Abstract
Sclerostin is a marker of low-turnover bone disease in end stage renal disease patients. The aim of this study was to evaluate serum sclerostin in uremic patients, analyzing its behavior during a single hemodialysis session. Twenty-one adult patients on intermittent hemodialysis treatment were enrolled. Acetate Free Bio-filtration (AFB) was the technique employed. Uremic patients were characterized by higher levels of serum sclerostin when compared with values observed in healthy subjects. Sclerostin assessed in pre-dialysis samples was 1.4 ± 1.02 ng/mL, whereas, in post dialysis samples, a reduction of sclerostin values was observed (0.8 ± 0.6 ng/mL; p: 0.008). Sclerostin correlated with parameters of dialysis adequacy, such as creatinine levels and Kt/V values, and it was significantly associated with atherosclerotic disease. Receiver operating characteristics analysis revealed a good diagnostic profile in identifying atherosclerotic disease. Sclerostin, a full dialyzable substance during AFB dialysis, is closely associated with atherosclerotic disease. Its reduction obtained through AFB could represent a defensive mechanism, improving vascular disease and renal osteodystrophy.
Introduction
Chronic kidney disease-mineral and bone disorder (CKD-MBD) represents a major complication of renal disease, involving patients from early stage of chronic kidney disease to uremic status. Recent studies have identified new molecules synthesized by osteocytes, such as fibroblast growth factor 23 (FGF 23), working as powerful predictors of mortality and cardio-vascular events.Citation1
Moreover, the majority of long-term hemodialysis patients are affected by cardiovascular calcification, representing one of the main causes of high cardiovascular mortality.Citation2
Numerous proteins have been identified to be significantly associated with the presence and amount of vascular calcification, such as osteoprotegerin, parathyroid hormone (PTH), several adipokines, some of which are also associated with morbidity and mortality.Citation3,Citation4
A novel candidate biomarker of bone and vascular disease is sclerostin. It is a 22 kDa mainly synthesized in osteocytes and represents a potent down-regulator of bone metabolism by reducing osteoblast differentiation and function via canonical Wnt-signaling inhibition.Citation5
Recent reports indicated that serum sclerostin levels may reflect reduced bone metabolism and may be useful as a marker for low-turnover bone disease and renal osteodystrophy in end-stage renal disease (ESRD) patients.Citation6
In vitro assays, sclerostin expression was revealed in vascular smooth muscle cells after a phenotypic transition to mineralizing osteoblast-like cells, due to calcifying environment exposition.Citation7
Furthermore, animal and human studies confirmed these data, highlighting sclerostin expression in calcified vessels and soft tissues. In particular, serum sclerostin was described in patients with chronic kidney disease and uremic patients, underlying its high levels, closely related to mortality risk.Citation8
The aim of this study was to evaluate the role of serum sclerostin in uremic patients, analyzing its behavior during a single hemodialysis session, through Acetate Free Bio-filtration (AFB) and high-efficiency convective technique. Moreover, we investigated the possible correlations between serum levels of sclerostin and different markers of uremic osteodystrophy and vascular calcification.
Material and methods
Patients and controls
Twenty-one adult patients on intermittent hemodialysis (HD) treatment, referred to the Nephrology and Dialysis Unit of Messina University Hospital, were enrolled for the study.
Patients with inflammatory disease or a diagnosis of cancer were excluded from the study. The HD sessions, of 3–4 h duration, took place three times per week; the blood flow was more than 250 mL/min, bicarbonate infusion 2000 mL/h. The AFB was the technique employed, by the Integra (Hospal, Bologna, Italy) monitor. The artero-venous fistula was the vascular access for hemodialysis in all patients.
The causes of renal failure were diabetes (n = 8), nephroangiosclerosis (n = 6), polycystic kidney (n = 2), and other causes (n = 5). All patients underwent electron beam computed tomography (EBCT) (Siemens, Forchheim, Germany). Images were performed with 100 ms scanning time and a single slice thickness of 3 mm. Thirty-six to 40 tomographic slices were obtained for each subject during two breath-holding sessions. Tomographic imaging was electronically triggered at 80% of the R–R interval, to minimize cardiac movement, and proceeded from the carina to the diaphragm. The intra-assay coefficient of variation for the scoring was <5%. The acquired images were scored in Hounsfield units (HU) with the use of dedicated software by a single radiologist blinded to the clinical or angiographic history of the patient.
As described by Agatston, the degree of coronary artery calcium (CAC) was calculated by multiplying the area of each calcified lesion by a weighting factor corresponding to the peak pixel intensity for each lesion to yield a lesion-specific calcification score.Citation9
The sum of the scores for each arterial segment, and for all arterial lesions, was used for analysis. We examined the proximal segments of four vessels: left main stem, left anterior descending artery, circumflex artery and right coronary artery.
Atherosclerotic disease was defined if cerebrovascular disease (ischemic stroke or transient ischemic attack), coronary heart disease (previous myocardial infarction, diagnosed stable or unstable angina, or coronary revascularization surgery), or ischemic peripheral arterial disease occurred.
Twenty healthy volunteers with a clinical history negative for arterious hypertension, diabetes mellitus, cancer, cardiovascular, pulmonary, inflammatory, renal, and endocrinal disease were also enrolled as the control group.
The Ethics Committee and the Institutional review board approved the study and fully informed consent was obtained in writing from all participants.
Collection of blood and sclerostin evaluation
In patients receiving HD, blood samples were collected in the morning at 08.00 h immediately before and just after hemodialysis from the arterial line of the HD, while in a healthy control group, blood samples were collected at 08:00 h following an overnight fast.
Dialysate effluent samples were analyzed through Quantiscan™ system collector (Hospal, Bologna, Italy). Blood samples were collected into chilled vacutainer tubes containing potassium ethylenediamine tetraacetate. Tubes were instantly cooled on ice and centrifuged at 3000 rpm for 10 min at 4 °C within 30 min. Serum was stored at −80 °C until analyzed.
Sclerostin was measured using a commercial available ELISA kit (reactive Teco Medical, Sissach, Switzerland), according to the manufacturer’s instructions. All specimens were diluted often to obtain concentration for the optima density according to the ELISA kit instruction. All measurements were made in a triplicate and blinded manner. Sclerostin levels were expressed as nanograms per milliliter (ng/mL).
Moreover, all values were normalized to volume. In particular, middle- and post-dialysis sclerostin levels were expressed after correction for hemo-concentration. In particular,
and
Sclerostin reduction ratio was calculated as
Dialysate samples were collected by the monitoring system Quantiscan™ (Hospal, Bologna, Italy). Quantiscan flow rate (Qs, expressed in mL/min) was calculated using the following formula:
where K is a coefficient that varies depending on the value of dialyzing flow (Qd) (when Qd is 500 mL/min as in our study, K is equal to 1.000), Quf is the weight loss, and Qinf is the value of infusion flow.
This formula can be found in the user guide of the Integra® monitor (Hospal, Bologna, Italy). The total amount of sclerostin removed by the hemodialyzer was calculated by multiplying the concentrations of the substance for the total of dialysate produced.
Statistical analysis
Statistical analyses were performed with NCSS for Windows (version 4.0), the MedCalc (version 11.0; MedCalc Software Acacialaan, Ostend, Belgium) software and the GraphPad Prism (version 5.0; GraphPad Software, Inc., San Diego, CA) package. Data were presented as mean ± SD for normally distributed values and median [IQ range] for non-normally distributed values. Differences between groups were established by unpaired t test or by ANOVA followed by Bonferroni’s test for normally distributed values and by the Kruskal–Wallis analysis followed by Dunn’s test for nonparametric values. Correlation coefficients were used as appropriate.
For the analyses of sclerostin as predictor of atherosclerotic disease, we performed univariate linear regression analyses with baseline sclerostin as the independent variable and atherosclerotic disease as dependent variable. Subsequently, this association was adjusted for covariates that potentially could be confounders using multivariable regression models. Multivariable models were built stepwise. The association between sclerostin and atherosclerotic disease was adjusted for age and dialysis vintage (model 1). Associations were adjusted for covariates that are causally linked to vascular disease and calcification in uremic patients (model 2), and additionally adjusted for comorbidities that may be a confounder in the association between sclerostin levels and outcome measures (dyslipidemia and diabetes mellitus; model 3). Receiver operating characteristics (ROC) analysis was employed to calculate the area-under-the-curve (AUC) for sclerostin and to find the best cut off values capable for identifying atherosclerotic disease. All results were considered significant if p was <0.05.
Results
The main characteristics of the study cohort are summarized in . The mean age of patients was 63.6 ± 14 years. Diabetes mellitus was observed in 5 patients (23%), whereas 18 patients (85%) suffered from high blood pressure. Dyslipidemia was revealed in 13 patients; all patients were treated with statins.
Table 1. Baseline demographic, clinical, and laboratory data of the study population.
Nine patients were affected by heart failure (43%), whereas history of acute myocardial infarction was positive in seven patients (33%). Fifteen subjects (71%) presented atheromatous disease involving one or more vascular districts.
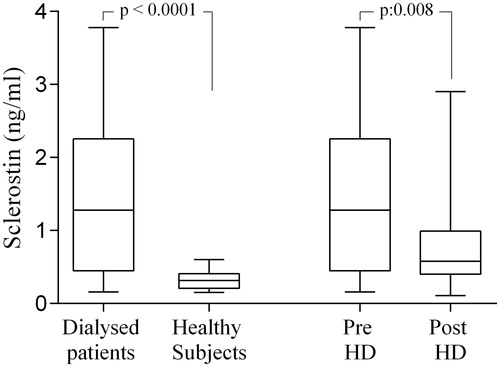
The mean dialysis vintage was 36 ± 37.4 months associated to a mean ultrafiltration (UF) of 0.70 ± 0.3 l/h and a mean weight loss of 3 ± 1.4 kg. Uremic patients were characterized by higher levels of serum sclerostin if compared with values observed in healthy subjects (1.4 ± 1.02 vs. 0.32 ± 0.12 ng/mL; p < 0.0001). No differences of sclerostin levels were assessed in the studied cohort, according to gender (males: 1.4 ± 1.04; females: 0.77 ± 0.78 ng/mL; p > 0.05). In particular, serum sclerostin levels assessed in pre-dialysis samples were 1.4 ± 1.02 ng/mL, whereas, in post dialysis samples, a significant reduction of sclerostin values was observed (0.8 ± 0.6 ng/mL; p: 0.008). In all dialysate samples, the presence of the sclerostin was detected with mean values of 11496 ± 44.7 ng/mL () The sclerostin reduction ratio observed in our dialyzed patients was 52.3 ± 17.4%.
Figure 1. Sclerostin levels in studied cohort sclerostin values were significantly higher in dialyzed patients than in healthy subjects; significant differences between the levels of sclerostin in uremic patients before and after a single dialysis session.
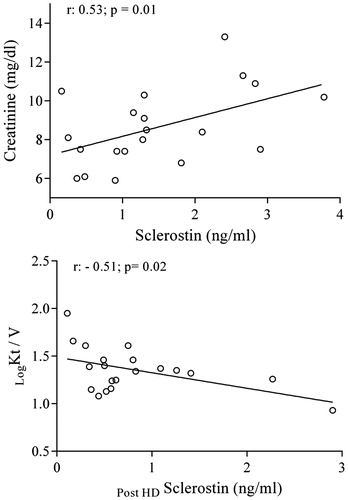
The mean value of calcium score was 762.791 ±44.65 HU. However, it was not found to be related to sclerostin levels. A positive correlation was revealed between sclerostin and creatinine levels in pre-dialysis sampling (r = 0.53, p = 0.01), whereas an inverse relation was detect between Kt/V values and post HD sclerostin (r : −0.52; p: 0.02). A negative correlation between sclerostin reduction ratio and vitamin D levels (r = −0.47; p =0.03) has also been found ().
The association between baseline sclerostin concentrations and atherosclerotic disease was tested for statistical significance in multivariable regression models. This relation remained significant after adjusting for age and dialysis vintage (model 1; p = 0.02), and after additional adjustment for risk factors for vascular disease and calcification risk in uremic patients (model 2; p = 0.01). Sclerostin level was associated significantly with atherosclerotic disease also after adjustment for comorbidities closely related with vascular impairment, such as diabetes mellitus and dyslipidemia (model 3; p = 0.02) ().
Table 2. Multiple logistic regression analysis of the association between baseline sclerostin and atherosclerotic disease in dialyzed patients.
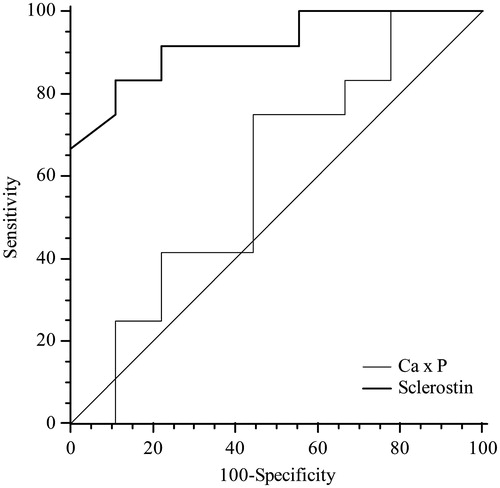
ROC analyses were performed in order to define the diagnostic profile of serum sclerostin in identifying atherosclerotic disease in uremic patients. The area-under-the-ROC curve for serum sclerostin was 0.921. When the cut off values of serum sclerostin was set at 1.15 ng/mL, sensitivity and specificity of the marker used for the diagnosis were 83.3% and 88.9%, respectively. ROC analysis showed an AUC for calcium phosphorus product of 0.602 with a best cut off level of 42.5 mg/dL (sensitivity 75%, specificity 55.6%). Sclerostin area was statistically different compared with that of CaxP (p = 0.03) ().
Discussion
Our data clearly demonstrated that sclerostin is a full dialyzable substance. We have in fact assessed that it was reduced after a single hemodialysis session, detecting it in effluent dialysate samples. This datum is apparently contradictory with results obtained by Bielesz who revealed that only in few patients sclerostin was detectable in dialysate fluid.Citation10
Acetate Free Biofiltration technique and polyacrylonitrile filters as hemodialyzers, due to their high porosity, provide a straightforward and better capacity to remove uremic toxins, such as β2-microglobulin, final products of the advanced glycation end products (AGEs), and several cytokines. Sclerostin, having a molecular weight of 22 kD and characterized by its complex tertiary structure based on glycosidic bonds, could be entirely eliminated only using high porosity membranes and convective techniques.
Furthermore, the closely and inverse relation with Kt/V values, a surrogate index of dialysis adequacy, revealed by our results links this peptide to dialysis kinetics. This hypothesis is also confirmed by a positive correlation found with creatinine values, underlying the lack or reduction of renal clearance of sclerostin.
It is well known that sclerostin, mainly secreted by osteocytes, reduces the bone formation process, inhibiting the proliferation and differentiation of osteoblasts and promoting apoptosis of osteocytes, through an antagonism of Wnt/β-catenin signaling pathway.Citation11
In uremic patients, sclerostin was related to renal osteodystrophy and parathyroid hormone levels, representing a potential biomarker of bone turnover.Citation12 However, it was assessed that sclerostin production did not exclusively derive from osteocyte cells from skeleton, but also by sclerostin up-regulation in vascular cells previously transformed to osteocytic phenotype after osteogenic regulation, such as has been shown in vascular smooth muscle cells under calcifying conditions.Citation7
In fact, experimental studies indicated that elevated calcium–phosphorus product had direct effects on vascular smooth muscle cells, promoting vascular calcification, osteogenic/chondrogenic differentiation, and extracellular matrix degradation, with osteocyte involvement in vascular and valvular calcification processes.Citation13
In our cohort, whereas we did not observe connections between sclerostin and biomarker of renal osteodystrophy, we revealed its strict association with atherosclerotic disease, independently of other risk factors, typically characterizing uremic patients, such as high coronary artery calcium score or high calcium phosphorus product, supposing that sclerostin action is not only based on the regulation of bone formation. We can hypothesize that sclerostin circulating levels represent an important modulator of Wnt signaling in hemodialyzed patients, implicating it in vascular disease and acting as a communicator between uremic bone and vasculature.
Moreover, physiopathological effects of sclerostin on atherosclerosis could be distinct from its effects on bone metabolism. Wnt pathway and accompanying β-catenin activation have been shown to be pro-proliferative in arterial and venous smooth muscle cells, both in vitro and in vivo, inducing a modification of blood vessel wall structure composition, leading to atherosclerosis.Citation14
Furthermore, it is in fact well known that vascular calcification associated with diffuse atherosclerotic disease is highly prevalent in hemodialysis populations and sclerostin could represent a promising biomarker, as also suggested by our analyses.
In fact, Wnt signaling and sclerostin activities are closely associated with vascular calcification processes beyond bone mineralization. Sclerostin has been demonstrated to be up-regulated during vascular smooth muscle cell calcification in vitro,Citation7whereas its high serum levels were associated with abdominal aortic calcification or the extent of valve calcification in dialysis patients, strongly co-localizing with areas of calcification.Citation6,Citation15
However, further studies are needed, also to test potential prognostic role of this peptide, regarding its effects on atherosclerosis and vascular calcifications.
It was in fact highlighted that high sclerostin levels were linked to inflammation, vascular lesions and prognostic biomarkers such as fibroblast growth factor-23.Citation16,Citation17
Consequently, further analyses are necessary in order to better establish the prognostic role of sclerostin in dialyzed patients, evaluating the potential effects of its elimination through specific filters and dialysis techniques. Its reduction obtained through AFB could represent a defensive mechanism, blocking or attenuating the canonical Wnt pathway, improving vascular disease and renal osteodystrophy.
The present study has some limitations that should be mentioned. It was a single-center and hypothesis generating study, involving a small cohort of patients. Confirmation in wider cohorts is indispensable to attribute general validity to our reports.
In conclusion, our study clearly demonstrates that a single AFB hemodialysis session reduces sclerostin levels. Moreover, high levels of sclerostin, characterizing uremic patients, are closely and independently related to atherosclerotic disease.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592.
- Buemi M, Lacquaniti A, Bolignano D, et al. Dialysis and the elderly: An underestimated problem. Kidney Blood Press Res. 2008;31:330–336.
- Lacquaniti A, Bolignano D, Donato V, et al. Obestatin: A new element for mineral metabolism and inflammation in patients on hemodialysis. Kidney Blood Press Res. 2011;34:104–110.
- Lacquaniti A, Bolignano D, Campo S, et al. Malnutrition in the elderly patient on dialysis. Ren Fail. 2009;31:239–245.
- Van Bezooijen RL, Ten Dijke P, Papapoulos SE, Löwik CW. SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev. 2005;16:319–327.
- Pelletier S, Confavreux CB, Haesebaert J, et al. Serum sclerostin: The missing link in the bone-vessel cross-talk in hemodialysis patients? Osteoporos Int. 2015;26:2165–2174.
- Zhu D, Mackenzie NC, Millan JL, Farquharson C, Mac Rae VE. The appearance and modulation of osteocyte marker expression during calcification of vascular smooth muscle cells. Plos One. 2011;6:e19595.
- Kanbay M, Siriopol D, Saglam M, et al. Serum sclerostin and adverse outcomes in nondialyzed chronic kidney disease patients. J Clin Endocrinol Metab. 2014;99:E1854–E1861.
- Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832.
- Bielesz BO, Hempfing T, Kieweg H, Marculescu R, Haas M, Cejka D. Sclerostin declines during hemodialysis and appears in dialysate. Blood Purif. 2014;38:30–36.
- Drüeke TB, Lafage-Proust MH. Sclerostin: Just one more player in renal bone disease? Clin J Am Soc Nephrol. 2011;6:700–703.
- Veverka V, Henry AJ, Slocombe PM, et al. Characterization of the structural features and interactions of sclerostin: Molecular insight into a key regulator of Wnt-mediated bone formation. J Biol Chem. 2009;284:10890.
- Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ Res. 2011;109:697–711.
- Claes KJ, Viaene L, Heye S, et al. Sclerostin: Another vascular calcification inhibitor? J Clin Endocrinol Metab. 2013;98:3221–3228.
- Qureshi AR, Olauson H, Witasp A, et al. Increased circulating sclerostin levels in end-stage renal disease predict biopsy-verified vascular medial calcification and coronary artery calcification. Kidney Int. 2015;88:1356–1364.
- Desjardins L, Liabeuf S, Oliveira RB, et al. Uremic toxicity and sclerostin in chronic kidney disease patients. Nephrol Ther. 2014;10:463–470.
- Yang CY, Chang ZF, Chau YP, et al. Circulating Wnt/β-catenin signalling inhibitors and uraemic vascular calcifications. Nephrol Dial Transplant. 2015;30:1356–1363.