases, including renal fibrosis.Citation24,Citation25 ItoCitation26 first studied pathogenic role of CTGF in DN and found that its expression was correlated with the severity and progression of kidney injury. Using transgenic mice, Yokoi et al.Citation27 provided direct evidence of CTGF in the pathogenesis of DN, especially in podocytes injury. CTGF is now considered to be an important biological marker and therapeutic target in DN, blockade of it by various means be able to delay the progression of DN.Citation28 This study confirmed that CTGF was implicated in EMT process of podocytes induced by high glucose, which was reversed by CTGF inhibition using anti-CTGF antibody.
Wnt/β-catenin signaling pathway plays an important role in the pathophysiological process of cell differentiation, proliferation and apoptosis, tumorigenesis, tumor invasion, and many other functions.Citation29 The activation of Wnt/β-catenin signaling was showed in DN, specific activation of β-catenin in podocytes resulted in proteinuria, conversely, specific deletion of it reduced podocytes lesion and proteinuria.Citation13,Citation14 Notably, β-catenin is regarded as a master switch inside cell that integrates signal inputs from multiple pathways and controls the EMT-related transcriptome.Citation8 Emerging evidence has suggested that inhibition of β-catenin could prevent the EMT of podocytes in DN.Citation13,Citation15 In line with these findings, our study further supported the involvement of β-catenin in high glucose induced EMT in podocytes.
Recently, the relation between CTGF and β-catenin was investigated in different studies, to the best of our knowledge, the role of CTGF on β-catenin expression in podocytes in diabetes remains to be elucidated. Mercurio et al.Citation30 found that CTGF could bind to low-density lipoprotein receptor-related protein (LRP)-6 to form a complex and regulate the activity of Wnt signaling. Similarity, it is indicated that blockade of Wnt/β-catenin with SERPINA3K (an endogenous inhibitor of LRP6) inhibited the overexpression of CTGF.Citation31 Another study in DN demonstrated that CTGF activated Wnt signaling in mesangial cells through LRP6, resulting in the activation of β-catenin promotion of DN.Citation32 In this research, we found that podocytes underwent EMT in high-glucose milieu; in addition, high glucose induced overexpression of CTGF and nuclear β-catenin in podocytes. Moreover, high glucose-induced EMT and β-catenin overexpression in podocytes were attenuated by anti-CTGF antibody.
Taken together, the study in vitro demonstrates that CTGF and β-catenin are involved in the EMT of podocytes in diabetes. CTGF mediates high-glucose induced EMT through the activation of β-catenin in podocytes. CTGF inhibition may protect podocytes from EMT in diabetes, which provides a new insight into the early inhibition of CTGF in the management and prevention of DN.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Funding
This work was supported by Chinese Society of Nephrology (No. 14050420579).
References
- Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14.
- Jefferson JA, Shankland SJ, Pichler RH. Proteinuria in diabetic kidney disease: A mechanistic viewpoint. Kidney Int. 2008;74:22–36.
- Li JJ, Kwak SJ, Jung DS, et al. Podocyte biology in diabetic nephropathy. Kidney Int. 2007;72:S36–S42.
- Reddy GR, Kotlyarevska K, Ransom RF, Menon RK. The podocyte and diabetes mellitus: Is the podocyte the key to the origins of diabetic nephropathy? Curr Opin Nephrol Hypertens. 2008;17:32–36.
- Shankland SJ. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147.
- Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: Podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005;54:1626–1634.
- Li Y, Kang YS, Dai C, Kiss LP, Wen X, Liu Y. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol. 2008;172:299–308.
- Liu Y. New insights into epithelial–mesenchymal transition in kidney fibrosis. J Am Soc Nephrol. 2010;21:212–222.
- Reidy K, Susztak K. Epithelial–mesenchymal transition and podocyte loss in diabetic kidney disease. Am J Kidney Dis. 2009;54:590–593.
- Yamaguchi Y, Iwano M, Suzuki D, et al. Epithelial–mesenchymal transition as a potential explanation for podocyte depletion in diabetic nephropathy. Am J Kidney Dis. 2009;54:653–664.
- Burns WC, Thomas MC. The molecular mediators of type 2 epithelial to mesenchymal transition (EMT) and their role in renal pathophysiology. Expert Rev Mol Med. 2010;12:e17.
- Dai HY, Zheng M, Lv LL, et al. The roles of connective tissue growth factor and integrin-linked kinase in high glucose-induced phenotypic alterations of podocytes. J Cell Biochem. 2012;113:293–301.
- Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y. Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol. 2009;20:1997–2008.
- Waters A, Koziell A. Activation of canonical Wnt signaling meets with podocytopathy. J Am Soc Nephrol. 2009;20:1864–1866.
- He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:765–776.
- Mundel P, Reiser J, Zuniga Mejia Borja A, et al. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258.
- Zhou G, Li C, Cai L. Advanced glycation end-products induce connective tissue growth factor-mediated renal fibrosis predominantly through transforming growth factor beta-independent pathway. Am J Pathol. 2004;165:2033–2043.
- Fink L, Seeger W, Ermert L, et al. Real-time quantitative RT-PCR after laser-assisted cell picking. Nat Med. 1998;4:1329–1333.
- Diez-Sampedro A, Lenz O, Fornoni A. Podocytopathy in diabetes: A metabolic and endocrine disorder. Am J Kidney Dis. 2011;58:637–646.
- Lv Z, Hu M, Zhen J, Lin J, Wang Q, Wang R. Rac1/PAK1 signaling promotes epithelial–mesenchymal transition of podocytes in vitro via triggering beta-catenin transcriptional activity under high glucose conditions. Int J Biochem Cell Biol. 2013;45:255–264.
- Bradham DM, Igarashi A, Potter RL, Grotendorst GR. Connective tissue growth factor: A cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991;114:1285–1294.
- Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41:771–783.
- Leask A, Abraham DJ. All in the CCN family: Essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810.
- Mason RM. Connective tissue growth factor(CCN2), a pathogenic factor in diabetic nephropathy. What does it do? How does it do it? J Cell Commun Signal. 2009;3:95–104.
- McLennan SV, Abdollahi M, Twigg SM. Connective tissue growth factor, matrix regulation, and diabetic kidney disease. Curr Opin Nephrol Hypertens. 2013;22:85–92.
- Ito Y, Aten J, Bende RJ, et al. Expression of connective tissue growth factor in human renal fibrosis. Kidney Int. 1998;53:853–861.
- Yokoi H, Mukoyama M, Mori K, et al. Overexpression of connective tissue growth factor in podocytes worsens diabetic nephropathy in mice. Kidney Int. 2008;73:446–455.
- Brigstock DR. Strategies for blocking the fibrogenic actions of connective tissue growth factor (CCN2): From pharmacological inhibition in vitro to targeted siRNA therapy in vivo. J Cell Commun Signal. 2009;3:5–18.
- Xiao L, Wang M, Yang S, Liu F, Sun L. A glimpse of the pathogenetic mechanisms of Wnt/β-catenin signaling in diabetic nephropathy BioMed Res Int. 2013:2013:987064
- Mercurio S, Latinkic B, Itasaki N, Krumlauf R, Smith JC. Connective-tissue growth factor modulates WNT signalling and interacts with the WNT receptor complex. Development (Cambridge, England). 2004;131:2137–2147.
- Zhang B, Zhou KK, Ma JX. Inhibition of connective tissue growth factor overexpression in diabetic retinopathy by SERPINA3K via blocking the WNT/beta-catenin pathway. Diabetes. 2010;59:1809–1816.
- Rooney B, O'Donovan H, Gaffney A, et al. CTGF/CCN2 activates canonical Wnt signalling in mesangial cells through LRP6: implications for the pathogenesis of diabetic nephropathy. FEBS Lett. 2011;585:531–538.
