Abstract
Context: Neutrophils are the primary effector cells in the pathogenesis of transfusion-related acute lung injury or multiple organ failure after blood transfusion.
Objective: We aimed to investigate the effect of fresh (1 day preparation) and aged (42 day preparation) PRBC-derived plasma on neutrophil morphology, migration and phagocytosis.
Materials and methods: We evaluated the production of reactive oxygen species (ROS) and the expression of non-muscle myosin heavy chain IIA (MYH9) in neutrophils treated with PRBC-derived plasma. We used western blots and antibody arrays to evaluate changes in signal transduction pathways in plasma-treated neutrophils.
Results: Aged PRBC-derived plasma elicited a stronger oxidative burst in neutrophils when compared with fresh PRBC-derived plasma (p < 0.05). Antibody arrays showed increased phosphorylation of NF-ĸB proteins (p105, p50 and Ikk) in aged PRBC-derived plasma-treated neutrophils. The expression of non-muscle myosin IIA (MYH9), a cytoskeleton protein involved in immune cell migration and morphological change, was also significantly upregulated in neutrophils treated with aged PRBC-derived plasma compared to fresh plasma (p < 0.05). Pretreatment of neutrophils with blebbistatin (a specific type II myosin inhibitor), ascorbic acid (an antioxidant), or staurosporine (a protein tyrosine kinase inhibitor), effectively abrogated the morphological changes, neutrophil migration, and phagocytosis induced by aged PRBC-derived plasma.
Conclusion: Upregulation of MYH9 in neutrophils treated with aged PRBC-derived plasma and abrogation of neutrophil migration in blebbistatin-treated neutrophils suggested a functional role of MYH9 in the directional migration of immune cells. Our data help elucidate the cellular and molecular mechanisms of transfusion-related injury.
Keywords::
| Abbreviations | ||
| NF-κB | = | nuclear factor-kappa B |
| STAT | = | the Signal Transducers and Activators of Transcription protein |
| MYH9 | = | Nonmuscle myosin IIA |
| TALI | = | transfusion-related acute lung injury |
| MOF | = | multiple organ failure |
| RBCs | = | red blood cells |
| ROS | = | reactive oxygen species |
| MAPK | = | mitogen-activated protein kinase |
| IL | = | interleukin |
| IFN | = | immunoreactive fibronectin |
| TNF | = | tumor necrosis factor |
| NADPH | = | reduced form of nicotinamide-adenine dinucleotide phosphate |
| UV | = | ultraviolet radiation |
| NMMHCA | = | non-muscle myosin heavy chain-A |
| AA | = | ascorbic acid |
| STS | = | tyrosine kinase inhibitor staurausporin |
Introduction
Neutrophils are the main effector cells of inflammation in the airway epithelium.(Citation1) Neutrophil priming has been reported to play a crucial role in the pathogenesis of post-injury hyper-inflammation leading to transfusion-related acute lung injury (TRALI) and multiple organ failure (MOF) in surgery patients.(Citation2) Although the pathogenesis of TRALI has been linked to the presence of antileukocyte antibodies, a significant number of cases do not have an immunologic etiology,(Citation3) leading to the postulation of the two-event model for TRALI. The two-event model suggests that TRALI is initiated by an immune priming step (consisting of the initial trauma), followed by exposure of neutrophils to specific biological response modifiers via transfusion, causing an interaction between neutrophils, platelets and lung endothelium.(Citation2,Citation4) Neutrophil priming is characterized by oxidative burst (release of reactive oxygen species (ROS) via activation of NADPH oxidase), morphological changes, delayed apoptosis and enhanced microbicidal activity during phagocytosis.(Citation2,Citation5) Stored blood was shown to cause more significant priming compared to freshly prepared blood and there is a direct correlation between the duration of storage time of red blood cells (RBCs) and neutrophil priming.(Citation6) Transfusion with stored plasma was also shown to delay neutrophil apoptosis(Citation7,Citation8) and to cause more tissue damage than fresh plasma.(Citation2)
ROS is a term that describes free oxygen radicals such as superoxide anion and hydroxyl radicals.(Citation9,Citation10) Oxidative burst and ROS are known to regulate protein phosphorylation in cells stimulated with growth factors/cytokines, leading to the activation of different signal transduction pathways such as the TNF, NF-kB, STAT, PKC and MAP kinase pathways,(Citation11,Citation12) A number of intracellular processes, such as modulation of cell function, inflammation and apoptosis are regulated by these signaling pathways.(Citation9,Citation10) Transfused blood induces an oxidative burst and upregulation of inflammation-related cytokines like IL-6, IFN, IL-18 and TNF in neutrophils, suggesting that transfusion plays a role in activation of these signaling pathways.(Citation13)
Neutrophil migration into tissues is the hallmark of all types of inflammatory responses. Neutrophil crawling is characterized by F-actin polymerization, cytoskeleton contraction and adhesion to surrounding tissue.(Citation14) We previously showed that non-muscle myosin heavy chain IIA (MYH9) formed the link between the integrin LFA-1 and the cytoskeleton and regulated T lymphocyte migration.(Citation15) Non-muscle myosin has also been shown to play a critical role in migration of T cells towards the site of inflammation.(Citation16–18) Interestingly, ROS was recently shown to be an important regulator of neutrophil chemotaxis.(Citation19,Citation20) However, the role of blood derivatives on the morphology and migration of neutrophils remains unclear.
The present study was therefore undertaken to investigate the effect of fresh (1 day preparation) and aged (42 day preparation) PRBC-derived plasma on ROS production, MYH9 expression and phagocytosis in neutrophils. We also investigated changes in the morphology and migration of neutrophils exposed to aged and fresh PRBC-derived plasma and explored the mechanisms underlying these changes.
Materials and methods
Blood leuko-reduction and neutrophil isolation
Ten healthy volunteers each donated 1 unit of whole blood (WB) which was collected in citrated 500 mL Triple Blood-Pack Unit bags (Baxter International Inc., Deerfield, IL) and processed per the standards of the American Association of Blood Banks, Bethesda, MD. One half of each packed red blood cell (PRBC) fraction was leuko-depleted using a gravity driven leukocyte reduction filter (Sepacell R-500 II; Baxter International Inc.) and all the PRBC units were stored at 4°C. At biweekly intervals, a portion of the unit was removed and centrifuged (at 6470 rpm for 7 min, then at 10,230 rpm for 10 min) to remove the RBCs, leaving a small plasma fraction that was divided into aliquots and stored at −80°C until assayed. This was done every other week until day 42. Prior to use, the plasma aliquots were thawed to room temperature and centrifuged at 4°C at 1000g for 15 min to ensure complete removal of residual platelets.
Neutrophils were isolated from EDTA (0.5%)-treated peripheral venous blood of healthy human volunteers using a 4-step discontinuous Percoll gradient (Sigma, St. Louis, MO). Erythrocytes were removed by hypotonic lysis, and neutrophils were resuspended in RPMI-1640 medium (Invitrogen, Carlsbad, CA). Neutrophil purity and viability were always higher than 99% and 96%, respectively. Neutrophils were incubated for 1 h at 37°C in the presence of 5% CO2, with the RBC plasmas prepared as described above (20% plasma/80% RPMI 1640).
This study was approved by the Institutional Review Board of the Lifespan Human Subject Research Committee, Providence, RI, USA and informed consent was obtained from all the volunteers.
Superoxide production
Superoxide production was measured by the O2− dismutase-inhibitable reduction of cytochrome c. Neutrophils (3.75 × 105/well) were incubated for 3 min with the different plasma preparations and immediately placed in a microplate reader (THERMOmax with Softmax software, Molecular Devices, Menlo Park, CA) for kinetic measurement of O2− production. Formyl-Met-Leu-Phe (fMLP; 1 mM/L) obtained from Sigma was used as positive control. Absorbance at 550–450 nm was measured every 20 s for 5 min. The maximal rate of O2− production (Vmax) was determined by the absorbance curve over 5 points. An extinction coefficient of 8.4 × 10−3 L/mol/min was used as determined for the 150 µL reaction volume and the 550-nm filter in the microplate reader. Data were recorded as Vmax, nmol O2−/3 × 106 neutrophils/min.
Antibody array analysis and western blot analysis
Four hundred commercial antibodies (20–40 ng in a volume of 0.4 mL or less) were spotted on a 5 × 4 cm nitrocellulose membrane (Bio-Rad) as previously described.(Citation21) The spots were less than 500 µm in diameter and all antibodies were obtained from Santa Cruz Biotech Inc., Santa Cruz, CA. Freshly prepared antibody arrays were immersed in PBS containing 3% bovine serum albumin for 2 h prior to use. Neutrophils were subjected to different PRBC-derived plasma treatments and whole cell extracts were prepared with RIPA lysis buffer (50 mM Tris [pH 8.0], 5 mM EDTA, 150 mM NaCl, 0.5% NP-40, 0.1% sodium dodecyl sulfate [SDS], 1 mM dithiothreitol, 1 mM Na3VO4, 0.5 mM phenylmethylsulfonyl fluoride, 1 mM NaF) containing a protease inhibitor cocktail (Roche, Indianapolis, IN). Whole cell extracts (1.5 mg) were incubated with the antibody arrays for 3 h at room temperature. The arrays were extensively washed with TBST and incubated with a 1:1000 dilution of HRP-conjugated phospho-tyrosine antibody pY20 (Santa Cruz Biotech Inc.) for 2 h at 37°C. Signals were detected using enhanced chemi-luminescence (Amersham Biosciences, Piscataway Township, NJ).
For western blots, whole cell extracts prepared from neutrophils were separated on 10% SDS-PAGE (100 µg/lane). All antibody incubations were performed as previously described(Citation22) and immune-blotting analysis was performed as described elsewhere.(Citation7) Whole cell extracts were also prepared from Jurkat and Daudio cells (ATCC, Manassas, VA) which were cultured in in RPMI media supplemented with 10% FBS (1 × 105–106 cells/mL). HeLa and HEK 293T cells (ATCC) were grown in DMEM media supplemented with 10% FBS (both from Invitrogen). Rabbit polyclonal antibodies against Myo IIA heavy chain were purchased from Sigma. Antibodies against actin were purchased from Santa Cruz Biotechnology. Neutrophils were pretreated with NADPH oxidase inhibitor, diphenylamine iodonium (DPI; Sigma) at a concentration of 3 µM/L for 30 min prior to incubation with PRBC-derived plasma.
Neutrophil migration, morphological changes and phagocytosis
Glass chamber slides (Nalge Nunc International) were coated for 1 h at 37°C, with ICAM-1-Fc (R&D Systems, Minneapolis, MN) at a concentration of 80 nM. Time-lapse video-microscopy was used to measure real time migration of human neutrophils on coated chamber slides. Time-lapse movies were taken every 5 s. Cells were maintained at 37°C in an FCS2 live cell imaging chamber (Bioptechs) in glucose medium containing IL-15 (2 mg/mL; Sigma) Zymosan-induced luminol-enhanced chemiluminescence (CL; Invivogen, 300 µg/30 µL PBS) was used to measure the phagocytic response of neutrophils. The CL signal produced by phagocytosing leukocytes was measured in a luminometer (Model Luminoscan-RT; Labsystems). Thirty cycles of measurements using a 5 s counting time and a 70 s interval time were performed for each sample. Neutrophil counts were determined in all the samples using a hemocytometer.(Citation8) Blebbistatin (Calbiochem), a specific type II myosin inhibitor, was used to inhibit myosin activity. Ascorbic acid (AA) and staurosporine (STS) were obtained from Sigma. Neutrophils (2 × 106/mL) were treated for 1 h at 37°C with 100 µM blebbistatin, 1 µM STS or 100 µM AA prepared in DMSO (Sigma).
Statistical analysis
Analysis of variance (ANOVA) followed by a multiple comparison test or Student’s t-test was performed to determine statistical significance. Values were taken to be statistically significant at p < 0.05.
Results
Differential oxidative burst and protein phosphorylation patterns in neutrophils treated with different PRBC-derived plasma preparations
We evaluated the effect of plasma on oxidative burst by comparing oxygen consumption in neutrophils incubated with PRBC-derived plasmas prepared under different conditions. There was an increase in superoxide production when neutrophils were incubated with the different PRBC-derived plasma preparations, suggesting that PRBC-derived plasma induced an oxidative burst in human neutrophils. fMLP was used as a positive control and untreated neutrophils were used as a control. Aged PRBC-derived plasma (42-day storage; NLR-42D) induced a significantly higher magnitude of oxidative burst when compared with fresh PRBC-derived plasma (1 day storage; NLR-1D) (p < 0.05; ). Preincubation of neutrophils with the NADPH oxidase inhibitor, DPI, resulted in a significant abrogation of superoxide production evoked by aged PRBC-derived plasmas (p < 0.05), suggesting the involvement of the NADPH oxidase machinery in aged PRBC-derived plasma-evoked superoxide production.
Figure 1. Fresh and aged plasmas trigger oxidative burst and protein phosphorylation in neutrophils. (A) Representative results of ROS generation in untreated normal human neutrophils (Control), and neutrophils treated for 1 h with fresh (1 day preparation) non-leukocyte-reduced plasma (NLR-1D), aged (42 day preparation) non-leukocyte-reduced plasma (NLR-42D), aged leukocyte-reduced plasma (LR-42D), and NLR-42D plus NADPH oxidase inhibitor DPI (NLR-42D + DPI). The results are expressed as means ± SD from 3 experiments. fMLP: formyl-Met-Leu-Phe (as a positive control). (*p < 0.05, compared with NLR-42D). (B) Western blot analysis of protein tyrosine phosphorylation in response to different preparations of plasma. Normal human neutrophils were incubated with the different plasma preparations for 1 h. Whole cell lysates were blotted with anti-pY20 antibody. (C) Normal human neutrophils were incubated with the different plasma preparations for 1 hr. Whole cell lysates prepared from these neutrophils were subjected to antibody array analysis with immunoblotted with anti-pY20-HRP. (D-E) Western blot analysis demonstrated tyrosine phosphorylation of IKK, p105 and p50 in response to plasma treatment (*p < 0.05, compared with NLR-42D). 1D represents the ratio of p-IKK value over IKK. 1E represents the ratio of p105 value over p50.
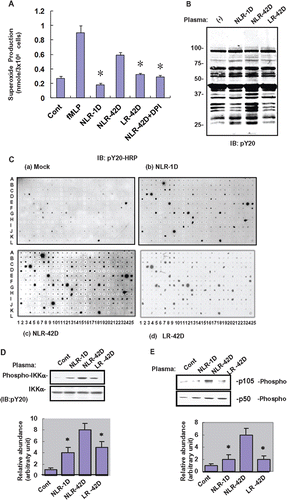
Since oxidative burst triggered by UV or cytokines is known to induce protein tyrosine phosphorylation, we evaluated the effect of plasma on protein tyrosine phosphorylation in neutrophils. We incubated whole cell neutrophil extracts with different plasma preparations for 1 h and immunoblotted with anti-pY20 antibody to show that aged PRBC-derived plasma induced higher levels of protein phosphorylation when compared with fresh PRBC-derived plasma (p < 0.05; ). We performed an antibody array analysis in order to identify the proteins that were tyrosine phosphorylated. Whole cell extracts were prepared from neutrophils which were incubated with different plasma preparations. The extracts were incubated with our antibody arrays immobilized with 400 different antibodies as previously described.(Citation21–24) The arrays immunoblotted with anti-pY20 antibody showed that freshly prepared and aged stored PRBC-derived plasmas induced differential protein tyrosine phosphorylation (). The criterion for differential protein tyrosine phosphorylation was a 3-fold difference in signal intensity between the spots in the NLR-42D group and the corresponding spots in the control group. Neutrophils incubated with aged PRBC-derived plasma preparations showed higher levels of phosphorylation of c-Abl (I11), c-Rel (C2), Hdac6 (E1), IKK (E16), Lyn (N6), p16 (G17), and RIP (I20, I21) when compared with neutrophils incubated with fresh PRBC-derived plasma (). We used western blots to confirm the phosphorylation of proteins involved in NF-kB pathway such as p105 and Ikk and p50 ().
Plasmas induce type II nonmuscle myosin expression in neutrophils
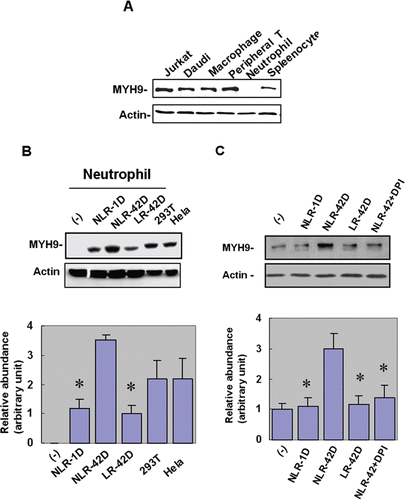
Since NF-kB activation is thought to play a role in cell migration via activation of the myosin-actin cytoskeletonsome,(Citation22) we evaluated the expression of MYH9 in plasma-treated neutrophils. Interestingly, although MYH9 was expressed in T cells, B cells, and macrophages, we were unable to detect MYH9 expression in untreated neutrophils (). However, we showed induction of MYH9 in neutrophils treated with different plasma preparations. Aged PRBC-derived plasma induced higher levels of MYH9 expression when compared with fresh PRBC-derived plasma (p < 0.05; ). Additionally, the NLR-42D plasma group had significantly higher levels of MYH9 compared to the LR-42D group (p < 0.05). Induction of MYH9 by plasma was abolished by NADPH oxidase inhibitor DPI (p < 0.05; ), suggesting that superoxide played a role in the regulation of MYH9 expression.
Figure 2. Fresh and aged plasmas induce nonmuscle type IIA myosin (MYH9) expression in neutrophils. (A) Of the immune cells tested, myosin type II was detected in T cells, B cells and macrophages, but not in neutrophils (B) The NLR-42D plasma group had significantly higher levels of MYH9 compared to the LR-42D plasma group (*p < 0.05). (C) NADPH oxidase inhibitor DPI abolished the plasma-induced expression of MYH9 in the NLR-42D group (*p < 0.05).
Plasmas induce morphological change, migration and phagocytosis in neutrophils
We investigated the effect of plasma on neutrophil migration. We showed that neutrophils incubated with different PRBC-derived plasma preparations, became polarized and migrated on ICAM-1 coated surfaces with a steady-state migration velocity of approximately 10µm/min. Analysis of 100 cells showed that the migrating neutrophils exhibited rapid changes in shape, formed constriction rings, and showed concomitant cytoplasmic streaming ().
Figure 3. Blebbastatin, ascorbic acid, and STS disrupt migration-related morphological change in neutrophils. (A) Fresh and aged plasma induced neutrophil morphological changes (n = 100 cells). (B) Blebbistatin, ascorbic acid, and STS abrogated plasma-induced morphological changes in neutrophils (n = 7 blood samples from 7 different volunteers; in triplicate). DMSO was used as a vehicle control. (C) Aged plasma induced a greater number of morphological changes than fresh plasma (*p < 0.05, compared with NLR-42D) (n = 5 blood samples from 5 different volunteers; in triplicate) (D) Blebbistatin, ascorbic acid, and STS significantly abrogated plasma-induced neutrophil morphological change, (*p < 0.05, compared with NLR-42D).
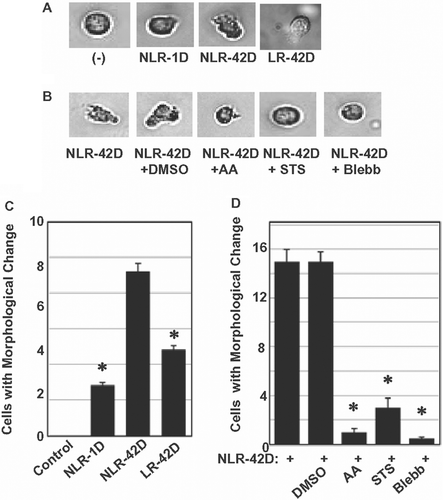
Based on the hypothesis that oxidative burst and tyrosine phosphorylation may play a critical role in MYH9 accumulation in plasma-treated neutrophils, we investigated the effect of anti-oxidant AA and tyrosine kinase inhibitor staurosporin (STS) on neutrophil migration and morphological changes. We also investigated if blebbistatin, a specific inhibitor of myosin type II, could inhibit neutrophil migration in vitro. Neutrophils were pre-treated with AA (an anti-oxidant), STS (a tyrosine kinase inhibitor) or blebbistatin for 1 h before incubating with different plasma preparations. We showed that incubation with aged PRBC-derived plasma induced more significant morphological changes when compared with fresh PRBC-derived plasma (p < 0.05; ). We also showed that pretreatment of neutrophils with AA, STS or blebbistatin abolished the morphological changes and cell migration induced by PRBC-derived plasma (p < 0.05; and ). DMSO was used as a vehicle control.
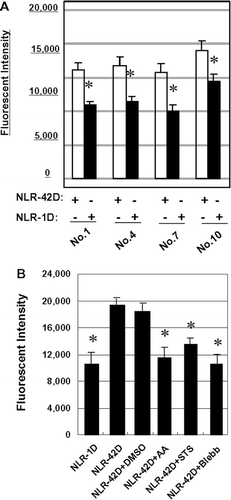
We investigated if plasma induced neutrophil phagocytosis. Our results showed that neutrophils exhibited more efficient phagocytosis of yeast in the presence of aged PRBC-derived plasma compared to fresh PRBC-derived plasma (p < 0.05; ). Pre-treatment of neutrophils with blebbistatin, AA or STS blocked this process efficiently (p < 0.05; ).
Figure 4. Blebbistatin, ascorbic acid, and STS suppress phagocytosis in plasma-treated neutrophils. Neutrophils were incubated with NLR-1D or NLR-42D from 4 different donors in triplicate (*p < 0.05, compared with NLR-42D). (B) Blebbistatin, ascorbic acid, and STS pretreatment significantly blocked plasmainduced phagocytosis in 6 different donors in triplicate (*p < 0.05, compared with NLR-42D).
Discussion
In this study, we showed that aged PRBC-derived plasma was more potent than fresh PRBC-derived plasma at inducing superoxide production in neutrophils. Antibody array analysis showed that this oxidative burst was accompanied by increased protein tyrosine phosphorylation. Some of the key proteins phosphorylated were members of the NF-kB family (p105, IKK and p50). We showed upregulation of non-muscle myosin IIA, MYH9, in plasma-treated neutrophils. MYH9 upregulation was reversed by NADPH oxidase inhibitor, DPI. We also showed that aged plasma promoted neutrophil migration and induced morphological changes and phagocytosis. These processes were reversed by pretreating neutrophils with blebbistatin, a myosin type II inhibitor.
TRALI is initiated by an immune priming step, followed by exposure to specific biological response modifiers via transfusion, causing an interaction between neutrophils, platelets and lung endothelium.(Citation4) Early research indicated the presence of a priming agent present in PRBCs, WB and platelet concentrates (PCs), which activated this enzyme system.(Citation25) This priming activity was observed in aged blood, but not in fresh blood. Further analysis showed the presence of lysophosphatidylcholines as well as a lipid priming activity in WB, PRBCs and PCs.(Citation2) Since fresh frozen plasma did not contain this lipid priming activity, such bioactive lipids which appeared after a few days of storage, were thought to be generated via cellular breakdown.(Citation2)
Our results agreed with these studies and showed higher levels of superoxide production in neutrophils incubated with aged PRBC-derived plasma when compared with fresh PRBC-derived plasma. Since phagocyte activation is characterized by oxidative burst (increased production of superoxide anions), these data suggested that aged plasma would be more efficient than fresh plasma at inducing phagocytosis. We used a yeast phagocytosis assay and showed higher levels of phagocytic activity in neutrophils exposed to aged plasma when compared to fresh plasma. Interestingly, Lyn, a member of the Src family tyrosine kinases, was previously shown to play an important role in phagocytosis by alveolar macrophages via regulation of respiratory burst.(Citation26) It will be interesting to more fully characterize the molecular mechanisms underlying increased phagocytosis in neutrophils treated with aged PRBC-derived plasma.
Migration of cells is a complex process, which results in the generation of motor forces through cytoskeleton reorganization. We previously showed that TNF induced migration of T cells by regulation of the myosin-actin cytoskeletonsome.(Citation22) There are conflicting reports on the effect of transfusion on cell migration and chemotaxis. Supernatant from standard RBC preparations was recently shown to prime polymorphonuclear cells (PMNs) and induce chemotaxis.(Citation27) These effects were directly correlated with storage time. Other reports indicated that supernatants from stored RBCs could inhibit fMLP-induced neutrophil chemotaxis as well as induce neutrophil migration.(Citation28) RBC transfusions were also recently shown to inhibit neutrophil chemotaxis.(Citation29)
Our data showed that aged PRBC-derived plasma was more efficient than fresh PRBC-derived plasma at inducing protein phosphorylation in neutrophils. Phosphorylation and activation of mitogen-activated protein kinase, p38, was previously shown to be required for priming of respiratory burst in neutrophils.(Citation30) Early studies also showed enhanced respiratory burst in neutrophils stimulated with granulocyte-colony stimulating factor (G-CSF), which resulted in activation of transcription factor p80c-rel.(Citation31) In this study, we showed that among the proteins that were phosphorylated, were members of the NF-kB family (p105, p50 and IKK). Since NF-kB activation has been long suspected to play a role in cell migration, presumably by transcriptional regulation of genes involved in migration,(Citation32) our data suggest a regulatory role for NF-kB in plasma-induced neutrophil migration.
Non-muscle myosin II has previously been implicated in the migration of tumor cells and leukocytes(Citation18,Citation33) and has been shown to participate in the redistribution of adhesion receptors in the immunological synapse.(Citation34,Citation35) Myosin IIA-deficient T-cells exhibit decreased interstitial migration.(Citation36) Furthermore, the H chain of myosin II is known to play a role in regulation of pseudopod formation and chemotaxis in Dictyostelium discoideum.(Citation37) Interestingly, MYH9, an isoform of the non-muscle type II myosin H chain, has been shown to be important for spontaneous (matrix-induced) as well as ligand-induced migration of leukocytes. In this study, we showed upregulation of MYH9 expression in neutrophils incubated with aged PRBC-derived plasma. These data are consistent with the increased migration observed in these cells. We used blebbistatin, a specific myosin type II inhibitor, to look at the role of MYH9 in neutrophil migration. Blebbistatin has previously been shown to inhibit cell migration and cell morphological changes during cytokinesis.(Citation16,Citation38) We showed a significant abrogation of plasma-induced cell migration and morphological changes in neutrophils which were pretreated with blebbistatin.
Taken together, our data showed that neutrophils treated with PRBC-derived plasma expressed significant amounts of MYH9 protein, while resting neutrophils did not. It will be interesting to investigate the kinetics of MYH9 upregulation and cell migration in response to PRBC-derived plasma. A number of studies have investigated possible mediators of neutrophil activation and transfusion-related immunomodulation. H2O2 was shown to amplify the innate immune response in certain inflammatory disorders, by stimulating NOX2-mediated superoxide production in neutrophils via Ca(2+)/c-Abl signaling pathway.(Citation39) Based on our results, we suggest that NLR-42D PRBCs induced superoxide production in neutrophils, leading to tyrosine phosphorylation and activation of transcription factors such as NF-kB, which may regulate MYH9 transcription. MYH9 and ICAM-1 were previously suggested to play a role in T cell migration.(Citation18) Our study, showing inhibition of neutrophil migration in response to blebbistatin, a myosin type II inhibitor is further validation that MYH9 plays a role in neutrophil migration. Furthermore, inhibition of MYH9 expression in response to DPI, an NADPH inhibitor, suggested that superoxide production plays a role in NLR-42D PRBC-induced MYH9 expression. The use of a no-plasma control group as well as the LR-42D negative control group in every experiment served to ensure that the difference between the fresh and aged plasma observed in our study were indeed authentic and not due to artifactual priming.
One limitation of our present study was that we did not use pooled donated blood samples. Individual differences may therefore play a role in the interpretation of the results. We would like to validate our results using larger sample sizes and pooled samples. We also did not explore if exposure of neutrophils to activators of oxidative burst could upregulate MYH9 expression. In our future studies, we would like to perform a finer dissection of the molecular mechanisms dictating the pathogenesis of transfusion-driven acute lung injury in vitro and in vivo.
To the best of our knowledge, we are the first to demonstrate a functional role of MYH9 in the directional migration of immune cells. Our findings indicate that transfusion with aged PBRC-derived plasmas resulted in a number of biologic effects such as (i) increased protein tyrosine phosphorylation, capable of triggering different signal transduction pathways in neutrophils, (ii) increased MYH9 expression in neutrophils, and (iii) modulation of phagocytosis, cell migration and morphological changes in neutrophils. Plasma-induced changes in migration and morphology were likely mediated via MYH9 and were abrogated by pretreatment of neutrophils with blebbistatin, a specific inhibitor of type II myosin. Our data provide an insight into the cellular and molecular mechanisms of transfusion-related injury and suggest that blebbistatin is a potential therapeutic agent for blood transfusion related disorders.
Declaration of interest
This research was sponsored by the National Natural Science Funds 30871181 and 81070547 (to Chen Yu). The authors declare no conflict of interest.
References
- Parker, D., Prince, A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol 2011, 45, 189–201.
- Fung, Y.L., Silliman, C.C. The role of neutrophils in the pathogenesis of transfusion-related acute lung injury. Transfus Med Rev 2009, 23, 266–283.
- Silliman, C.C., Ambruso, D.R., Boshkov, L.K. Transfusion-related acute lung injury. Blood 2005, 105, 2266–2273.
- Looney, M.R., Gilliss, B.M., Matthay, M.A. Pathophysiology of transfusion-related acute lung injury. Curr Opin Hematol 2010, 17, 418–423.
- Triulzi, D.J. Transfusion-related acute lung injury: an update. Hematology Am Soc Hematol Educ Program 2006, 497–501.
- Silliman, C.C., Boshkov, L.K., Mehdizadehkashi, Z., Elzi, D.J., Dickey, W.O., Podlosky, L., Clarke, G., Ambruso, D.R. Transfusion-related acute lung injury: epidemiology and a prospective analysis of etiologic factors. Blood 2003, 101, 454–462.
- Biffl, W.L., West, K.E., Moore, E.E., Gonzalez, R.J., Carnaggio, R., Offner, P.J., Silliman, C.C. Neutrophil apoptosis is delayed by trauma patients’ plasma via a mechanism involving proinflammatory phospholipids and protein kinase C. Surg Infect (Larchmt) 2001, 2, 289–93; discussion 294.
- Biffl, W.L., Carnaggio, R., Moore, E.E., Ciesla, D.J., Johnson, J.L., Silliman, C.C. Clinically relevant hypertonicity prevents stored blood- and lipid-mediated delayed neutrophil apoptosis independent of p38 MAPK or caspase-3 activation. Surgery 2003, 134, 86–91.
- Circu, M.L., Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 2010, 48, 749–762.
- Xiang M, Fan J. Association of Toll-like receptor signaling and reactive oxygen species: a potential therapeutic target for posttrauma acute lung injury. Mediators Inflamm. 2010;2010.
- Thannickal, V.J., Fanburg, B.L. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 2000, 279, L1005–L1028.
- Frey, R.S., Ushio-Fukai, M., Malik, A.B. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal 2009, 11, 791–810.
- Galley, H.F., El Sakka, N.E., Webster, N.R., Lowes, D.A., Cuthbertson, B.H. Activated protein C inhibits chemotaxis and interleukin-6 release by human neutrophils without affecting other neutrophil functions. Br J Anaesth 2008, 100, 815–819.
- Chodniewicz, D., Zhelev, D.V. Chemoattractant receptor-stimulated F-actin polymerization in the human neutrophil is signaled by 2 distinct pathways. Blood 2003, 101, 1181–1184.
- Morin, N.A., Oakes, P.W., Hyun, Y.M., Lee, D., Chin, Y.E., Chin, E.Y., King, M.R., Springer, T.A., Shimaoka, M., Tang, J.X., Reichner, J.S., Kim, M. Nonmuscle myosin heavy chain IIA mediates integrin LFA-1 de-adhesion during T lymphocyte migration. J Exp Med 2008, 205, 195–205.
- Straight, A.F., Cheung, A., Limouze, J., Chen, I., Westwood, N.J., Sellers, J.R., Mitchison, T.J. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science 2003, 299, 1743–1747.
- Jacobelli, J., Chmura, S.A., Buxton, D.B., Davis, M.M., Krummel, M.F. A single class II myosin modulates T cell motility and stopping, but not synapse formation. Nat Immunol 2004, 5, 531–538.
- Jacobelli, J., Bennett, F.C., Pandurangi, P., Tooley, A.J., Krummel, M.F. Myosin-IIA and ICAM-1 regulate the interchange between two distinct modes of T cell migration. J Immunol 2009, 182, 2041–2050.
- Hattori, H., Subramanian, K.K., Sakai, J., Jia, Y., Li, Y., Porter, T.F., Loison, F., Sarraj, B., Kasorn, A., Jo, H., Blanchard, C., Zirkle, D., McDonald, D., Pai, S.Y., Serhan, C.N., Luo, H.R. Small-molecule screen identifies reactive oxygen species as key regulators of neutrophil chemotaxis. Proc Natl Acad Sci USA 2010, 107, 3546–3551.
- Hattori, H., Subramanian, K.K., Sakai, J., Luo, H.R. Reactive oxygen species as signaling molecules in neutrophil chemotaxis. Commun Integr Biol 2010, 3, 278–281.
- Chung, A.S., Chin, Y.E. Antibody array platform to monitor protein tyrosine phosphorylation in mammalian cells. Methods Mol Biol 2009, 527, 247–55, ix.
- Chung, A.S., Guan, Y.J., Yuan, Z.L., Albina, J.E., Chin, Y.E. Ankyrin repeat and SOCS box 3 (ASB3) mediates ubiquitination and degradation of tumor necrosis factor receptor II. Mol Cell Biol 2005, 25, 4716–4726.
- Ivanov, S.S., Chung, A.S., Yuan, Z.L., Guan, Y.J., Sachs, K.V., Reichner, J.S., Chin, Y.E. Antibodies immobilized as arrays to profile protein post-translational modifications in mammalian cells. Mol Cell Proteomics 2004, 3, 788–795.
- Yuan, Z.L., Guan, Y.J., Wang, L., Wei, W., Kane, A.B., Chin, Y.E. Central role of the threonine residue within the p+1 loop of receptor tyrosine kinase in STAT3 constitutive phosphorylation in metastatic cancer cells. Mol Cell Biol 2004, 24, 9390–9400.
- Silliman, C.C., Thurman, G.W., Ambruso, D.R. Stored blood components contain agents that prime the neutrophil NADPH oxidase through the platelet-activating-factor receptor. Vox Sang 1992, 63, 133–136.
- Kannan, S., Audet, A., Huang, H., Chen, L.J., Wu, M. Cholesterol-rich membrane rafts and Lyn are involved in phagocytosis during Pseudomonas aeruginosa infection. J Immunol 2008, 180, 2396–2408.
- Sparrow, R.L., Patton, K.A. Supernatant from stored red blood cell primes inflammatory cells: influence of prestorage white cell reduction. Transfusion 2004, 44, 722–730.
- Ghio, M., Ottonello, L., Contini, P., Amelotti, M., Mazzei, C., Indiveri, F., Puppo, F., Dallegri, F. Transforming growth factor-beta1 in supernatants from stored red blood cells inhibits neutrophil locomotion. Blood 2003, 102, 1100–1107.
- Ottonello, L., Ghio, M., Contini, P., Bertolotto, M., Bianchi, G., Montecucco, F., Colonna, M., Mazzei, C., Dallegri, F., Indiveri, F. Nonleukoreduced red blood cell transfusion induces a sustained inhibition of neutrophil chemotaxis by stimulating in vivo production of transforming growth factor-beta1 by neutrophils: role of the immunoglobulinlike transcript 1, sFasL, and sHLA-I. Transfusion 2007, 47, 1395–1404.
- Yan, S.R., Al-Hertani, W., Byers, D., Bortolussi, R. Lipopolysaccharide-binding protein- and CD14-dependent activation of mitogen-activated protein kinase p38 by lipopolysaccharide in human neutrophils is associated with priming of respiratory burst. Infect Immun 2002, 70, 4068–4074.
- Druker, B.J., Neumann, M., Okuda, K., Franza, B.R. Jr, Griffin, J.D. rel Is rapidly tyrosine-phosphorylated following granulocyte-colony stimulating factor treatment of human neutrophils. J Biol Chem 1994, 269, 5387–5390.
- Hsieh, H.L., Wang, H.H., Wu, W.B., Chu, P.J., Yang, C.M. Transforming growth factor-ß1 induces matrix metalloproteinase-9 and cell migration in astrocytes: roles of ROS-dependent ERK- and JNK-NF-?B pathways. J Neuroinflammation 2010, 7, 88.
- Bastian, P., Lang, K., Niggemann, B., Zaenker, K.S., Entschladen, F. Myosin regulation in the migration of tumor cells and leukocytes within a three-dimensional collagen matrix. Cell Mol Life Sci 2005, 62, 65–76.
- Ilani, T., Vasiliver-Shamis, G., Vardhana, S., Bretscher, A., Dustin, M.L. T cell antigen receptor signaling and immunological synapse stability require myosin IIA. Nat Immunol 2009, 10, 531–539.
- Ryu, J., Liu, L., Wong, T.P., Wu, D.C., Burette, A., Weinberg, R., Wang, Y.T., Sheng, M. A critical role for myosin IIb in dendritic spine morphology and synaptic function. Neuron 2006, 49, 175–182.
- Jacobelli, J., Friedman, R.S., Conti, M.A., Lennon-Dumenil, A.M., Piel, M., Sorensen, C.M., Adelstein, R.S., Krummel, M.F. Confinement-optimized three-dimensional T cell amoeboid motility is modulated via myosin IIA-regulated adhesions. Nat Immunol 2010, 11, 953–961.
- Steimle, P.A., Licate, L., Côté, G.P., Egelhoff, T.T. Lamellipodial localization of Dictyostelium myosin heavy chain kinase A is mediated via F-actin binding by the coiled-coil domain. FEBS Lett 2002, 516, 58–62.
- Shu, S., Liu, X., Korn, E.D. Blebbistatin and blebbistatin-inactivated myosin II inhibit myosin II-independent processes in Dictyostelium. Proc Natl Acad Sci USA 2005, 102, 1472–1477.
- El Jamali, A., Valente, A.J., Clark, R.A. Regulation of phagocyte NADPH oxidase by hydrogen peroxide through a Ca(2+)/c-Abl signaling pathway. Free Radic Biol Med 2010, 48, 798–810.