Abstract
Objectives: Extracorporeal shock wave (ESW) lithotripsy is the preferred treatment modality for uncomplicated kidney stones. More recently free oxygen radical production following ESW application has been considered to be crucial in shock wave-induced renal damage. It has been shown that ozone therapy (OT) has ameliorative and preventive effects against various pathological conditions due to increased nitro-oxidative stress. In current study, we aimed to evaluate the efficacy of OT against ESW-induced renal injury. Methods: Twenty-four male Sprague–Dawley rats were divided into three groups: sham-operated, ESW, and ESW + OT groups. All groups except sham-operated group were subjected to ESW procedure. ESW + OT group received 1 mg/kg/day of oxygen/ozone mixture intraperitoneally at 2 h before ESW, and OT was continued once a day for consecutive three days. The animals were killed at the 4th day, and kidney tissue and blood samples were harvested for biochemical and histopathologic analysis. Results: Serum ALT and AST levels, serum neopterin, tissue nitrite/nitrate levels, and tissue oxidative stress parameters were increased in the ESW group and almost came close to control values in the treatment group (p < 0.05, ESW vs. ESW + OT). Histopathological injury scores were significantly lower in treatment group than the ESW group (p < 0.05, ESW vs. ESW + OT). Immunohistochemical iNOS staining scores in ESW group were higher than those of sham-operated group (p < 0.05, ESW vs. sham-operated), iNOS staining scores in OT group were significantly lower than the ESW group (p < 0.05, ESW + OT vs. ESW). Conclusion: OT ameliorates nitro-oxidative stress and reduces the severity of pathological changes in the experimental ESW-induced renal injury of rat model.
Introduction
Extracorporeal shock wave (ESW) lithotripsy, which produces fragmentation of the calculi in the renal pelvis and facilitates calculi elimination from renal pelvis through the excretory system, is the preferred treatment modality for uncomplicated kidney stones, because it is noninvasive and has a high success rate for stone removal.Citation1,Citation2 However, it is not completely free of complication. After ESW, the most frequently observed sign of kidney trauma is hematuria, while less often perirenal, subcapsular, or intrarenal hematomas or other symptomatic fluid collections are observed.Citation3,Citation4
Several clinical and experimental studies have reported that sound waves on the kidney cause damage to tissue in exposed kidney.Citation1,Citation5–9 Although the mechanism of renal injury after ESW is still not well-defined, more recently free oxygen radical production originating from transient ischemia due to ESW application has been considered to be an integral element in shock wave-induced renal damage.Citation7,Citation10,Citation11 Studies, measuring renal resistive index or followed renal blood velocity in the kidney subjected to SWT, showed that an increase in resistive index or a reduction in blood velocity indicative of vasoconstriction occurred.Citation4,Citation12,Citation13 It was also reported that there is a decrease in renal perfusion in kidney following SWT procedure by using dynamic gadolinium-DTPA-enhanced magnetic resonance imaging.Citation12 Two possible explanations for the impairment of renal flow put forward by researchers; either renal sympathetic nerve may be activated by shock waves or vasoconstriction may be occurred by vasoconstrictors released from kidneys in response to the shock waves. It is assumed that this transient ischemia and following reperfusion causes release of cytokines/inflammatory cellular mediators, production of oxygen-derived free radicals and nitrogen-derived free radicals, and infiltration of tissue by inflammatory response cells, and this process initiates the cellular damage and death.Citation6,Citation8,Citation9,Citation14 Moreover, many experimental studies have shown that renal injury following ESW can be prevented by antioxidant treatment modalities such as melatonin, coenzyme Q10, curcumin, and vitamin E;Citation5,Citation6,Citation14 the main purpose of these studies was to inhibit inflammatory reaction at the early phase so as to prevent renal damage. Our recent works also showed that SWT causes renal injury, especially at the proximal tubular system, and we demonstrated that this injury is due to excessive production of oxygen free radicals and nitric oxide (NO) production. In addition, we demonstrated that iNOS inhibitors or peroxynitrite scavengers might protect the kidneys against SWT-induced morphological and functional injury.Citation8,Citation9
Ozone (O3), a gas composed of three oxygen atoms, is continuously produced in the stratosphere by UV radiation or during the electric discharge of lightning from atmospheric oxygen. A gas mixture comprising ozone/oxygen used in medicine is known as medical ozone therapy (OT). Ozone/oxygen mixture exhibits various effects on the immune system, such as the modulation of phagocytic activity of peritoneal and alveolar macrophages.Citation15–17 Clinical studies have so far shown that OT appears useful in diseases including peritonitis, infected wounds, and advanced ischemic diseases.Citation17,Citation18 It was also demonstrated that ozone increases antioxidant enzyme activities such as glutathione peroxidase (GSH-Px), SOD, and catalase (CAT), preparing the host to face physiopathological conditions mediated by ROS/RNS.Citation16,Citation19 Ameliorative effects of OT on oxidative and nitrosative stress have been reported in different experimental models such as renal ischemia/reperfusion injury,Citation20 necrotizing pancreatitis,Citation21 caustic esophageal burn,Citation22 and necrotizing enterocolitisCitation23 by our research group.
Based on these data and observations, we designed this study to find out whether OT has an ameliorative effect on ESW-induced renal injury in an experimental model of rats.
Materials and methods
Animals and study groups
All animal procedures were approved by the Institutional Committee on the Care and Use of Animals of our institution (Issue; 11/34, 1 July 2011). Twenty-four male Sprague–Dawley rats (200–250 g) provided by the animal laboratory of our institute were randomly assigned into three groups containing eight rats each: Sham-operated, ESW, and ESW + OT groups. Before the experiment, animals were fed standard rat chow and water ad libitum and housed in cages with controlled temperature and 12-h light/dark cycle for at least 1 week.
Induction of ESW-induced renal injury
To provide standardization for injury, ESW was administrated to right kidney only for each animal. Therefore, ESW-free left kidneys were nephrectomized to avoid the concealing properties of intact kidney in terms of biochemical parameters indicating renal functions 2 weeks earlier the ESW procedure. All animals were fasted overnight and anesthetized by an intramuscular injection of 50 mg/kg ketamine (Ketalar; Parke Davis, Eczacibasi, Istanbul, Turkey) and 10 mg/kg xylazine (Rompun; Bayer AG, Leverkusen, Germany), and were operated at room temperature (24 °C). Through a midline laparotomy, left kidneys of all rats removed, and then radiopaque materials were placed in the rear wall of the abdomen on the back of remaining right kidneys in ESW and ESW + OTgroups in order to focus renal tissue. The control group was underwent laparotomy with just nephrectomy. Finally, the abdominal incision was closed, and for the adaptation of animals to single kidney after nephrectomy, the animals were returned to their cages to recover for 2 weeks. Water and food were available ad libitum. The weights of rats were recorded throughout the experimental period.
Under anesthesia, rats in the ESW and ESW + OT groups were fixed in supine position on the platform of lithotriptor and applied with shock wave lithotripsy at the right kidney under the guidance of X-Rays. Each rat received 2000 shocks, 18 kiloVolt, and total 15 joule energy delivered with a Siemens LithoskopTM (Germany). The focal size is 16 mm; the pressure range is not known according to technical brochure; and the focal depth is 16 cm. The shockwave rate was 60 shock waves/min. The shock waves were applied with ramping similar to human application. The number of shock waves applied and the level of energy were decided based on previous studies and our preliminary studies.Citation8,Citation9,Citation24,Citation25 After ESW procedure, rats were returned to their cages.
Treatment modalities
Two hours before the ESW procedure, the rats in the ESW + OT group were administered ozone/oxygen mixture at a single dose of 1 mg/kg via intraperitoneal route, and the dose was repeated for three consecutive days.
Ozone (O3) was generated by the ozone generator (OZONOSAN Photonik 1014, Hansler GmbH, Nordring 8, Iffezheim, Germany) allowing control of the gas flow rate and ozone concentration in real time by a built-in UV spectrometer. The ozone flow rate was kept constant at 3 L/min, representing a concentration of 60 μg/mL and gas mixture of 97% O2 + 3% O3. Tygon polymer tubes and single-use silicon-treated polypropylene syringes (ozone resistant) were used throughout the reaction to ensure containment of O3 and consistency of concentrations.
Harvesting the samples
After 24 h of last dose of treatment, animals were sacrificed by cervical dislocation. At the time of death, blood was collected by heart puncture for biochemical analyses, and right kidneys were harvested for histopathological evaluation and biochemical examination. Harvested renal tissue samples stored in 10% formalin solution for histopathological evaluation and in −80 °C for antioxidant enzyme activity and tissue lipid peroxidation levels valuation. Blood samples also obtained for biochemical analysis and centrifuged to study other biochemical parameters in serum.
Tissue preparation and biochemical analysis
The frozen tissues were homogenized in phosphate buffer (pH 7.4) by means of a homogenization (Heidolph Diax 900; Heidolph Elektro GmbH, Kelhaim, Germany) on an ice cube. The supernatant was used for entire assay. Initially, the protein content of tissue homogenates was measured by the method of Lowry with bovine serum albumin as the standardCitation26 which was used for all assays.
Lipid peroxidation level was measured with the thiobarbituric acid (TBA) reaction by the method of Ohkawa.Citation27 This method was used to obtain a spectrophotometric measurement of the color produced during the reaction to thiobarbituric acid (TBA) with malondialdehyde (MDA) at 535 nm. The calculated MDA levels were expressed as mmol/g-protein.
Superoxide dismutase (SOD) activity was assayed using the nitroblue tetrazolium (NBT) method of Sun et al and modified by Durak et al.Citation28 In this method, NBT was reduced to blue formazan by O2−, which has a strong absorbance at 560 nm. One unit (U) of SOD is defined as the amount of protein that inhibits the rate of NBT reduction by 50%. The estimated SOD activity was expressed as Units per gram protein.
The glutathione peroxidase (GSH-Px) activity was measured using the method described by Paglia and ValentineCitation29 in which GSH-Px activity was coupled with the oxidation of NADPH by glutathione reductase. The oxidation of NADPH was spectrophotometrically followed up at 340 nm at 37 °C. The absorbance at 340 nm was recorded for 5 min. The activity was the slope of the lines which presented as mmol of NADPH oxidized per minute. GSH-Px activity was presented as U/g-protein.
Serum creatinine, uric acid, alkaline phosphatase (ALP), and electrolytes (sodium, potassium, and chloride) concentrations were measured with a spectrophotometric technique by the Olympus AU-2700 autoanalyzer using commercial kits (Olympus, Hamburg, Germany) and presented as mg/dL, mg/dL, IU/L, and mEq/dL, respectively.
Serum neopterin (NP) levels were determined by using a high-pressure liquid chromatography (HPLC) system with a fluorescence detector (AgilentTechnologies 1200 Series System, Santa Clara, CA) as prescribed previouslyCitation30,Citation31 and presented as nmol/L.
Serum NOx levels were detected by means of an ion chromatograph (Dionex ICS-1000, Sunnyvale, CA). Before NOx analysis, serum samples were passed through 0.45-μm pore membrane nitrocellulose filters. Anion and guard columns (AS-9HC/AG-9HC, CS12A/CG12A, Sunnyvale, CA) and automated suppression were used. NOx levels were quantified using separate standard solutions for each ion and expressed as mg/L.Citation32
Histopathologic evaluation
One-half of each kidney was taken for histopathologic evaluation. In all groups, samples of kidney were placed in 10% tamponed formalin and send to pathology for routine automatic tissue processing. After paraffin embedding, blocks were subsequently sectioned at 5-μm thickness and stained with hematoxylin and eosin (H&E) and iNOS immunohistochemical staining.
The sections were scored with a semiquantitative scale designed to evaluate the degree of renal damage. The kidneys were evaluated in terms of glomerular changes (congestion, hemorrhage, necrosis, intracapillary, and extracapillary proliferation [crescent formation]), tubular changes (epithelial vacuolar degeneration, necrosis, and regeneration) interstitial changes (edema, peritubular capillary congestion, hemorrhage, and inflammation), and vascular changes (fibrinoid necrosis and fibrointimal thickening). All section areas for each kidney slide were examined and assigned for severity of changes. The scoring system used was 0, normal; 1, focal and mild; 2, focal and severe; 3, diffuse and mild; 4, diffuse and severe. Total histopathologic injury score per kidney was calculated by addition of all scores. Blind analysis of the histological samples was performed by two independent experts.
For demonstrating the iNOS expression, standard 5-mm sections were obtained and deparaffinized with xylene and rehydrated in graded alcohols. Then, the kidney sections were stained with primary antibodies against iNOS (1:50, monoclonal, Enzo, Enzo Life Sciences Ltd, Plymouth, PA) using the standard streptavidin–biotin peroxidase technique on an automatic device (Ventana, Benchmark XT, Ventana Medical Systems, Tucson, AZ). The expression of iNOS was evaluated qualitatively by a pathologist using a light microscope.
Statistical analysis
Results were expressed as median ± (standard deviation [SD]). In the other analyses, differences among the groups were analyzed by the Kruskal–Wallis test. Dual comparisons among groups with significant values were evaluated with the Mann–Whitney U test. p < 0.05 were considered significant. All analyses were performed with the Statistical Package for the Social Sciences (SPSS) statistical program (Software version 11.0, SPSS Inc., Chicago, IL).
Results
Serum biochemical values
All subjects were survived throughout the study period and there were any statistically differentbetween animal and group in terms of body weight.
All serum biochemical values are summarized in . Serum AST and ALP levels were significantly higher in the ESW group than the other groups suggesting increased renal injury (p < 0.05). Serum creatinine and serum electrolyte levels (Na+, K+, Cl−) were significantly decreased in the ESW and ESW + OT groups than the sham-operated group (p < 0.05). Serum NP level was significantly increased in the ESW group than the sham-operated group (p < 0.05).
Table 1. Biochemical values in serum.
Tissue lipid peroxidation levels
The MDA levels in the ESW group were significantly increased than the other groups, but it was decreased in the ESW + OT group than the ESW group (p < 0.05; ESW vs. the other groups and ESW vs. ESW + OT; ).
Table 2. Values of lipid peroxidation and antioxidant enzymes.
Tissue antioxidant enzyme activities
The tissue SOD and GSH-Px activity were significantly increased in ESW and ESW + OT group (p < 0.05, ESW and ESW + OT groups vs. sham-operated group). These enzyme activities were decreased in the ESW + OT group when compared the ESW group (p < 0.05, ESW + OT vs. ESW). However, antioxidant enzyme activities were still high in the ESW + OT group when compared to the sham-operated group (p < 0.05; ESW + OT groups vs. sham-operated group; ).
Tissue NOx level (nitrite/nitrite concentration)
The tissue NOx levels were significantly increased in the SWT group, suggesting increased nitric oxide and peroxynitrite production (p < 0.05, SWT group vs. sham-operated group). The NOx levels in the SWT + OT group were significantly decreased when compared to ESW group, but it was still higher than the sham-operated group (p < 0.05; SWT + OT group vs. SWT groups and sham-operated group; ).
Histological findings
Histopathological injury scores were summarized in . The score was the highest in the ESW group, and microscopic examination of the kidneys for this group revealed obvious mild to moderate glomerular congestion, and severe tubular injury, which are consisted of epithelial vacuolization and necrosis with necrotic luminal debris, diminishing or loss of brush borders, and moderate to severe interstitial edema, inflammation, and peritubular capillary congestion (). In the SWT + OT group, glomerular, tubular, and interstitial changes were better than the SWT group ().
Figure 1. Light microscopic examination of the kidneys. (A) shows the kidney of sham-operated group. There is a mild to moderate glomerular congestion, and severe tubular dilatation and injury in ESW group (B). Tubular structures are seen preserved in the kidneys of ESW + OT groups (C) compared with the ESW group.
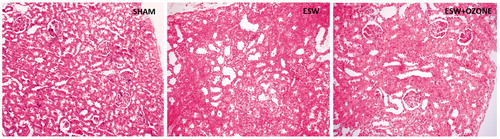
Table 3. Pathologic scores.
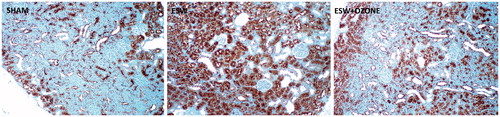
Immunostaining of kidneys for iNOS in SWT group revealed that iNOS is expressed mostly in the cortex, especially among the proximal tubular epithelium (). This intensity was observed as decrease in the kidneys of SWT + OT group when compared to the SWT group ().
Figure 2. Immunohistochemical evaluation of kidney. (A) shows the sham-operated group. Immunostaining of the iNOS protein in the kidneys reveals intense positive staining (brown) in the tubular structure of kidneys subjected to SWT procedure (2B). Immunostaining for iNOS is lower in the SWT + OT group (2C) when compared with the SWT group.
Discussion
To the best of our knowledge, this is the first report evaluating the effect of the medical OT on ESW-induced renal injury in an experimental rat model. Our biochemical data showed that OT reduces the oxidative and nitrosative stress in the kidney subjected to ESW. In addition, histopathologic evaluation demonstrated that OT protects the kidney from the damage caused by ESW.
We found that serum creatinine, serum Na+, and serum K+ levels are decreased in rats subjected to ESW procedure. It is known that following the glomerular filtration, water, electrolytes, and other molecules (aminoacits, glucose etc.) are handled at different rates depending on tubular regions. In addition, creatinine is secreted from proximal tubulus and not reabsorbed in distal tubules. Renal injury due to ESW is especially and intensively occurred in proximal tubulus as shown our histopathologic evaluation and our iNOS immunohistochemical staining in our previous studies.Citation8,Citation9 Therefore, we assume that creatinine and electrolytes are secreted more than normally from proximal tubules in rats subjected to ESW procedure resulting in lower serum creatinine level. Another explanation for this situation may be related to different protein channels for each reabsorbed molecules and this needs further studies.
We evaluated the tissue oxidative stress marker (MDA), nitrosative stress marker (NOx), and antioxidant system (SOD and GSH-Px) in the kidney. Our data reveal that oxidative/nitrosative stress clearly has a pivotal role in the pathogenesis of ESW-induced renal injury as indicated and discussed in our previous studies and the others.Citation8,Citation9,Citation13,Citation14,Citation33,Citation34
Our data also displayed that OT administration decreases oxidative stress status by decreasing MDA and increasing SOD and GSH-Px in the kidneys subjected to ESW. The researches have shown that OT acts as a prodrug and formed a transient and miniaturized oxidative stress resulting in a sort of therapeutic shock for the injured cells. It is assumed that ozone, after administration, is dissolved in biological fluids such as plasma, lymph, and urine; it immediately reacts with macromolecular glycoproteins composed of carbohydrates and polypeptide chains, namely proteoglycans and collagen types II and IV. All of these compounds act as electron donors and undergo oxidation, resulting in the formation of hydrogen peroxide (H2O2) and lipid oxidation products (LOPs). H2O2, an essential ROS molecule, is able to act as an ozone messenger for eliciting several biological and therapeutic effects.Citation15,Citation16,Citation35,Citation36 In contrast to the conventional idea that H2O2 is harmful, it has been widely revised that it acts as a regulator of signal transduction and is an important mediator of host defense and immune responses. While H2O2 acts immediately and disappears (early and short-acting messenger), LOPs, via the circulation, distribute throughout the tissues and become late and long-lasting messengers.Citation16 This process stimulates the innate immune system and helps the cell to survive when an injury occurs. It has also been shown that ozone administration stimulates oxidative preconditioning or enhances adaptation to oxidative stress involving glutathione, SOD, and catalase and enzymatic reactions, preparing the host to face physiopathological conditions mediated by ROS/RNS.Citation37,Citation38 In light of recent pharmacological knowledge, our consideration is that ozone acts as a pro-drug and induces a rearrangement of the biochemical pathways with the activation of a second messenger in a cascade with a multiple system action.Citation39 Ameliorative effect of OT on oxidative stress in our study is also in agreement with those displayed in researches related with ischemia/reperfusion injury in kidney.Citation37,Citation38,Citation40,Citation41 In conclusion, OT may promote a moderate oxidative stress which, in turn, increases antioxidant endogenous systems protecting kidney against ESW-induced renal injury. Although our results indicate that OT is effective to improve ESW-induced renal injury in rats, it needs well-designed and documented clinical studies examining the OT renal and the other system function.
This study also revealed that OT reduces the tissue NOx levels in kidneys subjected to SWT procedure. The tissue NOx level gives information about nitrosative stress. Nitric oxide (NO) and peroxynitrite (ONOO−) are eventually converted to nitrite (NO2) and/or nitrate (NO3), that is, NOx, therefore NOx levels are used as an indirect but reliable indicator for NO and O NOO− formation in vivo.Citation42 We also correlate our observation about nitrosative stress with iNOS immunohistochemical staining of renal tissue. And, iNOS expression of tissue showed a clear correlation with tissue NOx levels. In the kidneys of rats subjected to SWT procedure, extensive and prominent iNOS immunoreactivity was noted in the tubular structure, whereas iNOS immunostaining in the glomerular structure was none. These findings show correlation with light microscopic evaluations showing severe tubular injury in SWT group. On the other hand, iNOS immunostaining expression was significantly lower in the ESW + OT group that the EWS group. There are a couple of studies showing the role of NO on kidney subjected to ESW procedure. One of them reported that shock waves releases NO from renal cells.Citation34 The other one reported by Sheng et al.Citation43 reported that plasma NO levels increased in patients with nephrolithiasis after SWT. On the other hand, recent studies clearly demonstrated that excessive NO formation causes renal injury following ischemia reperfusion insults.Citation8,Citation23,Citation40,Citation44,Citation45 Our findings are also consistent with these reports, in which some of them found that OT protected kidney against I/R injury through blocking formation of endogenous NO. So, it can be concluded that renal injury following SWT originates from excessive production of NO and ONOO− as seen at renal I/R injury. Thus, although our results suggested the OT protects kidney against SWT by inhibiting iNOS or scavenging of ONOO− in the kidney, additional experiments, like the use of knockout mice for iNOS, should be performed.
In conclusion, OT ameliorates nitro-oxidative stress and reduces the severity of pathological changes in the experimental ESW-induced renal injury of rat model. Therefore, OT might be considered as a novel treatment modalities by itself or a positive complement to the actual pharmacological therapies protecting the kidney from possible renal injury following ESW.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Skolarikos A, Alivizatos G, de la Rosette J. Extracorporeal shock wave lithotripsy 25 years later: Complications and their prevention. Eur Urol. 2006;50:981–990; discussion 990.
- Sarica K, Yencilek F. Prevention of shockwave induced functional and morphological alterations: An overview. Arch Ital Urol Androl. 2008;80:27–33.
- D’Addessi A, Vittori M, Racioppi M, Pinto F, Sacco E, Bassi P. Complications of extracorporeal shock wave lithotripsy for urinary stones: to know and to manage them-a review. ScientificWorldJournal 2012;2012:619820.
- Connors BA, Evan AP, Willis LR, Blomgren PM, Lingeman JE, Fineberg NS. The effect of discharge voltage on renal injury and impairment caused by lithotripsy in the pig. J Am Soc Nephrol. 2000;11:310–318.
- Munver R, Delvecchio FC, Kuo RL, Brown SA, Zhong P, Preminger GM. In vivo assessment of free radical activity during shock wave lithotripsy using a microdialysis system: the renoprotective action of allopurinol. J Urol. 2002;167:327–334.
- Serel TA, Ozguner F, Soyupek S. Prevention of shock wave-induced renal oxidative stress by melatonin: An experimental study. Urol Res. 2004;32:69–71.
- Williams JC, Jr., Stonehill MA, Colmenares K, et al. Effect of macroscopic air bubbles on cell lysis by shock wave lithotripsy in vitro. Ultrasound Med Biol. 1999;25:473–479.
- Alp BF, Malkoc E, Demirer Z, et al. Inhibition of inducible nitric oxide synthase prevents shock wave therapy induced renal injury. Ren Fail. 2014;36:774–780.
- Malkoc E, Alp BF, Demirer Z, et al. Efficacy of poly(adenosine diphosphate-ribose) polymerase inhibition in extracorporeal shock wave-induced renal injury. Ren Fail. 2014;36:1564–1569.
- Suhr D, Brummer F, Hulser DF. Cavitation-generated free radicals during shock wave exposure: Investigations with cell-free solutions and suspended cells. Ultrasound Med Biol. 1991;17:761–768.
- Li X, Long Q, Cheng X, He D. Shock wave induces biological renal damage by activating excessive inflammatory responses in rat model. Inflammation 2014;37:1317–1325.
- Mostafavi MR, Chavez DR, Cannillo J, Saltzman B, Prasad PV. Redistribution of renal blood flow after SWL evaluated by Gd-DTPA-enhanced magnetic resonance imaging. J Endourol. 1998;12:9–12.
- Yilmaz E, Mert C, Keskil Z, Tuglu D, Batislam E. Effect of SWL on renal hemodynamics: Could a change in renal artery contraction-relaxation responses be the cause? Urol Res. 2012;40:775–780.
- Bas M, Tugcu V, Kemahli E, et al. Curcumin prevents shock-wave lithotripsy-induced renal injury through inhibition of nuclear factor kappa-B and inducible nitric oxide synthase activity in rats. Urol Res. 2009;37:159–164.
- Bocci V. Ozone as Janus: This controversial gas can be either toxic or medically useful. Mediators Inflamm. 2004;13:3–11.
- Bocci VA. Scientific and medical aspects of ozone therapy. State of the art. Arch Med Res. 2006;37:425–435.
- Oter S, Korkmaz A. Relevance of hyperbaric oxygen to ozone therapy. Arch Med Res. 2006;37:917–918.
- Re L, Mawsouf MN, Menendez S, Leon OS, Sanchez GM, Hernandez F. Ozone therapy: Clinical and basic evidence of its therapeutic potential. Arch Med Res. 2008;39:17–26.
- Bocci V. Does ozone therapy normalize the cellular redox balance? Implications for therapy of human immunodeficiency virus infection and several other diseases. Med Hypotheses 1996;46:150–154.
- Chen H, Xing B, Liu X, et al. Similarities between ozone oxidative preconditioning and ischemic preconditioning in renal ischemia/reperfusion injury. Arch Med Res. 2008;39:169–178.
- Uysal B, Yasar M, Ersoz N, et al. Efficacy of hyperbaric oxygen therapy and medical ozone therapy in experimental acute necrotizing pancreatitis. Pancreas 2010;39:9–15.
- Guven A, Demirbag S, Uysal B, et al. Effect of 3-amino benzamide, a poly (adenosine diphosphate-ribose) polymerase inhibitor, in experimental caustic esophageal burn. J Pediatric Surg. 2008;43:1474–1479.
- Kesik V, Guven A, Vurucu S, et al. Melatonin and 1400 W ameliorate both intestinal and remote organ injury following mesenteric ischemia/reperfusion. J Surg Res. 2009;157:e97–e105.
- Carvalho M, Freitas Filho LG, Fagundes DJ, Ortiz V. Effects of repeated extracorporeal shock wave in urinary biochemical markers of rats. Acta Cir Bras. 2009;24:496–501.
- Kira VM, Fagundes DJ, Bandeira CO, Kaufman O, Fagundes AT, Ortiz V. Effects of repeated extracorporeal shock wave on kidney apoptosis of normal and diabetic rat. Int Braz J Urol. 2008;34:91–96.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358.
- Durak I, Yurtarslanl Z, Canbolat O, Akyol O. A methodological approach to superoxide dismutase (SOD) activity assay based on inhibition of nitroblue tetrazolium (NBT) reduction. Clin Chim Acta. 1993;214:103–104.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169.
- Cakir E, Akgul OE, Aydin I, et al. The association between neopterin and acetaminophen-induced nephrotoxicity. Ren Fail. 2010;32:740–746.
- Agilli M, Yaman H, Cayci T, et al. Comparison of Two Different HPLC Methods and Elisa Method For Measurement of Serum Neopterin. J Invest Biochem. 2012;1:43–47.
- Gocgeldi E, Uysal B, Korkmaz A, et al. Establishing the use of melatonin as an adjuvant therapeutic against paraquat-induced lung toxicity in rats. Exp Biol Med. 2008;233:1133–1141.
- Clark DL, Connors BA, Evan AP, Handa RK, Gao S. Effect of shock wave number on renal oxidative stress and inflammation. BJU Int. 2011;107:318–322.
- Park JK, Cui Y, Kim MK, et al. Effects of extracorporeal shock wave lithotripsy on plasma levels of nitric oxide and cyclic nucleotides in human subjects. J Urol. 2002;168:38–42.
- Halliwell B, Clement MV, Long LH. Hydrogen peroxide in the human body. FEBS Lett. 2000;486:10–13.
- Zamora ZB, Borrego A, Lopez OY, et al. Effects of ozone oxidative preconditioning on TNF-alpha release and antioxidant-prooxidant intracellular balance in mice during endotoxic shock. Mediators Inflamm. 2005;2005:16–22.
- Xing B, Chen H, Wang L, Weng X, Chen Z, Li X. Ozone oxidative preconditioning protects the rat kidney from reperfusion injury via modulation of the TLR4-NF-kappaB pathway. Acta Cir Bras. 2015;30:60–66.
- Chen H, Xing B, Liu X, et al. Ozone oxidative preconditioning inhibits inflammation and apoptosis in a rat model of renal ischemia/reperfusion injury. Eur J Pharmacol. 2008;581:306–314.
- Bocci V, Zanardi I, Travagli V. Has oxygen-ozonetherapy a future in medicine? J Exp Integr Med. 2011;1:5–11.
- Chen H, Xing B, Liu X, et al. Ozone oxidative preconditioning protects the rat kidney from reperfusion injury: The role of nitric oxide. J Surg Res. 2008;149:287–295.
- Oztosun M, Akgul EO, Cakir E, et al. The effects of medical ozone therapy on renal ischemia/reperfusion injury. Ren Fail. 2012;34:921–925.
- Guven A, Gundogdu G, Uysal B, et al. Hyperbaric oxygen therapy reduces the severity of necrotizing enterocolitis in a neonatal rat model. J Pediatr Surg. 2009;44:534–540.
- Sheng B, He D, Zhao J, Chen X, Nan X. The protective effects of the traditional Chinese herbs against renal damage induced by extracorporeal shock wave lithotripsy: A clinical study. Urol Res. 2011;39:89–97.
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142.
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437.
