Abstract
Introduction: Patients with diabetic kidney disease (DKD) are more prone to contrast-induced nephropathy (CN). Apoptosis and autophagy were found to be essential in the pathogenesis of DKD. Interleukin-33 (IL-33) is a cytokine, but its role in DKD and CN is unknown. As IL-33 is modulated by apoptosis, we aimed to determine the relationship between IL-33 apoptosis and autophagy in DKD with CN. Materials and methods: Thirty male Sprague–Dawley rats were enrolled and randomly allocated into three groups. The first group was comprised of healthy rats (HRs), whereas the other two groups were made up of diabetic rats (DRs) and diabetic rats with CN (DRs + CN). All groups except the HRs received 50 mg/kg/day of streptozotocin (STZ). The DRs + CN group was induced by administering 1.5 mg/kg of intravenous radiocontrast dye on the 35th day. Results: We observed increased IL-33 in the kidney tissue following induction of CN in the DRs. The DRs showed moderate immunopositivity, and the DRs + CN showed severe immunopositivity for caspase-3, cleaved caspase-3, caspase-8, caspase-9, LC3B, and Beclin-1 in tubular cells and glomeruli. The DRs also showed moderate immunopositivity in tubular cells, and the DRs + CN group showed severe immunopositivity for IL-33 in tubular cells. Increased caspase-3 was found in both glomeruli and tubuli; however, we could not demonstrate IL-33 in glomeruli. This could be secondary to inactivation of IL-33 via increased caspase-3 activity. Conclusion: The release of IL-33 from necrotic cells might induce autophagy, which can further balance the effects of increased apoptosis secondary to CN in DKD.
Introduction
Despite improvements in their contents, contrast dye agents are still the third leading cause of hospital-acquired acute kidney injuries. A reduction in the incidence of contrast-induced nephropathy (CN) can lead to decreased morbidity, mortality (up to 36%), and length of hospital stay.Citation1 Patients with diabetes are more prone to CN than are healthy individuals, which might further enhance the progression of DKD.Citation2 The exact pathophysiologic mechanisms of CN are not fully understood; however, inflammation, hypoxia, and oxidative stress constitute the main responsible pathways, especially in DKD.Citation3–4 Recently, interleukin-33 (IL-33), as a new interleukin-1 (IL-1) family member, was presented as an alarming molecule that invites inflammatory cells to the kidney in cisplatin-induced nephropathy.Citation5 Accumulating evidence suggests that IL-33 is one of the most important cytokines in the pathogenesis of various disorders, including autoimmune diseases, myocardial infarction, heart failure, and allergic pulmonary diseases.Citation6 However, in the literature, data regarding the role of IL-33 in DKD and CN are scant. Hence, further experimental data are needed to characterize the role of cytokines in CN and DKD.
Two important pathways, apoptosis and autophagy, have been found to be essential in the pathogenesis of DKD. The role of autophagy in metabolic disorders, such as diabetes, is currently under intense investigation, and it has been revealed that autophagy plays a protective role in pancreatic β cells against various deleterious stresses.Citation7 Activation of autophagy might be beneficial in the novel treatment of diabetes mellitus.Citation8
As IL-33 is modulated by necrosis and apoptosis and increased necrosis and apoptosis are features of DKD, we aimed to determine the effects of IL-33 on CN in diabetic rats. If a role for IL-33 is confirmed, preventing such inflammation, and/or the immediate consequences of such inflammation, may represent the key target for preventing CN in DKD.
Material and methods
The study protocol was approved by the Medical Ethics Committee of Erzincan University (Mengucek Gazi Training and Research Hospital, Erzincan, Turkey).
Thirty male Sprague–Dawley rats were obtained from Ataturk University Medical Experimental Application and Research Center. The rats were housed in a standard environment with a room temperature of 22 ± 2 °C, a humidity level of 40–60%, and a 12/12-h light/dark cycle. Before starting the experimental procedure, the rats were acclimated to the facilities for one week. After completion of the acclimation period, the rats were randomly allocated into three groups. The first group was comprised of healthy rats (HRs), whereas the other two groups were diabetic rats (DRs) and diabetic rats with contrast-induced nephropathy (DRs + CN). All groups except the HRs received 50 mg/kg/day of STZ (Sigma, St. Louis, MO) within 0.1 M of sodium citrate solution (pH 4.50). Daily blood sugar measurements were performed through tail-end punctures using a Glucomax Ultra TD 4227 (Germany) blood sugar measurement device. In all STZ-administered groups, blood sugar levels were >400 mg/dL at the end of the fifth day. In the DRs + CN group, the diabetic rats were given 1.5 mg/kg of intravenous radiocontrast dye (Omnipaque, Amersham, Ireland) on the 35th day. All rats were sacrificed under thiopental sodium (5 mg/kg) anesthesia on the 36th day using blood evacuation via cardiac puncture and cervical dislocation. Kidney tissue samples were collected for histopathological, immunohistochemical, and biochemical analyses.
Blood samples preparation and analyses
Blood-containing tubes (Vacutainer SST, Becton Dickinson, NJ) were centrifuged for 7 min at 3000 rpm. Serum samples were collected into 2-mL Eppendorf tubes (Eppendorf, Germany) and stored at −80 °C until the time of analysis. IL-6 (eBioscience, BMS625, San Diego, CA), IL-33 (Cusabio, CSB-E14077r, China), and IL-1β (eBioscience, BMS630) levels were measured using ELISA kits appropriate to the species in both the kidney tissues and the serum samples. Serum blood urea nitrogen, creatinine, glucose, sodium, potassium, and calcium levels were measured with an auto-analyzer (AU 5800, Beckman Coulter, Japan) using original kits.
Histopathological examination
At the end of the experiment, necropsies of the rats were performed, and kidney tissue samples were fixed in 10% neutral buffered formalin. Paraffin-embedded blocks were routinely processed, and 5 μm thick sections were stained with hematoxylin–eosin and examined under a microscope. Ten microscopic fields were randomly examined at 20x magnification. The histopathological findings in the sections were graded as 0 (none), 1 (mild), 2 (moderate), or 3 (severe). The slides were covered, and photographs were taken using a light microscope with a camera attachment (Nikon Eclipse E600, Japan).
Immunohistochemical examination
Apoptosis was determined by caspase-3, cleaved caspase-3, caspase-8, caspase-9, Bax, Bcl-2, and autophagic flux assays to measure the autophagy markers including LC3B, ATG-5, and Beclin-1. Inflammation was determined by IL-1β, and IL-33 was studied as an alarming and proinflammatory cytokine.
Kidney sections on polylysine-coated slides were fixed in 10% neutral buffered formalin, embedded in paraffin, and treated with IL-33 antibody (Bioss, Catalog No. bs-2633R, dilution 1/200) for immunohistochemical analysis. An EXPOSE mouse- and rabbit-specific HRP/DAB detection IHC kit (Abcam, Catalog No. ab80436) was used as follows: sections were incubated with goat anti-mouse antibody, then with streptavidin peroxidase, and finally with 3,30 diaminobenzidine chromogen. Slides were counterstained with hematoxylin. The slides were visualized under light microscopy, and the number of cells with immunopositivity was assessed.
The kidney sections were also treated for IL-1β, Bax, Bcl-2, caspase-3, cleaved caspase-3, caspase-8, caspase-9, light-chain 3B (LC3B), Beclin-1, and ATG-5 antibodies for immunohistochemical analysis.
The procedures were performed according to the manufacturer’s protocol for the primary antibodies with slight modifications. Negative controls included staining tissue sections with omission of the primary antibody. The sections were graded as 0 (no staining), 1 (staining <25%), 2 (staining between 25% and 50%), or 3 (staining >50%).
Statistical analysis
Statistical analyses were carried out using SPSS statistical software (SPSS for Windows, version 20.0). All data were presented as mean (±) standard error (SE). Differences in measured parameters among the four groups were analyzed with a nonparametric test (Kruskal–Wallis). Dual comparisons between groups exhibiting significant values were evaluated with the Mann–Whitney U-test (p < 0.05).
Results
Serum levels of IL-33, creatinine, and urea in HRs, DRs, and DRs + CN
Serum levels of IL-33, IL-6, and IL-1β, as well as of serum creatinine and urea, were significantly increased in the DRs + CN group when compared with the HRs and DRs (all p < 0.05) ().
Table 1. The serum and kidney levels of IL-33, IL-6, and IL-1β of groups.
Kidney tissue levels of interleukins in HRs, DRs, and DRs + CN
Kidney tissue levels of IL-33, IL-6, and IL-1β were significantly increased in the DRs + CN group when compared with the HRs and DRs ().
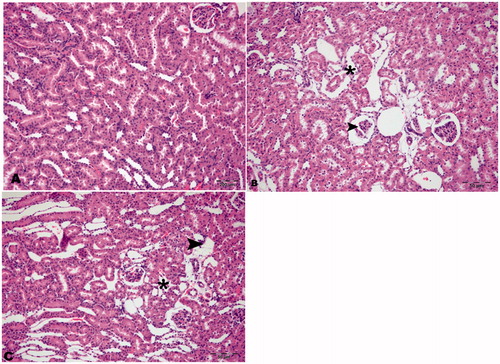
Histopathological features of HRs, DRs, and DRs + CN
The histopathological sections of kidney tissues of the HRs were found to be normal in appearance. Necrotic and degenerative changes were found to be similar in the DRs and the DRs + CN but were absent in the HRs ().
Immunohistochemical staining of inflammation markers in all groups
To assess the inflammatory status of the rats, we performed immunohistochemical staining of IL-1β and IL-33. The HRs showed no immunopositivity for IL-33 and IL-1β. The DRs showed moderate immunopositivity in tubular cells, and the DRs + CN group showed severe immunopositivity in tubular cells for IL-33 (). However, both the DRs and the DRs + CN showed moderate staining for IL-1β ().
Figure 2. Immunohistochemical staining of IL-33 in rat kidney (200×). (A) HRs showed no immunopositivity, (B) DRs showed moderate immunopositivity in tubular cells (arrowhead), and (C) DRs + CN showed severe immunopositivity in tubular cells for IL-33 (arrowhead).

Table 2. Immunohistochemical staining of markers of inflammation, apoptosis, and autophagy in groups.
Immunohistochemical staining of apoptosis markers in all groups
To assess apoptosis, we analyzed Bax, caspase-3, cleaved caspase-3 (as the main executioner caspase), caspase-8 (as a marker of the extrinsic apoptotic pathway), and caspase-9 (as a marker of the intrinsic apoptotic pathway). The DRs showed moderate immunopositivity, and the DRs + CN showed severe immunopositivity for caspase-3, cleaved caspase-3, caspase-8, and caspase-9 in tubular cells and glomeruli. The HRs were not found to be stained for cleaved caspase-3 and caspase-8; however, they were slightly positive for caspase-9 ( and , respectively). We also assessed Bcl-2 as an antiapoptotic marker in all groups and found that the HRs were slightly stained with Bcl-2, while the DRs and DRs + CN were moderately stained ().
Figure 3. Immunohistochemical staining of cleaved caspase-3 in rat kidney (200×). (A) HRs showed no immunopositivity, (B) DRs showed moderate immunopositivity in tubular cells (arrowhead) and glomeruli (arrow), and (C) DRs + CN showed severe immunopositivity for cleaved caspase-3 in tubular cells (arrowhead) and glomeruli (arrow).
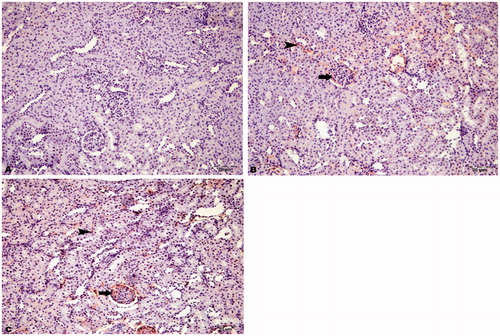
Figure 4. Immunohistochemical staining of caspase-8 in rat kidney (200×). (A) HRs showed no immunopositivity, (B) DRs showed moderate immunopositivity in tubular cells (arrowhead) and glomeruli (arrow), and (C) DRs + CN showed severe immunopositivity for caspase-8 in tubular cells (arrowhead) and glomeruli (arrow).
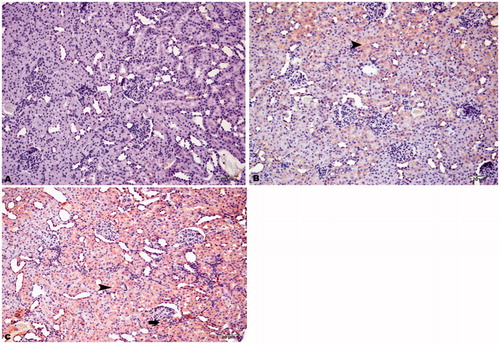
Figure 5. Immunohistochemical staining of caspase-9 in rat kidney (200×). (A) HRs showed slight immunopositivity in intertubular areas, (B) DRs showed moderate immunopositivity in tubular cells (arrowhead) and glomeruli (arrow), and (C) DRs + CN showed severe immunopositivity for caspase-9 in tubular cells (arrowhead) and glomeruli (arrow).
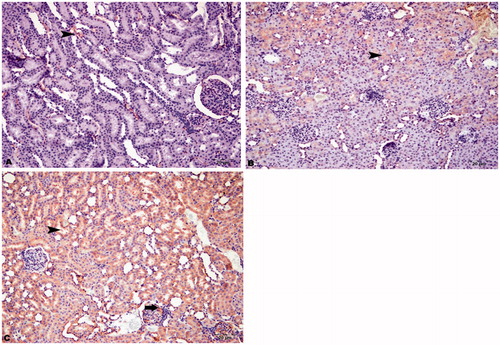
Immunohistochemical staining of autophagy markers in all groups
The immunohistochemical staining results of LC3B and Beclin-1 are demonstrated in and . The HRs showed no immunopositivity. In contrast, the DRs showed moderate immunopositivity and the DRs + CN showed severe immunopositivity for LC3B and Beclin-1 in intertubular areas and glomeruli. We also showed that the DRs and DRs + CN had moderately positive ATG5 staining in intertubular areas and glomeruli ().
Figure 6. Immunohistochemical staining of LC3B in rat kidney (200×). (A) HRs showed no immunopositivity, (B) DRs showed moderate immunopositivity in intertubular areas (arrowhead) and glomeruli (arrow), and (C) DRs + CN showed severe immunopositivity for LC3B in intertubular areas (arrowhead) and glomeruli (arrow).
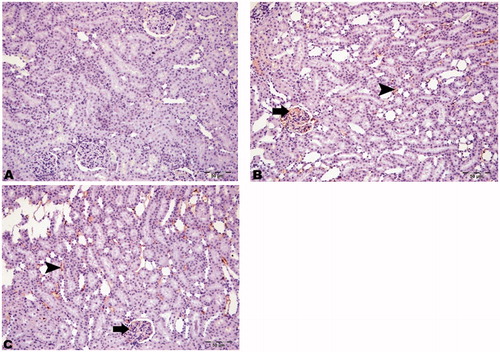
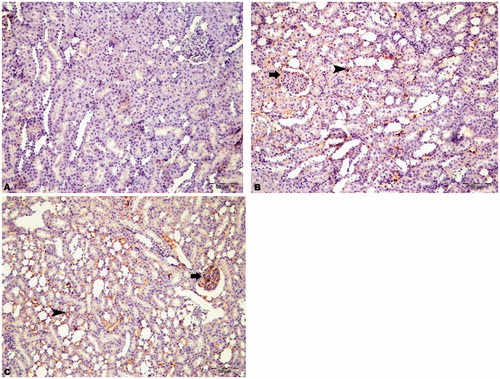
Figure 7. Immunohistochemical staining of Beclin-1 in rat kidney (200×). (A) HRs showed no immunopositivity, (B) DRs showed moderate immunopositivity in intertubular areas (arrowhead) and glomeruli (arrow), and (C) DRs + CN showed severe immunopositivity for Beclin-1 in intertubular areas (arrowhead) and glomeruli (arrow).
Discussion
There are four main findings of the present study. First, both kidney tissues and serum levels of IL-33, as well as other inflammatory cytokines, including IL-6 and IL-1β, were found to be significantly increased in the DRs. Further increases were also determined in the DRs with CN. Second, these findings are also supported by the immunohistochemical staining of the proinflammatory cytokines mentioned above. Third, the markers of apoptosis, including cleaved caspase-3, caspase-8, and caspase-9, were also found to be increased in glomeruli and tubuli of both the DRs and the DRs + CN. Fourth, autophagy markers, such as LC3B and Beclin-1, were evident in glomeruli and intertubular areas of both the DRs and the DRs + CN. This study is the first to demonstrate the possible role of IL-33 along with apoptosis and autophagy in the pathogenesis of CN in DRs.
The prevalence of CN has increased according to widely used imaging and interventional procedures, especially in patients with advanced age and comorbidities, including diabetic nephropathy. The exact trigger of CN remains mysterious. In this regard, we hypothesize that IL-33, also named alarming, might be a candidate as a trigger cytokine in DRs with CN. IL-33 is a proinflammatory cytokine that can be released from necrotic cells, binds the ST2R on immune cells, and increases secretion of cytokines, with resultant inflammation.Citation9–10 Recently, Karatas et al.Citation11 showed that IL-33 expression was typically located in the peritubular and intraglomerular capillaries of diabetic mice. They also found 6-fold (diabetic mice at the 4th month versus healthy mice; p < 0.01) and 5.8-fold (diabetic mice at the 6th month versus healthy mice; p < 0.01) increases in IL-33 protein expression in western blots of whole-kidney tissues from diabetic mice. Assessment of IL-33 with enzyme-linked immunosorbent assay (ELISA) also confirmed their results (IL-33 levels of 0.409 pg/μg in diabetic mice at the 6th month versus 0.164 pg/μg in healthy mice; p < 0.01). These results also support our hypothesis that IL-33 may participate in the pathogenesis of DKD.
Apoptosis has also been shown to play an important role in the pathogenesis of diabetes.Citation12 Caspase-3 is the “executioner caspase” that is centrally important in apoptotic cell death in vivo. Some studies have demonstrated that contrast dye has a cytotoxic or caspase-based apoptotic effect on renal tubular cells.Citation13 Different caspases, in particular caspase-3 and caspase-9, play a crucial role in high-glucose-induced apoptosis of proximal tubular epithelial cells.Citation14 Our previous work demonstrated a significant increase in cleaved caspase-3 as a marker of apoptosis in rats with CN compared with a control group.Citation15 The present study showed similar results regarding increased levels of cleaved caspase-3 in a different model of CN, compared with our previous study.Citation15
Autophagy is a tightly regulated, highly conserved physiologic process that involves recycling cytoplasmic proteins and molecules within autophagolysosomes.Citation16 Autophagosome generation begins with the induction of several autophagy-related genes, including LC3, ATG-5, and Beclin-1.Citation17 LC3 II, which is associated with the autophagosomal membrane, has been used as a marker for the presence of autophagosomes.Citation18 The accumulation of LC3B within a cell can indicate the presence of increased autophagy (increased autophagic flux) or inhibition of autophagosome breakdown (decreased autophagic flux).Citation16
In a recent study, it was stated that autophagy is present in ischemia-associated acute kidney injury.Citation19 However, the data are scant in terms of examining the relationship between CN and autophagic flux in the kidneys of DRs. We observed an increase in LC3B and Beclin-1 protein expression in the kidneys of the DRs and DRs + CN compared with the kidneys of the HRs, suggesting increased autophagy. In accordance with these results, we also demonstrated LC3B and Beclin-1 immunostaining in glomeruli and intertubular areas in both the DRs and the DRs + CN. These results indicate that increased autophagy might be protective against increased apoptosis in DRs with CN.
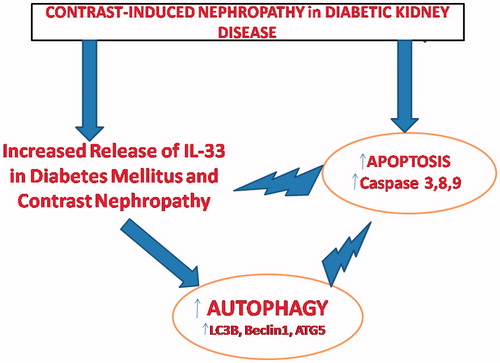
Here, the main question is: which mechanism organizes the two-faced effect of autophagy and apoptosis in DKD with CN? In this regard, the relationship between IL-33, apoptosis, and autophagy might highlight the mystery. According to the results of the present study, we advocate that the release of IL-33 from necrotic cells could induce autophagy, which can further balance the effects of increased apoptosis secondary to CN in DKD. As demonstrated previouslyCitation20–21, increased caspase-3 and caspase-7 activity might inactivate IL-33. Our results confirmed that increased caspase-3 was found in both glomeruli and tubuli; however, we could not demonstrate IL-33 in glomeruli. This could be secondary to inactivation of IL-33 via increased caspase-3 activity secondary to CN. In this context, autophagy activated via IL-33 may be cytoprotective in DKD and CN. A recent study done by Lim et al.Citation22 suggested that loss of autophagy may foster the progression from obesity to DM, since autophagy haploinsufficiency in murine animal models of obesity can lead to increased insulin resistance with elevated lipids and inflammation. The pathways of autophagy and apoptosis also may complement each other to control cell survival. For instance, autophagy inhibition has been associated with an increased apoptosis in the studies of nutrient-deprived cells.Citation23 Conversely, induction of autophagy may protect cardiomyocytes from apoptotic cell death during DM.Citation24 This could also be true for DKD and CN. Hence, it is plausible that there might be a precise balance between apoptosis and autophagy in our study. The heterogeneity of possible outcomes after the induction or inhibition of autophagy may be explained by common upstream signaling events that trigger both autophagy and apoptosis concurrently or in a mutually exclusive fashion. We illustrated this proposed mechanism between IL-33, apoptosis, and autophagy in .
Figure 8. Schematic presentation of the relationship between IL-33, apoptosis, and autophagy in diabetic patients with contrast-induced nephropathy.
In summary, we have shown that autophagy and apoptosis are increased in the contrast-exposed diabetic kidneys of rats. Interleukin-33 might play an important role in the apoptosis- and autophagy-related pathways of CN in DKD. Hence, activation of IL-33 might provide a therapeutic rationale in DKD patients with CN.
Disclosure statement
None of the authors declare a conflict of interest.
The authors do not have any relationships with pharmaceutical companies or other entities, such as employment contracts, consultancies, advisory board positions, speaker bureaus, membership on the Board of Directors, or stock ownership.
References
- Sanaei-Ardekani M, Movahed MR, Movafagh S, Ghahramani N. Contrast-induced nephropathy: a review. Cardiovasc Revasc Med. 2005;6:82–88.
- Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399.
- Goldenberg I, Matetzky S. Nephropathy induced by contrast media: pathogenesis, risk factors and preventive strategies. CMAJ. 2005;172:1461–1471.
- Elmarakby AA, Sullivan JC. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc Ther. 2012;30:49–59.
- Akcay A, Nguyen Q, He Z, et al. IL-33 exacerbates acute kidney injury. J Am Soc Nephrol. 2011;22:2057–2067.
- Lu J, Kang J, Zhang C, Zhang X. The role of IL-33/ST2L signals in the immune cells. Immunol Lett. 2015;164:11–17.
- Fujitani Y, Kawamori R, Watada H. The role of autophagy in pancreatic beta-cell and diabetes. Autophagy. 2009;5:280–282.
- Tanaka Y, Kume S, Kitada M, et al. Autophagy as a therapeutic target in diabetic nephropathy. Exp Diabetes Res. 2012;2012:628978.
- Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel 'alarmin'? PLoS One. 2008;3:e3331.
- Lamkanfi M, Dixit VM. IL-33 raises alarm. Immunity. 2009;31:5–7.
- Karatas SG, Yilmaz H, Firat Z, et al. Role of the inflammasome and interleukin-33 in diabetic nephropathy pathogenesis. JASN Abstract Supplement. 2014:25–26.
- Maiese K, Shang YC, Chong ZZ, Hou J. Diabetes mellitus: Channeling care through cellular discovery. Curr Neurovasc Res. 2010;7:59–64.
- Yano T, Itoh Y, Sendo T, Kubota T, Oishi R. Cyclic AMP reverses radiocontrast media-induced apoptosis in LLC-PK1 cells by activating a kinase/PI3 kinase. Kidney Int. 2003;64:2052–2063.
- Allen DA, Harwood S, Varagunam M, Raftery MJ, Yaqoob MM. High glucose-induced oxidative stress causes apoptosis in proximal tubular epithelial cells and is mediated by multiple caspases. FASEB J. 2003;17:908–910.
- Buyuklu M, Kandemir FM, Ozkaraca M, et al. Benefical effects of lycopene against contrast medium-induced oxidative stress, inflammation, autophagy, and apoptosis in rat kidney. Hum Exp Toxicol. 2015;34:487–496.
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326.
- Mizushima N. Autophagy: Process and function. Genes Dev. 2007;21:2861–2873.
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42.
- Kimura T, Takabatake Y, Takahashi A, et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol. 2011;22:902–913.
- Luthi AU, Cullen SP, McNeela EA, et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98.
- Ali S, Nguyen DQ, Falk W, Martin MU. Caspase 3 inactivates biologically active full length interleukin-33 as a classical cytokine but does not prohibit nuclear translocation. Biochem Biophys Res Commun. 2010;391:1512–1516.
- Lim YM, Lim H, Hur KY, Quan W, Lee HY, Cheon H, et al. Systemic autophagy insufficiency compromises adaptation to metabolic stress and facilitates progression from obesity to diabetes. Nat Commun. 2014;5:4934.
- Boya P, Gonzalez-Polo RA, Casares N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040.
- He C, Zhu H, Li H, Zou MH, Xie Z. Dissociation of Bcl-2–Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes. 2013;62:1270–1281.