Abstract
Background: Chronic kidney disease-mineral and bone disorders (CKD-MBD) have been associated with poor health outcomes, including diminished quality and length of life. Standard management for CKD-MBD includes phosphate restricted diet, vitamin D and phosphate binders. Persistently elevated parathyroid hormone levels may require the addition of cinacalcet hydrochloride (cinacalcet), which sensitizes calcium receptors in the parathyroid gland.
Purpose: The objective of this systematic review is to compare, in patients with CKD-MBD the effect of cinacalcet versus standard treatment on patient-important outcomes, including parathyroidectomy, fractures, hospitalizations due to cardiovascular events, cardiovascular mortality, all-cause mortality, and intermediate outcomes, in particular Kidney Disease Outcome Quality Initiative targets.
Methods: Data sources included MEDLINE, EMBASE, the Cochrane Register of Controlled Trials and Web of Science from 1996 to June 2015. Teams of two reviewers, independently and in duplicate, screened titles and abstracts and potentially eligible full text reports to determine eligibility, and subsequently abstracted data and assessed risk of bias in eligible trials. We calculated the effect estimates (risk ratios or mean differences) and 95% confidence intervals, as well as statistical measures of variability in results across studies using random effect models. We used the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) approach to rate quality of evidence about estimates of effect on an outcome-by-outcome basis for all outcomes. We presented our results with a GRADE summary table.
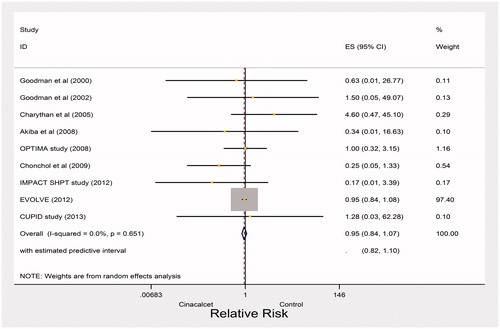
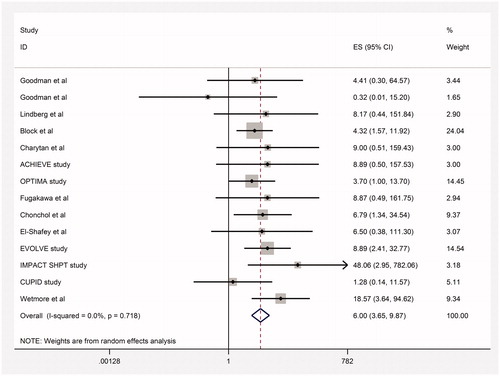
Results: Twenty-four trials including 8311 CKD patients proved eligible. The results left considerable uncertainty regarding the impact of cinacalcet on reducing fractures (relative risk [RR] 0.59, 95% confidence interval [CI] 0.13–2.60; heterogeneity: p = 0.03, I2= 78%; very low quality evidence), and indicated that cinacalcet did not reduce hospitalizations due to cardiovascular events (RR 0.93, 95% CI 0.85–1.02, moderate quality of evidence), cardiovascular mortality (RR 0.95, 95% CI 0.84–1.07; heterogeneity p= 0.61, high quality evidence) or all-cause mortality (RR 0.96, 95% CI 0.89–1.04; heterogeneity: p= 0.98, I2= 0%; moderate quality evidence). Cinacalcet reduced the need for parathyroidectomy (RR 0.30, 95% CI 0.22–0.42; heterogeneity: p= 0.70, I2= 0%; absolute effect 55 fewer per 1000 [95% CI 61 fewer to 45 fewer], high quality of evidence). The most common adverse event associated with cinacalcet therapy was gastrointestinal side effects. Cinacalcet increased nausea (RR 2.16, 95% CI 1.46–3.21, absolute effect 158 more per 1000 [95% CI 82 more to 302 more]) and vomiting (RR 2.15, 95% CI 1.66–2.80, absolute effect 63 more per 1000 [95% CI 109 more to 171 more]). Cinacalcet treatment increased the rate of hypocalcemia (RR 6.0, 95% CI 3.65–9.87; heterogeneity: p= 0.71, I2= 0%, absolute effect 20 more per 1000 [95% CI 11 more to 36 more], high quality of evidence).
Conclusions: In the hands of clinicians participating in these studies, cinacalcet decreased the rate of parathyroidectomy but had no influence on mortality. Patients and clinicians can trade of the benefit of fewer parathyroidectomies against the adverse effects.
Introduction
Chronic kidney disease-mineral and bone disorder (CKD-MBD) involves abnormal serum concentrations of calcium, phosphorus, parathyroid hormone (PTH) and vitamin D.Citation1 These biochemical abnormalities lead to abnormal bone metabolism as well as cardiovascular and soft tissue calcifications.Citation1 Cardiovascular calcifications have been linked to cardiovascular events and mortality, which is the leading cause of death in patients with CKD.Citation2,Citation3
Persistently elevated serum PTH concentrations in those with CKD-MBD indicate the presence of secondary hyperparathyroidism (SHPT). In severe forms of the disease (serum PTH >300 pg/mL), medical management requires combination therapy: the use of vitamin D, which works through vitamin D receptors, and calcimimetic agents that work through calcium sensing receptors.Citation4–6 The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (K/DOQI) clinical practice guidelines provide weak recommendations based on moderate quality evidence in favor of surgical parathyroidectomy if PTH remains constantly above 1000 pg/mL.Citation4
Cinacalcet hydrochloride (cinacalcet) is a second generation calcimimetic agent used to sensitize calcium receptors on the parathyroid glands.Citation7,Citation8 This leads to decreased PTH synthesis and secretion.Citation7,Citation8 Cinacalcet is a positive allosteric modulator that interacts with non-calcium binding sites.Citation5 Although the medication has a very wide applicability, including parathyroid cancers and primary and SHPT, cost is a major drawback that limits its utilization. The total cost of cinacalcet per patient with CKD-MBD ranges from $4000 to $23,500 per year in Canada. The cost of the unit price of the medication in the USA is $0.42 per mg. A patient who needs 100 mg cinacalcet per day would need to pay $42 dollars per day, and approximately $14,400 per year.
A consensus exists regarding the need for CKD-MBD treatment to maintain guideline recommended targets for serum PTH, calcium, and phosphorus concentrations in the presumption that meeting these targets will improve quality and quantity of life.Citation6 Prior systematic reviews have suggested that cinacalcet treatment reduces the rate of parathyroidectomy and fractureCitation9,Citation10; the effect on mortality has not been established.Citation11–13 However, prior reviews have not included trials published after February 2013, searched limited databases, only included adult CKD patients, and did not assess the quality of evidence. We completed a systematic review that addresses these limitations.
The objective of this systematic review was to evaluate the impact of cinacalcet treatment in patients with CKD-MBD and uncontrolled SHPT on patient-important outcomes, including parathyroidectomy, fractures, hospitalization due to cardiovascular events, cardiovascular mortality, all-cause mortality, and intermediate outcomes—specifically, whether patients achieved Kidney Disease Outcome Quality Initiative (K/DOQI) targets.
We hypothesized that reduction in PTH levels may be associated with regression of left ventricular hypertrophy and consequently lowers cardiovascular events, cardiovascular calcifications and mortality. We also addressed the effect of cinacalcet for achieving PTH targets.
Methods
Data sources and search strategy
We searched MEDLINE, EMBASE, the Cochrane Register of Controlled Trials, and web of Science from 1996 until June 2015 without language restrictions. We used controlled vocabulary and text words to search all databases (Supplemental Table S1 available online at http://informahealthcare.com/doi/suppl/10.3109/0886022X.2016.1172468). We searched for conference abstracts from 2003 to 2014 presented at recent annual meetings of the American Society of Nephrology, National Kidney Foundation (from 2006 to 2014: conference abstracts are provided from 2006 onwards) and European Renal Association-European Dialysis and Transplant Association and International Society of Nephrology. We scanned the bibliographies of all prior systematic reviews and meta-analyses as well as all eligible primary studies for additional relevant articles.
Eligibility criteria
We included studies, which enrolled patients with CKD stages 3–5. We limited the studies included in this review to randomized controlled trials (RCTs) evaluating the effectiveness and safety of cinacalcet for the treatment of secondary HPT. We explored the effectiveness of cinacalcet treatment on the following outcomes: parathyroidectomy, fractures, hospitalization due to cardiovascular events, cardiovascular mortality, all-cause mortality, PTH levels (any measure), serum calcium concentrations (mg/dL or mmol/L), serum phosphorus concentrations (mg/dL or mmol/L) and calcium × phosphorus product (mg2/dL2). We also collected data regarding cinacalcet related adverse events, such as hypocalcemia, nausea, and vomiting. We did not employ any restrictions for patient age. We excluded studies with a primary objective of optimal dosing or economic evaluation of cinacalcet treatment.
Study selection
Teams of two investigators independently screened each unique title and abstract identified in our literature search. If either reviewer identified a citation as potentially relevant, we obtained the full text of the article. Two reviewers independently determined the eligibility of all studies that underwent full text evaluation.
We measured the inter-rater agreement for full text eligibility and assessment of risk of bias using the kappa statistic.Citation14 Values of kappa between 0.40 and 0.59 reflect fair agreement, between 0.60 and 0.74 reflect good agreement and ≥0.75 reflects excellent agreement.Citation14 Disagreements were resolved through discussion between reviewers or through adjudication with a third party if necessary.
One of our eligibility criteria was having CKD stages 3–5. CKD is described as kidney damage caused by structural or functional abnormalities that persist for at least 3 months.Citation15 We employed estimated glomerular filtration rate (eGFR) to define CKD from a functional perspective and included those with an eGFR below 60 mL/min/1.73 m2, including dialysis CKD patients (CKD stage 5D) and non-dialysis CKD patients (stages 3–5).Citation15,Citation16
Data abstraction
Using a standardized data collection form, two reviewers abstracted the following information from each study: author, date of publication, eligibility criteria, summary of baseline characteristics of the participants, number of participants in each arm at study onset and completion, duration of the trial and treatment effects, including effectiveness and safety. We resolved disagreements by discussion.
Quality assessment and risk of bias of included studies
Two independent reviewers employed a modified version of the Cochrane risk for bias tool (http://distillercer.com/resources/) to assess risk of bias for all eligible trials. The assessment included the following components: adequacy of sequence generation, allocation sequence concealment, level of blinding, incomplete outcome data, loss to follow-up and stopping early for benefit or futility.Citation17 Reviewers chose among response options of “definitely yes”, “probably yes”, “probably no”, and “definitely no” for each of the domains, with “definitely yes” and “probably yes” ultimately assigned low risk of bias and “definitely no” and “probably no” assigned high risk of bias.Citation18 The reviewers resolved disagreements by discussion.
We used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach to evaluate the quality of evidence, which considers the overall risk of bias, precision, consistency, directness and publication bias, and summarized the results with a GRADE summary findings table.Citation19 With respect to directness, we considered differences in population, intervention, outcomes and settings (primary vs. secondary vs. tertiary care settings). With respect to consistency, we considered statistical heterogeneity and also the visual inspection of forest plots. With respect to precision, we assessed the optimal information size (OIS; the number of patients generated by a conventional sample size calculation for a single trial) and the width of the 95% CIs. For the purposes of calculating the OIS we assumed, for all-cause mortality a relative risk reduction (delta) of 25%, alpha of 0.05, beta of 0.20, and median baseline risk from the largest cohort study of 0.3. We assessed selective reporting in terms of failure to report planned outcomes by comparing published protocols with the manuscripts (when available) as well as methods and results sections of the manuscript.
After considering these reasons for rating down, the overall quality of evidence in estimates of effect for each outcome was reported as follows: “high” quality of evidence (we are very confident that the true effect lies close to that of the estimate of the effect); “moderate” quality of evidence (we are moderately confident in the effect estimate and the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different); “low” quality of evidence (our confidence in the effect estimate is limited and the true effect may be substantially different from the estimate of the effect); and “very low” quality of evidence (we have very little confidence in the effect estimate and the true effect is likely to be substantially different from the estimate of effect).Citation20
Data synthesis and statistical analysis: measures of treatment effect
We used contrast-level summary data to perform pair-wise meta-analysis based on normal models. We reported descriptive statistics as proportions for categorical variables and mean or medians for continuous variables. We calculated pooled risk ratios (RRs) and the associated 95% CI for each outcome using random effects models by applying the maximum likelihood method. We also calculated absolute effects and the associated 95% CIs by multiplying pooled RRs and 95% CI by the control rate of outcomes from the RCT at low risk of bias and with the largest sample size.Citation21 All analyses were performed using Stata (StataCorp. 2013, Stata Statistical Software: Release 13, StataCorp LP, College Station, TX).
Dealing with missing participant data for dichotomous outcomes: sensitivity analyses
We employed a complete-case analysis for our primary analysis and planned to conduct sensitivity analyses to address the robustness of our findings with respect to missing data. We planned to use plausible worst-case scenario for missing trial-level data.
Assessment of heterogeneity and dealing with heterogeneity
We formally assessed heterogeneity using Cochran’s Q test, chi-square test of homogeneity and the I2 statistic for which 0–40% may be unimportant heterogeneity, 30–60% moderate, 50–90% substantial, and 75–100% considerable heterogeneity.Citation22
For cardiovascular mortality, we employed random effects meta-regression and included mean age, the mean baseline serum PTH concentration, trial duration and stages of CKD (dialysis vs. non-dialysis) in our univariate linear models. For all-cause mortality, we employed random effects meta-regression and included trial duration and stages of CKD (dialysis vs. non-dialysis) in our univariate linear models.
Assessment of publication bias
In order to identify publication bias, we employed a funnel plot of the log risk ratio of the treatment effect against its precision for each outcome.Citation23 Publication bias is considered unlikely unless the effect measure is asymmetrically distributed around the pooled effect.Citation23 We also employed Begg’s and Mazumdar’s rank correlation test as well as Egger’s regression to address small study effects and quantify publication bias.Citation24 These tests employ the rank or actual value of effect sizes and their precisions.Citation24
Results
Trial identification
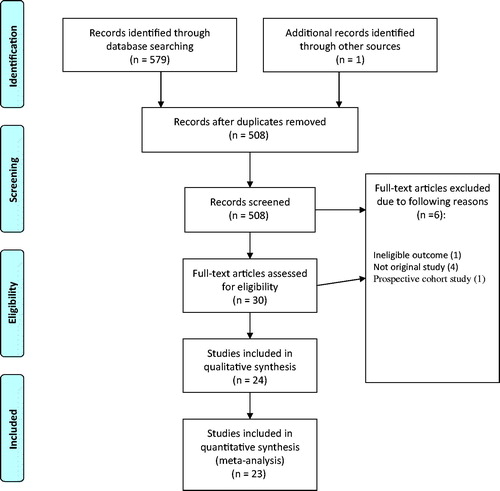
Our search yielded 579 citations, of which 30 were retrieved for full review and 24 RCTs enrolling 8311 patients were eligible for our review (). Agreement for full text eligibility was excellent (kappa = 0.80). We assessed selective reporting in all included studies methods and results sections of the manuscript.
Trial and total population characteristics
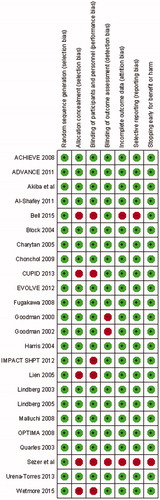
presents the characteristics of the 24 eligible studies. In 3 of the 24 studies non-dialysis patients were included. All studies excluded patients with unstable conditions, and enrolled patients with uncontrolled SHPT. Risk of bias was low in most studies across components, including allocation concealment and blinding of participants and personnel, which was adequately reported in about 75% of the trials ( and ).
Figure 2. Risk of bias summary of included studies of cinacalcet plus standard treatment versus placebo or no standard treatment in patients with chronic kidney disease.
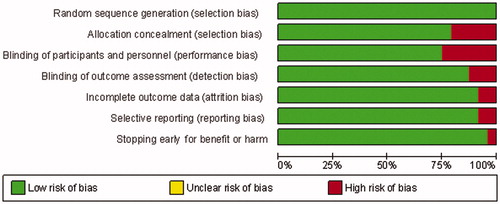
Table 1. Characteristics of included studies.
Outcomes assessment
Parathyroidectomy
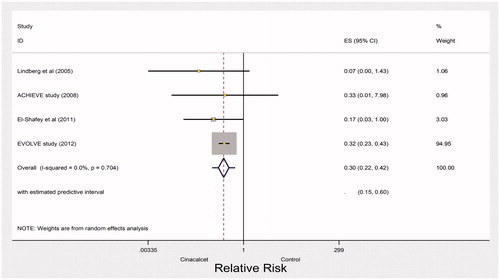
High quality evidence from four trials found a large effect of cinacalcet for preventing parathyroidectomy (RR 0.30, 95% CI 0.22–0.42; heterogeneity: p = 0.70, I2= 0%) (). The point estimate of the absolute effect is 55 fewer events in 1000 over the period of 1 year (CI 61 fewer to 45 fewer, ).
Fractures and hospitalization due to cardiovascular events
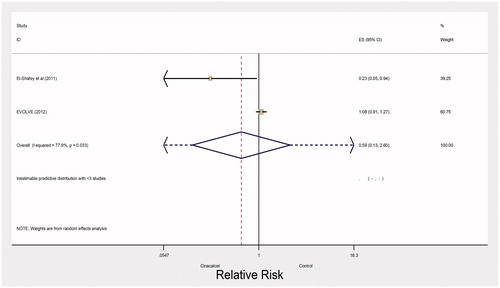
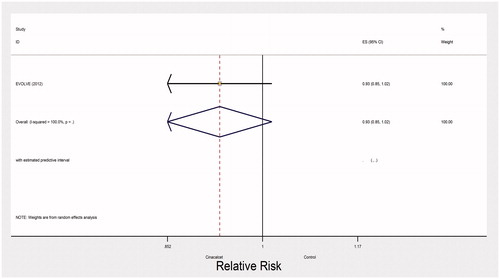
Very low quality evidence from two trials with 3965 participants and 500 events left the impact of cinacalcet on fractures uncertain (RR 0.59, 95% CI 0.13–2.60; heterogeneity: I2= 77%, very low quality evidence) (). The evidence was rated down due to inconsistency, indirectness and imprecision. One large trial (EVOLVE trial) reported no effect of cinacalcet on hospitalization due to cardiovascular events (RR 0.93, 95% CI 0.85–1.02, moderate quality of evidence) ().
Cardiovascular mortality
High quality evidence from nine studies showed 382 versus 399 deaths due to cardiac causes in the cinacalcet and control arms, respectively (RR 0.95, 95% CI 0.84–1.07; heterogeneity: p = 0.61, I2= 0%) (). Cardiovascular mortality may be associated with 5 fewer cardiac deaths in 1000 patients treated with cinacalcet (CI 26 fewer to 16 more, ). Mean age, the mean baseline serum PTH concentration, trial duration, and the percentage of patients receiving dialysis were not associated with magnitude of effect in our univariable linear models (Supplemental Table S2 available online at http://informahealthcare.com/doi/suppl/10.3109/0886022X.2016.1172468).
All-cause mortality
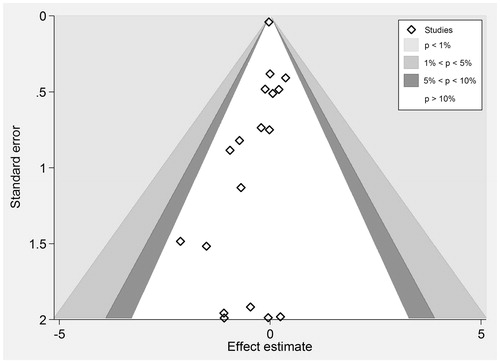
There were 766 deaths in the cinacalcet arm versus 775 deaths in the control arm of 18 studies that reported this outcome (RR 0.96, 95% CI 0.89–1.04; heterogeneity: p = 0.98, I2= 0%; moderate quality of evidence about effects because of inconsistency and publication bias, ) (). We also evaluated heterogeneity using the L’abbe and Galbraith plots both of which did not indicate heterogeneity ( and , respectively). The vertical distance of ±2 above or below the regression through the origin line included all trials in the Galbraith plot ().
Figure 7. Pooled risk ratio of all-cause mortality with cinacalcet plus standard treatment versus placebo or no standard treatment.
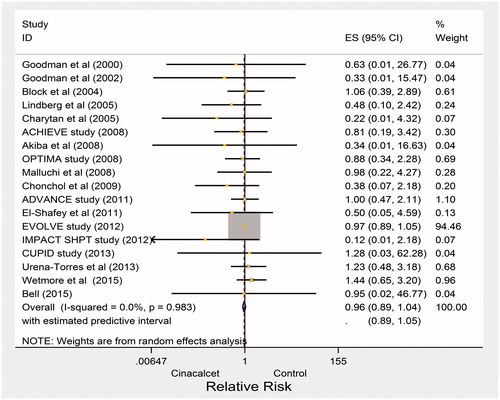
Figure 9. Galbraith plot of standardized effect estimate against inverse standard error for all-cause mortality.
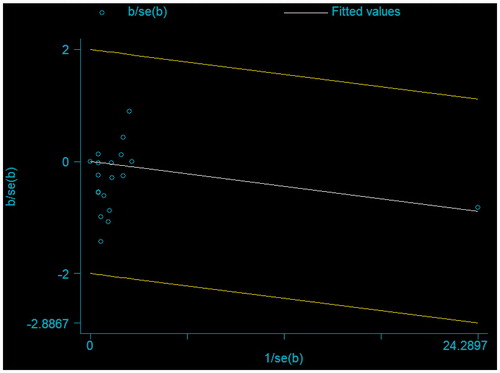
We needed a total sample size of 1200 for all-cause mortality to meet the OIS, which was met. We achieved both targets in our study. We rated down quality of evidence to moderate for a publication bias () (p values for the Mazumdar’s rank correlation test = 0.04; and p values for Egger’s regression = 0.06). Visual inspection of the funnel plot also indicated that small studies favored cinacalcet (). Trial duration and the percentage of patients receiving dialysis were not associated with magnitude of effect in our univariable linear models (Supplemental Table S3 available online at http://informahealthcare.com/doi/suppl/10.3109/0886022X.2016.1172468).
Hypocalcemia
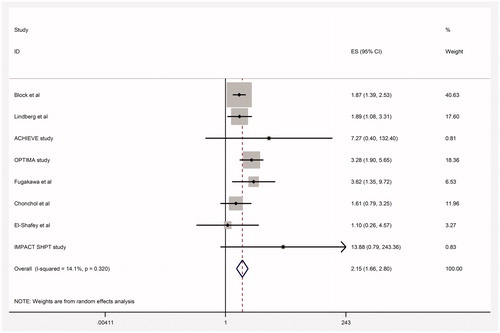
There were 164 cases of hypocalcemia in the cinacalcet arm versus 11 in the control arm of 14 studies (RR 6.0, 95% CI 3.65–9.87; heterogeneity: p = 0.71, I2 = 0%) (95% CI around absolute difference 11 more to 36 more per 1000, high quality of evidence, , ).
Nausea
Cinacalcet increased the risk of nausea (RR 2.16, 95% CI 1.46–3.21; heterogeneity: p = 0.002, I2= 64%). There were 425 nausea in the cinacalcet arm versus 129 nausea in the control arm of 11 studies (158 more cases, 95% CI 82 more to 302 more, moderate quality of evidence due to unexplained heterogeneity, , ).
Vomiting
There were 314 reported cases of vomiting in the cinacalcet arm versus 91 in the control arm of the nine studies (RR 2.15, 95% CI 1.66–2.80; heterogeneity: p = 0.32, I2= 14%, high quality of evidence). Cinacalcet may have an effect on vomiting (CI 63 more to 171 more, , ).
End of treatment serum PTH, Ca and P concentrations
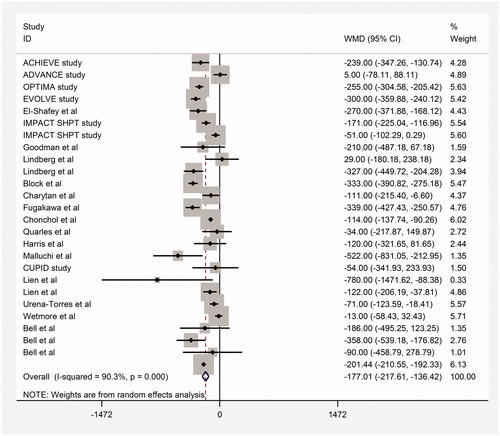
Moderate quality evidence from 21 trials found a significant effect of cinacalcet for lowering serum PTH concentration (WMD −177 pg/mL, 95% CI −227 to −127; heterogeneity: p = 0.001, I2= 90%, , Supplemental Table S4 available online at http://informahealthcare.com/doi/suppl/10.3109/0886022X.2016.1172468). Moderate quality evidence found that cinacalcet lowered serum calcium (17 studies included) and calcium phosphorus product (9 studies included) (WMD −0.66 mg/dL, 95% CI −1.07 to −0.25 for calcium; WMD −4.03 mg2/dL2, 95% CI −7.7 to −0.34 for calcium phosphorus product). Very low quality evidence found no effect on serum phosphorus concentration (WMD −0.12 mg/dL, 95% CI −0.32 to 0.07) () (Supplemental Table S4 available online at http://informahealthcare.com/doi/suppl/10.3109/0886022X.2016.1172468).
Sensitivity analyses
The majority of the studies reported numbers and reasons for missing data in each arm. We planned to employ sensitivity analysis for benefit outcomes showing significant effects—in this case, parathyroidectomy—using the plausible worst case scenario. Three of four studies reported no loss to follow-up. As a result, we did not perform the sensitivity analysis.
Discussion
Our systematic review found high quality evidence that cinacalcet therapy reduces the risk of parathyroidectomy (RR 0.30, 95% CI 0.22–0.42) and moderate quality of evidence for most variables (very low for phosphorus) in achieving K/DOQI targets (PTH WMD −177 pg/mL, 95% CI −227 to −127; calcium WMD -0.66 mg/dL, 95% CI −1.07 to −0.25; phosphorus WMD −0.12 mg/dL, 95% CI −0.32 to 0.07; calcium-phosphorus product WMD −4.03 mg2/dL2, 95% CI −7.7 to −0.34). If our estimates of effect on outcomes of parathyroidectomy were accurate, the impact of cinacalcet would represent an important benefit (point estimates of absolute effects: 55 fewer parathyroidectomies per 1000 patients).
We found no effect on fractures, hospitalization due to cardiovascular events, cardiovascular mortality and all-cause mortality (RR 0.95, 95% CI 0.84–1.07 for cardiovascular mortality; RR 0.96, 95% CI 0.89–1.04 for all-cause mortality). The most common adverse events related to cinacalcet included gastrointestinal side effects, such as nausea and vomiting. Moreover, cinacalcet was associated with an increased risk for hypocalcemia.
Even though our pooled estimate failed to show the mortality benefit of cinacalcet, several observational studies indicated a reduced risk of cardiovascular and all-cause mortality in those who were on cinacalcet treatment (HR = 0.78, 95% CI 0.71–0.86; HR = 0.73, 95% CI 0.68–0.78, cardiovascular and all-cause mortality, respectively) and those with well-controlled biochemical markers.Citation25,Citation26 A subgroup analysis (age <65 vs. ≥65) of the EVOLVE trial indicated mortality benefit of cinacalcet treatment in those who were older than 65 (HR =0.73, 95% CI 0.62–086). The test of significance for the subgroup effect was not reported.Citation27
In addition to study level findings, a recent systematic review explored the correlation between CKD-MBD biochemical markers and mortality.Citation28 The results indicated a significant negative correlation between PTH and all-cause mortality.Citation28 Nevertheless, the correlation between serum calcium and phosphorus concentration and mortality did not prove significant.Citation28 We found that cinacalcet reduces PTH but has no effect on mortality. A mortality benefit seems implausible since the damage in the cardiovascular system is irreversible and progressive at this advanced stage of CKD-MBD.
Economic evaluation of cinacalcet in CKD-MBD indicated mixed results in terms of cost-effectiveness both in the US and European healthcare settings.Citation29–32 The reduction in the cost was attributable to lower rates of parathyroidectomy, cardiovascular events and fractures in an economic evaluation from Japan.Citation31 The effectiveness data were received from observational studies. This analysis is not valid given that our results show that cinacalcet does not change hospitalizations due to cardiovascular events and may not reduce fractures.Citation31 In any case, access to cinacalcet care is restricted to those at the upper end of the socioeconomic spectrum unless publicly funded health care systems provide price subsidies for eligible patients.
Strengths and limitations of this study
Strengths of our systematic review and meta-analysis include explicit eligibility criteria, a comprehensive search, independent duplicate assessment of eligibility, and use of the GRADE approach to assessing quality of evidence an outcome-by-outcome basis. This is the first systematic review that includes a search for both pediatric and adult CKD patient populations in the assessment of cinacalcet and addresses patient important outcomes available in the literature. Our search identified one study in the pediatric patient population, which proved ineligible since it was a dose-finding study.Citation33 Limitations of our review included low quality evidence for some outcomes ().
Table 2. GRADE assessment of quality of evidence about effectiveness of cinacalcet plus standard treatment versus placebo or no standard treatment in patients with chronic kidney disease.
Conclusions
Our findings did not indicate apparent benefits of cinacalcet therapy on mortality which may preclude the use of cinacalcet as the first line therapy in CKD patients with uncontrolled hyperparathyroidism. A systematic review with individual patient data may provide an avenue for subgroup analyses to verify potential benefits of cinacalcet in selected patient populations. In order to guide future work in this area, another recommendation would be a large multicenter RCT with a long trial period, for instance 2 years, by focusing on cardiovascular events, mortality and fractures.
In the interval, clinicians should seriously consider benefits and harms of standard treatment versus the use of cinacalcet to control persistently elevated PTH levels due to CKD-MBD. Patient values, preferences and ability to pay are other considerations in the decision-making process.
Disclosure statement
The authors declare that they have no financial or non-financial competing interests.
References
- Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: A position statement from kidney disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006;69:1945–1953.
- Block GA, Zaun D, Smits G, et al. Cinacalcet hydrochloride treatment significantly improves all-cause and cardiovascular survival in a large cohort of hemodialysis patients. Kidney Int. 2010;78:578–589.
- Noordzij M, Korevaar JC, Boeschoten EW, Dekker FW, Bos WJ, Krediet RT. The Kidney Disease Outcomes Quality Initiative (K/DOQI) guideline for bone metabolism and disease in CKD: Association with mortality in dialysis patients. Am J Kidney Dis. 2005;46:925–932.
- Arenas MD, Alvarez-Ude F, Gil MT, et al. Implementation of ‘K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease’ after the introduction of cinacalcet in a population of patients on chronic hemodialysis. Nephrol Dial Transplant. 2007;22:1639–1644.
- Wetmore JB, Quarles LD. Calcimimetics or vitamin D analogs for suppressing parathyroid hormone in end-stage renal disease: Time for a paradigm shift? Nat Clin Pract Nephrol. 2009;5:24–33.
- Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: Pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6:913–921.
- Hammerland LG, Garrett JE, Hung BC, Levinthal C, Nemeth EF. Allosteric activation of the Ca2+ receptor expressed in Xenopus laevis oocytes by NPS 467 or NPS 568. Mol Pharmacol. 1998;53:1083–1088.
- Nemeth EF, Steffey ME, Hammerland LG, et al. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci USA. 1998;95:4040–4045.
- Palmer SC, Nistor I, Craig JC, et al. Cinacalcet in patients with chronic kidney disease: A cumulative meta-analysis of randomized controlled trials. PLoS Med. 2013;10:e1001436.
- Strippoli GFM, Tong A, Palmer SC, Elder G, Craig JC. Calcimimetics for secondary hyperparathyroidism in chronic kidney disease patients. Cochrane Database Syst Rev. 2006;CD006254.
- Fishbane S, Shapiro WB, Corry DB, et al. Cinacalcet HCl and concurrent low-dose vitamin D improves treatment of secondary hyperparathyroidism in dialysis patients compared with vitamin D alone: The ACHIEVE study results. Clin J Am Soc Nephrol. 2008;3:1718–1725.
- Messa P, Macario F, Yaqoob M, et al. The OPTIMA study: Assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2008;3:36–45.
- Raggi P, Chertow GM, Torres PU, et al. The ADVANCE study: A randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant. 2011;26:1327–1339.
- Orwin RG. Evaluating coding decisions. In: Cooper H, Hedges LV, eds. The Handbook of Research Synthesis. New York, NY: Russell Sage Foundation; 1994;50–100.
- Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;113:S1–S130. doi: 10.1038/ki.2009.188.
- Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–159.
- Akl EA, Sun X, Busse JW, et al. Specific instructions for estimating unclearly reported blinding status in randomized trials were reliable and valid. J Clin Epidemiol. 2012;65:262–267.
- Guyatt GH, Oxman AD, Santesso N, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013;66:158–172.
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical Research Ed). 2008;336:924–926.
- EVOLVE Trial Investigators, Chertow GM, Block GA, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482–2494.
- Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence-inconsistency. J Clin Epidemiol. 2011;64:1294–1302.
- Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence–publication bias. J Clin Epidemiol. 2011;64:1277–1282.
- Borenstein M. Software for publication bias. In: Rothstein HR, Sutton AJ, Borenstein M, eds. ublication bias in meta-analysis, prevention, assessment and adjustment. Hoboken, NJ: John Wiley & Sons; 2005:195–219.
- Tentori F, Wang M, Bieber BA, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: The DOPPS study. Clin J Am Soc Nephrol. 2015;10:98–109.
- Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis. 1998;31:607–617.
- Parfrey PS, Drueke TB, Block GA, et al. The effects of cinacalcet in older and younger patients on hemodialysis: The evaluation of cinacalcet HCl therapy to lower cardiovascular events (EVOLVE) trial. Clin J Am Soc Nephrol. 2015;10:791–799.
- Palmer SC, Teixeira-Pinto A, Saglimbene V, et al. Association of drug effects on serum parathyroid hormone, phosphorus, and calcium levels with mortality in CKD: A meta-analysis. Am J Kidney Dis. 2015;66:962–971.
- Belozeroff V, Chertow GM, Graham CN, Dehmel B, Parfrey PS, Briggs AH. Economic evaluation of cinacalcet in the United States: The EVOLVE trial. Value Health. 2015;18:1079–1087.
- Iannazzo S, Carsi M, Chiroli S. A cost-utility analysis of cinacalcet in secondary hyperparathyroidism in five European countries. Appl Health Econ Health Policy. 2012;10:127–138.
- Komaba H, Moriwaki K, Goto S, et al. Cost-effectiveness of cinacalcet hydrochloride for hemodialysis patients with severe secondary hyperparathyroidism in Japan. Am J Kidney Dis. 2012;60:262–271.
- Boer R, Lalla AM, Belozeroff V. Cost-effectiveness of cinacalcet in secondary hyperparathyroidism in the United States. J Med Econ. 2012;15:509–520.
- Padhi D, Langman CB, Fathallah-Shaykh S, et al. An open-label study to evaluate a single-dose of cinacalcet in pediatric dialysis subjects. Pediatr Nephrol (Berlin, Germany). 2012;27:1953–1959.
- Goodman WG, Hladik GA, Turner SA, et al, The calcimimetic agent AMG 073 lowers plasma parathyroid hormone levels in hemodialysis patients with secondary hyperparathyroidism. J Am Soc Nephrol. 2002;13:1017–1024.
- Goodman WG, Frazao JM, Goodkin DA, Turner SA, Liu W, Coburn JW. A calcimimetic agent lowers plasma parathyroid hormone levels in patients with secondary hyperparathyroidism. Kidney Int. 2000;58:436–445.
- Block GA, Martin KJ, de Francisco ALM, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516–1525.
- Lindberg JS, Culleton B, Wong G, et al. Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: A randomized, double-blind, multicenter study. J Am Soc Nephrol. 2005;16:800–807.
- Charytan C, Coburn JW, Chonchol M, et al. Cinacalcet hydrochloride is an effective treatment for secondary hyperparathyroidism in patients with CKD not receiving dialysis. Am J Kidney Dis. 2005;46:58–67.
- Malluche HH, Monier-Faugere MC, Wang G. An assessment of cinacalcet HCI effects on bone histology in dialysis patients with secondary hyperparathyroidism. Clin Nephrol. 2008;69:269–277.
- Quarles LD, Sherrard DJ, Adler S, et al. The calcimimetic AMG 073 as a potential treatment for secondary hyperparathyroidism of end-stage renal disease. J Am Soc Nephrol. 2003;14:575–583.
- El-Shafey EM, Alsahow AE, Alsaran K, Sabry AA, Atia M. Cinacalcet hydrochloride therapy for secondary hyperparathyroidism in hemodialysis patients. Ther Apher Dial. 2011;15:547–555.
- Chonchol M, Locatelli F, Abboud HE, et al. A randomized, double-blind, placebo-controlled study to assess the efficacy and safety of cinacalcet HCl in participants with CKD not receiving dialysis. Am. J. Kidney Dis. 2009;53:197–207.
- Urena-Torres P, Bridges I, Christiano C, et al. Efficacy of cinacalcet with low-dose vitamin D in incident haemodialysis subjects with secondary hyperparathyroidism. Nephrol. Dial. Transplant. 2013;28:1241–1254.
- Wetmore JB, Gurevich K, Sprague S, et al. A randomized trial of cinacalcet versus vitamin D analogs as monotherapy in secondary hyperparathyroidism (PARADIGM). Clin J Am Soc Nephrol. 2015;10:1031–1040.
- Kim HJ, Kim H, Shin N, et al. Cinacalcet lowering of serum fibroblast growth factor-23 concentration may be independent from serum Ca, P, PTH and dose of active vitamin D in peritoneal dialysis patients: a randomized controlled study. BMC Nephrol. 2013;14:112.
- Akiba T, Akizawa T, Tsukamoto Y, Uchida E, Iwasaki M, Koshikawa S. Dose determination of cinacalcet hydrochloride in Japanese hemodialysis patients with secondary hyperparathyroidism. Ther Apher Dial. 2008;12:117–125.
- Lien Y-HH, Silva AL, Whittman D. Effects of cinacalcet on bone mineral density in patients with secondary hyperparathyroidism. Nephrol. Dial. Transplant.2005;20:1232–1237.
- Fukagawa M, Yumita S, Akizawa T, et al. Cinacalcet (KRN1493) effectively decreases the serum intact PTH level with favorable control of the serum phosphorus and calcium levels in Japanese dialysis patients. Nephrol Dial Transplant. 2008;23:328–335.
- Ketteler M, Martin KJ, Wolf M, et al. Paricalcitol versus cinacalcet plus low-dose vitamin D therapy for the treatment of secondary hyperparathyroidism in patients receiving haemodialysis: Results of the IMPACT SHPT study. Nephrol Dial Transplant. 2012, 27:3270–3278.
- Bell G, Huang S, Martin KJ, Block GA. A randomized, double-blind, phase 2 study evaluating the safety and efficacy of AMG 416 for the treatment of secondary hyperparathyroidism in hemodialysis patients. Curr Med Res Opin. 2015;31:943–952.
- Harris RZ, Padhi D, Marbury TC, et al. Pharmacokinetics, pharmacodynamics, and safety of cinacalcet hydrochloride in hemodialysis patients at doses up to 200 mg once daily. Am J Kidney Dis. 2004;44:1070–1076.
- Sezer S, Tutal E, Bal Z, Ozelsancak, R et al. Treatment of secondary hyperparathyroidism in the maintanence hemodialysis patients: A randomized clinical trial comparing paricalcitol, calcitriol and sinacalcet. Nephrol Dial Transplant. 2012;27(2):ii509.
- Lindberg JS, Moe SM, Goodman WG, et al. The calcimimetic AMG 073 reduces parathyroid hormone and calcium phosphorus in secondary hyperparathyroidism. Kidney Int. 2003;63:248–254.