Abstract
Background: Acute kidney injury (AKI) affects up to 60% of severely asphyxiated neonates. The diagnosis of AKI can be and is further challenged by a lack of good biomarkers. We studied the role of novel markers for AKI, neutrophil gelatinase-associated lipocalin (NGAL), interleukin-8 (IL-18), Netrin-1 (NTN-1), and sodium hydrogen exchanger isoform 3 (NHE3) on development and early diagnosis of AKI in newborns with perinatal asphyxia (PA). Methods: Forty-one newborns with a diagnosis of PA (15 with AKI and 26 without AKI) and 20 healthy matched controls were involved to the study. Urinary samples were obtained on postnatal days 1 and 4 for patients with PA and on postnatal day 1 for the control subjects. AKI was defined using a serum creatinine-based modification of the acute kidney injury network criteria. Results: The levels of NGAL, NTN-1, NHE3, and IL-18 on the first postnatal day urine samples were higher in patients compared to controls (p < 0.001, p <0.001, p <0.02, p <0.001, respectively). In patients with AKI, the levels of NGAL and IL-18 were higher when compared to patients without AKI (p = 0.002, p <0.001, respectively). The levels of NTN-1 and NHE3 were similar in both groups. For the samples obtained on postnatal day 4, only NGAL levels were significantly higher in patients with AKI (p = 0.004) compared to those without AKI. Conclusion: To our knowledge, this is the largest study, which evaluated the utility of urinary biomarkers in the diagnosis of AKI in newborns with PA. First day, urine NGAL and IL-18 levels have an important diagnostic power in such patients.
Introduction
Perinatal asphyxia (PA) is defined as abnormal neurological incident resulting in neonatal hypoxic ischemic encephalopathy (HIE), which occurs usually due to brain hypoxia and ischemic incidents.Citation1 PA remains to be a significant cause of neonatal morbidity and mortality, despite the advances in management of PA.Citation2 The PA can result in multiorgan dysfunction through redirection of cardiac output to maintain cerebral, cardiac, and adrenal perfusion while potentially compromising perfusion to nonvital organs including kidneys, this causing AKI.Citation3,Citation4 Currently, moderate therapeutic hypothermia is the only proven neuroprotective therapy in HIE caused by PA.Citation2 Therapeutic hypothermia has side effects including arrhythmia, thrombocytopenia, and subcutaneous/visceral fat necrosis.Citation5–7
The incidence of AKI after PA in term neonates was shown to be around 30% to 56%.Citation8–10 This number is probably an underestimate given the limitations of the diagnostic criteria as mentioned below. Early detection of AKI could optimize and improve patient outcomes;Citation11 therefore, the use of biomarkers to predict renal damage has been of interest. Serum creatinine (Cr) is the most commonly used clinical measure of renal function; however, it is a poor diagnostic marker and its utility is further questionable in neonates since kidneys undergo maturational changes in postnatal period.Citation10 Moreover, the levels of serum Cr do not increase until several days after renal injury; thus, there has been a search to find better biomarkers of renal damage.Citation12 Four of the potential biomarkers in AKI are urinary neutrophil gelatinase-associated lipocalin (uNGAL), interleukin-18 (uIL-18), netrin-1 (uNTN-1), and Na+/H+ exchanger isoform 3 (uNHE3).
NGAL is a 25 kDa lipocalin secreted by activated neutrophils and expressed in a variety of cells. Cellular injury in the form of infection, malignancy, direct renal tubular injury can up regulate NGAL via induction of NGAL mRNA.Citation13,Citation14 In some studies, in the setting of renal epithelial injury, uNGAL levels increase by 25–100-fold.Citation15 NGAL protein was easily detected in the blood and urine soon after AKI development in animal models.Citation15,Citation16
Another urinary biomarker, IL-18 is also being promoted as biomarker for the early detection of AKI.Citation17 Interleukin-18 is an 18-kDa pro-inflammatory cytokine and subsequently cleaved by caspase-1 in the epithelial cells of the proximal tubules into its active form. It gets detected in urine following ischemic AKI in mice models.Citation17 Urinary NGAL and IL-18 were shown to predict early phase of AKI in children undergoing cardiac surgery.Citation18
The other biomarker of interest, NTN-1 is predominantly produced by renal tubule cells and appears in urine early on, usually within 1 to 3 h of AKI caused by ischemia or other insults. Indeed, administration of recombinant NTN-1 before renal ischemia/reperfusion injury was shown to prevent further renal dysfunction and inflammation.Citation19 In children undergoing cardiopulmonary by-pass surgery, subjects who developed AKI had significantly elevated uNTN-1 levels within the first 2 h after the initiation of cardiopulmonary by-pass, and this elevation preceded the rise in serum Cr by 48 to 72 h.Citation20
Finally, NHE3 is the most abundant sodium transporter in renal tubule and it is localized in the apical membrane and subapical endosomes of renal proximal tubular cells and in the apical membrane of thick ascending limb cells.Citation21 NHE3 is in charge of reabsorbing 60–70% of filtered sodium. NHE3 is critical in proximal tubule perfusion and a hallmark of ischemic injury to proximal tubule.Citation22,Citation23
We hypothesized that urinary biomarkers might be applicable to detect PA with AKI. To test this hypothesis, we compared uNGAL, uIL-18, uNTN-1, and uNHE3 in infants with PA versus healthy controls and PA with AKI versus PA without AKI.
Materials and methods
Study design and patient selection
This prospective nested case–control study was conducted in the neonatal intensive care unit (NICU) of Zekai Tahir Burak Maternity Teaching Hospital, Ankara, Turkey, between May 2011 and May 2014. Approval of the local ethics committee was obtained. The NICU at our hospital is a referral Level III facility with 130 incubators, treating approximately 4000 newborn patients per year. A total number of 112 patients were assessed for enrollment in the study. We excluded patients for whom a parental consent was not obtained (n = 37), newborns with Stage I HIE (n = 20), known congenital renal abnormalities (n = 1), sepsis (n = 5), and those who had less than two serum Cr measurements (n = 8). A final number of 41 subjects were included in analysis. shows the flowchart of the participants. Control group included 20 healthy newborns. Twenty healthy newborns of comparable gestational age born after an uncomplicated pregnancy and labor comprised the control group. Informed consent was obtained from all parents of patients who were eligible for the study.
General supportive care was applied to all patients according to the NICU’s protocol. Demographic characteristics for all newborns were recorded. Newborns with asphyxia underwent following measurements; blood gases were obtained upon admission to the NICU, urine output was monitored and anuria was defined as absence of any urine or oliguria was defined as urine output <1 mL/kg per hour for more than 24 h, severity assessment of HIE at day of life 1 was made according to Sarnat and Sarnat.Citation24
Needs for institution of hypothermia protocol was determined according to the recommendations from “Total Body Hypothermia for Perinatal Asphyxia (TOBY)” study group.Citation2 Hypothermia was performed to all of the patients in the study group according to these criteria. Amplitude-integrated electroencephalography (aEEG) records were obtained for all patients, using an Olympic CFM 6000 (Natus, Seattle, WA) device.
Whole body cooling
The whole body cooling procedure involved induction of hypothermia to maintain a rectal temperature of 33–34 °C for 72 h, for which a Tecotherm TS med 200 N (Inspiration Healthcare Ltd, Leicester, UK) device was used. This was followed by a rewarming period, with an increase in temperature rate not exceeding 0.5 °C per hour, eventually achieving a normal body temperature within 8 h.
Acute kidney injury
Categorization of the enrolled asphyxiated neonates into the asphyxia subgroups (asphyxia with or without AKI) was based on daily measurements of serum Cr up to third day of life. Modified acute kidney injury network (AKIN) criteria were used to classify AKI based on absolute rise in serum Cr level from a previous trough (Stage I, rise in serum Cr of 0.3 mg/dL or serum Cr 150–<200%; Stage II, rise in serum Cr of 200–<300%; Stage III, rise in serum Cr of ≥300%, serum Cr 2.5 mg/dL, or dialysis).Citation25 Maternal renal status of all patients was normal before delivery.
Laboratory analyses
Urinary samples were obtained from newborn with PA on postnatal days 1 (after 6 h) and 4 and samples were obtained on postnatal day 1 from control subjects. Urine samples were collected using bags or through a bladder catheter that was placed for clinical reasons. Urine samples were centrifuged immediately at 3000 rpm for 10 min at room temperature. The aliquots of supernatants were stored at −80 °C until the time of analysis.
NGAL measurements were performed with enzyme-linked immunosorbent assay (ELISA) kit method (Biovendor R&D Products, Heidelberg, Germany) on urine samples. NGAL concentration was measured at 450 nm wavelength. The supernatants were aliquoted and measured using a human IL-18 ELISA kit (eBioscience BMS267/2, Vienna, Austria) that specifically detects the mature form of IL-18. IL-18 concentration was measured at 450 nm wavelength. Human NTN-1 and NHE3 measurements were performed with ELISA kit method (Eastbiopharm Co. Ltd., Hangzhou, China) on urine samples. NTN-1 and NHE3 concentrations were measured at 450 nm wavelength.
Statistical analyses
Statistical analyses were performed using the SPSS for windows (ver. 17.0) statistical package (SPSS Inc., Chicago, IL). Shapiro–Wilk test was used to test for the normality of data. Difference between two groups was examined by independent samples t-test for normally distributed variables and Mann–Whitney U-test for non-normally distributed variables. The chi-square test was used for categorical variables. Continuous variables are presented as the median (min–max), while categorical variables are given as frequencies and percentages. Discriminatory performance of variables was determined by area under the ROC curve and best cutoff values were calculated using Youden index. Spearman correlation coefficient was used to examine the relationship between AKIN stages and urine markers. A p value of less than 0.05 was considered indicative of statistical significance.
Results
A total of 41 neonates with PA and 20 healthy controls were included in the study. The demographic and clinical features of the infants are summarized in . There was no statistically significant difference between groups in terms of gestational age, birth weight, gender, and mode of delivery. All patients in the control group had normal APGAR scores and cord blood analysis. In the PA group, hypothermia was induced within 181 ± 121 min after birth. According to Sarnat and Sarnat, 53.6% of PA patients had Stage II HIE (n = 22) and 46.4% had Stage III HIE (n = 19). Median duration of the hospital stay in the PA group was 14 days (6–26.5), while 10 patients (24.4%) died during hospitalization.
Table 1. The demographic and clinical features of the perinatal asphyxia and control groups.
Fifteen (36.5%) of newborns with PA had AKI, whereas 26 (63.5%) did not have AKI. Based on the modified AKIN criteria, five newborns (33.3%) had Stage I AKI, seven newborns (46.7%) had Stage II AKI, and three newborns (20%) had Stage III AKI. Among 15 patients with AKI, 12 (80%) had oliguria. But none of our patients developed anuria. Renal replacement therapy was not required in any patient.
When compared to control group, newborns with PA had significantly higher levels of first day NGAL, NTN-1, NHE3, and IL-18 levels (p <0.001, p < 0.001, p < 0.02, p < 0.001, respectively). When compared to patients without AKI, patients with AKI had significantly higher levels of NGAL and IL-18 (p = 0.002 and p < 0.001, respectively). However, levels of NTN-1 and NHE3 were similar in both groups. On samples obtained on postnatal day 4, only NGAL levels were significantly elevated in patients compared to controls (p = 0.004) (). AKIN stages was not found to be correlated with NGAL (r = 0.334; p = 0.224) and IL-18 (r = 0.310; p = 0.261).
Table 2. Serum creatinine and urine biomarkers on the first and fourth day in groups and subgroups (with or without AKI).
Receiver operating characteristic (ROC) analysis was used to determine the power of variables to differentiate groups, and the area under the curve (AUC) was calculated. The optimal cutoff values for urine NGAL and IL-18 on first day were identified by plotting ROC curves. ROC curve of PA with AKI versus healthy controls and PA with AKI versus PA without AKI are depicted in . The AUC, cutoff levels, sensitivity, specificity, positive predictive values and negative predictive values are listed in . When patients with AKI and controls subjects were compared using ROC analysis, first day cutoff level for urine NGAL and IL-18 was, 81.9 ng/mL and 918 pg/mL, respectively; the sensitivity was 100% and 93.3%; specificity was 76.2% and 90.5%, respectively.
Figure 2. ROC curves for asphyxia with AKI versus control group (A) and asphyxia with AKI versus asphyxia without AKI (B) for urine NGAL and IL-18 on first day.
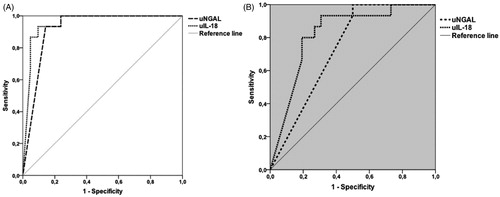
Table 3. The area under the curve, cutoff level, sensitivity, specificity, PPV, and NPV of urine NGAL and IL-18.
Discussion
In this study, we found that uNGAL and uIL-18 levels measured on the first day and uNGAL levels on the fourth day of life were associated with the development of AKI in newborns with PA.
AKI is a common manifestation of PA in the term neonate. Despite significant improvements in neonatal care, the morbidity and mortality rates of renal failure induced by PA remain high. The combination of functional (AKIN/RIFLE) and injury biomarkers (Cr, NGAL, IL-18) are used to diagnose AKI. However, the diagnosis of AKI can be and is further challenged by a lack of good biomarkers.Citation26,Citation27
Several studies showed a rise in NGAL in patients with AKI but not in controls.Citation19,Citation28 Furthermore, this rise in NGAL occurs at 24 to 48 h before the rise in serum Cr is observed. NGAL both in urine and in plasma has been demonstrated as an excellent early marker of AKI in children undergoing cardiopulmonary by-pass.Citation28 Sarafidis et al. showed that serum and uNGAL levels were significantly higher in asphyxiated neonates compared to controls on day of life 1, 3, and 10. The levels of serum NGAL and uNGAL were significantly elevated compared to controls regardless of the presence of AKI based on the elevation of serum Cr, which hints at the spectrum of AKI that is being missed when diagnosis is based solely on rising serum Cr. Within the subgroup of asphyxiated infants, serum NGAL was significantly elevated in the asphyxia with AKI group compared to the asphyxia without AKI group on day of life 1 and 3 (p = 0.016 and 0.001, respectively). This underlines the fact that defining AKI by serum Cr alone is inadequate and an important number of patients with AKI can be missed. Serum and uNGAL showed good diagnostic performance as predictors of AKI on day of life 1.Citation29 Our study demonstrated that uNGAL levels were higher in patients with AKI compared to control subjects on postnatal day 1 and 4, similar to current literature. Several cutoff points ranging from 80 to 550 ng/mL have been used to determine the best sensitivity and specificity of urine NGAL. Cutoff for uNGAL levels have been reported as 18.6 ng/mL by Sarafidis et al.,Citation29 and 250 ng/mL by Essajee F et al.Citation30 Our cutoff for uNGAL was 75.1 ng/mL.
Urinary NGAL and uIL-18 were recently shown to represent early, predictive, sequential AKI biomarkers in children undergoing cardiac surgery.Citation18 During development of postoperative AKI, uNGAL was induced within 2 h (25-fold increase) and peaked at 6 h. Urinary IL-18 levels increased at around 6th hour, peaked at over 25-fold at 12 h and remained elevated up to 48 h after cardiopulmonary bypass. Urinary IL-18 performed best as a predictor of AKI at 12 h compared to other time points. Both uNGAL and uIL-18 were independently associated with duration of AKI.Citation18 In another study, a group of 62 critically ill neonates including eight term neonates with PA were recently evaluated for urinary biomarkers of AKI by Li et al.Citation31 Urinary IL-18 was significantly higher in nonseptic critically ill neonates with AKI compared to controls.Citation31 Our study showed the presence of higher IL-18 levels in newborns with PA compared to controls. The sensitivity of uIL-18 was 93.3%; and the specificity was 90.5% in predicting AKI in newborns with PA.
In preclinical studies of mouse models, NTN-1 is not expressed in normal tubular epithelial cells and is undetectable in normal urine.Citation32 However, NTN-1 protein expression is highly induced within a few hours of ischemia-reperfusion injury and is easily detected in the urine. Ramesh et al. demonstrate that urinary excretion of NTN-1 is an early predictive biomarker of human AKI.Citation20 In pediatric patients undergoing cardiopulmonary bypass, subjects that developed AKI displayed significantly increased urinary NTN-1 levels within the first 2 h of the initiation of cardiopulmonary bypass, preceding the rise in serum Cr by 48 to 72 h. An adult study in critically ill patients with AKI study suggests that urinary NHE3 protein may be a novel, sensitive, and specific marker of human renal tubular injury in AKI. Urinary NHE3 protein may be useful to differentiate acute tubular necrosis, prerenal azotemia, and intrinsic AKI other than acute tubular necrosis.Citation33 In current literature, NTN-1 and NHE3 levels were shown to predict development of AKI after cardiopulmonary bypass, but our study did not show a significant difference among newborns with PA and controls.
Our study has few limitations. First, it was performed in a single center; however, number of patients is sufficient compared to similar studies in the literature. Second, the definition of AKI in our study was based on rise in serum Cr. Although serum Cr remains an accepted and widely used method for evaluating renal function in NICU, it has several limitations as discussed previously. Third, urine Cr levels could not be measured because of technical reasons. Therefore, we cannot calculate standardized urine values. Fourth, hypothermia was performed to all of the patients in our study group since all of the patients were Stages II–III HIE. Therefore, the effect of hypothermia on AKI markers alone was undetermined. Finally, a better trend would have been obtained if the biomarkers were analyzed on a daily basis.
Conclusion
To our knowledge, our study is the largest clinical study that shows utility of novel biomarkers in the diagnosis of AKI in newborns with PA. This study indicates that uNGAL and uIL-18 on the first day of life may have an important diagnostic role as a noninvasive biomarker to independently predict AKI development in newborns with PA.
Acknowledgements
We are grateful to Sevilay Karahan from the Department of Biostatistics, Hacettepe University, for her help in the statistical analysis.
Disclosure statement
All the authors have declared no competing interest.
References
- Bona E, Hagberg H, Loberg EM, Bågenholm R, Thoresen M. Protective effects of moderate hypothermia after neonatal hypoxia-ischemia: Short- and long-term outcome. Pediatr Res. 1998;43:738–745.
- Azzopardi DV, Strohm B, Edwards AD. TOBY Study Group, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–1358.
- Perlman JM, Tack ED, Martin T, Shackelford G, Amon E. Acute systemic organ injury in term infants after asphyxia. Am J Dis Child. 1989;143:617–620.
- Durkan AM, Alexander RT. Acute kidney injury post neonatal asphyxia. J Pediatr. 2011;158:e29–e33.
- Oncel MY, Erdeve O, Calisici E, et al. The effect of whole-body cooling on hematological and coagulation parameters in asphyxic newborns. Pediatr Hematol Oncol. 2013;30:246–252.
- Akcay A, Akar M, Oncel MY, et al. Hypercalcemia due to subcutaneous fat necrosis in a newborn after total body cooling. Pediatr Dermatol. 2013;30:120–123.
- Sahin S, Oncel MY, Alkan M, et al. Visceral fat necrosis in a newborn after whole body hypothermia. J Pediatr. 2015;166:1545.
- Agras PI, Tarcan A, Baskin E, Cengiz N, Gurakan B, Saatci U. Acute renal failure in the neonatal period. Ren Fail. 2004;26:305–309.
- Gupta BD, Sharma P, Bagla J, Parakh M, Soni JP. Renal failure in asphyxiated neonates. Indian Pediatr. 2005;42:928–934.
- Askenazi DJ, Ambalavanan N, Goldstein SL. Acute kidney injury in critically ill newborns: what do we know? what do we need to learn? Pediatr Nephrol. 2009;24:265–274.
- Bagshaw SM, Bellomo R. Early diagnosis of acute kidney injury. Curr Opin Crit Care. 2007;13:638–644.
- Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: Why drugs haven't worked and what is on the horizon. Clin J Am Soc Nephrol. 2007;2:356–365.
- Mishra J, Ma Q, Kelly C, et al. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol. 2006;21:856–863.
- Wagener G, Jan M, Kim M, et al. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. 2006;105:485–491.
- Schmidt-Ott KM, Mori K, Kalandadze A, et al. Neutrophil gelatinaseassociated lipocalin-mediated iron traffic in kidney epithelia. Curr Opin Nephrol Hypertens. 2006;15:442–449.
- Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003;63:1714–1724.
- Melnikov VY, Ecder T, Fantuzzi G, et al. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest. 2001;107:1145–1152.
- Parikh CR, Mishra J, Thiessen-Philbrook H, et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70:199–203.
- Mussap M, Degrandi R, Fravega M, Fanos V. Acute kidney injury in critically ill infants: the role of urine neutrophil gelatinase-associated lipocalin (NGAL). J Matern Fetal Neonatal Med. 2010;23:70–72.
- Ramesh G, Krawczeski CD, Woo JG, Wang Y, Devarajan P. Urinary netrin-1 is an early predictive biomarker of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2010;5:395–401.
- Biemesderfer D, Rutherford PA, Nagy T, Pizzonia JH, Abu-Alfa AK, Aronson P. Monoclonal antibodies for high-resolution localization of NHE3 in adult and neonatal rat kidney. Am J Physiol Ren Physiol. 1997;273:F289–F299.
- Wang T, Yang CL, Abbiati T, et al. Mechanism of proximal tubule bicarbonate absorption in NHE3 null mice. Am J Physiol. 1999;277:F298–F302.
- Sutton TA, Molitoris BA. Mechanisms of cellular injury in ischemic acute renal failure. Semin Nephrol. 1998;18:490–497.
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: A clinical and electroencephalographic study. Arch Neurol. 1976;33:695–706.
- Selewski DT, Jordan BK, Askenazi DJ, Dechert RE, Sarkar S. Acute kidney injury in asphyxiated newborns treated with therapeutic hypothermia. J Pediatr. 2013;162:725–729.
- Sweetman DU, Molloy EJ. Biomarkers of acute kidney injury in neonatal encephalopathy. Eur J Pediatr. 2013;172:305–316.
- McCullough PA, Shaw AD, Haase M, et al. Diagnosis of acute kidney injury using functional and injury biomarkers: workgroup statements from the tenth acute dialysis quality initiative consensus conference. Contrib Nephrol. 2013;182:13–29.
- Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238.
- Sarafidis K, Tsepkentzi E, Agakidou E, et al. Serum and urine acute kidney injury biomarkers in asphyxiated neonates. Pediatr Nephrol. 2012;27:1575–1582.
- Essajee F, Were F, Admani B. Urine neutrophil gelatinase-associated lipocalin in asphyxiated neonates: a prospective cohort study. Pediatr Nephrol. 2015;30:1189–1196.
- Li Y, Fu C, Zhou X, et al. Urine interleukin-18 and cystatin-C as biomarkers of acute kidney injury in critically ill neonates. Pediatr Nephrol. 2012;27:851–860.
- Reeves WB, Kwon O, Ramesh G. Netrin-1 and kidney injury. II. Netrin-1 is an early biomarker of acute kidney injury. Am J Physiol Renal Physiol. 2008;294:F731–F738.
- du Cheyron D, Daubin C, Poggioli J, et al. Urinary measurement of Na+/H + exchanger isoform 3 (NHE3) protein as new marker of tubule injury in critically ill patients with ARF. Am J Kidney Dis. 2003;42:497–506.