Abstract
Over the past decade, the off-label use of biologic agents such as TNF-α antagonists, including infliximab and adalimumab, has improved the treatment armamentarium for refractory immune-mediated uveitis, with particular success in Behçet disease-associated uveitis. Golimumab is a novel fully human anti-TNF-α monoclonal antibody that has been approved for the treatment of rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis, with very promising results. Herein, the authors present the use of GLM in a case of Behçet uveitis refractory to other TNF-α blockers. There are only two reports in the literature about the use of GLM in uveitis, describing four patients with JIA-associated uveitis and a case of idiopathic retinal vasculitis. To the authors’ knowledge, this is the first report about the use of GLM in Behçet uveitis.
Dear Editor,
Over the past decade, the off-label use of biologic agents, especially tumor necrosis factor (TNF) antagonists, such as infliximab (IFX) and adalimumab (ADA), has improved the treatment armamentarium for refractory immune-mediated uveitis, with particular success in Behçet disease (BD)-associated uveitis.Citation1,Citation2 Golimumab (GLM) is a novel fully human anti-TNF monoclonal antibody, recently approved for a variety of rheumatic conditions,Citation3–5 with very promising results. Herein we describe a case that exemplifies the efficacy of GLM in a patient with refractory Behçet uveitis, and represents, to our knowledge, the first report about the use of GLM in Behçet uveitis.
Case report
A 28-year-old man was diagnosed with Behçet disease (BD) in 2007 when presenting with oral and genital ulcers and uveitis. Initially he was treated with oral prednisone (10 mg/day) and cyclosporine A (CyA) (300 mg/day), but was unable to enter drug-induced remission. In 2008 he was referred to us because of poor control of the uveitis. At the first visit the best-corrected visual acuity (BCVA) was 20/25 in the right eye (OD) and 20/20 in the left eye (OS). Slit-lamp examination demonstrated 1+ anterior chamber cells OD (based on Standard Uveitis Nomenclature criteria)Citation6 and posterior synechia. Fundus examination demonstrated OD 1+ cells in the vitreous chamber, peripheral retinal vasculitis, retinal infiltrates, and retinal haemorrhages. Ocular examination of his left eye was unremarkable. Fluorescein angiography confirmed peripheral occlusive vasculitis with retinal vein sheathing. In October 2010 the patient suffered a relapse of his panuveitis while he was on CyA and oral prednisone. Biological therapy with IFX at a dosage of 5 mg/kg at weeks 0, 2, and 6 and then every 8 weeks was then added to his therapy while his CyA was maintained, which achieved control of the intraocular inflammation. In December 2011, while the patient was on IFX every 8 weeks, a further flare of posterior uveitis occurred (). The relapse occurred 4 weeks after the last IFX infusion and, although rescue was attempted with high-dose oral steroids (prednisone 0.5 mg/kg/day), remission was not achieved. At this time and because of the sight-threatening nature of his uveitis, we decided to switch IFX to GLM (50 mg subcutaneously every 4 weeks) in a further attempt to control his condition. We could not determine if lack of efficacy was secondary to anti-IFX antibody formation as a means of evaluating this was at that time not available at our hospital. The rationale for instituting GLM was achieving Cmax equivalent to IFX but with a longer half-life.Citation7 Soon after starting GLM, the ocular inflammation improved and remission was achieved. Moreover, CyA dose could be reduced to 150 mg/day and oral prednisone to 5 mg/day. Currently, after 6 months of treatment with GLM, the patient remains asymptomatic and ophthalmic examination reveals no sign of active inflammation. Current visual acuity is 20/20 in both eyes. Systemic disease was also quiescent. No remarkable side effects were reported.
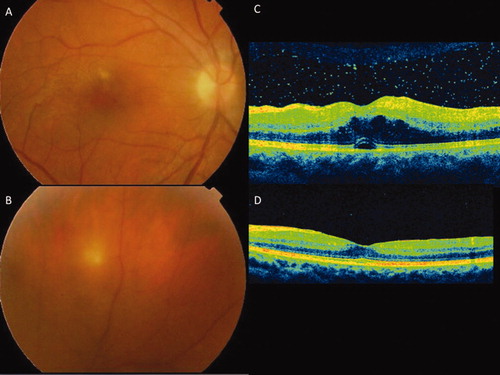
Figure 1. Active Behçet uveitis showing vitritis and retinal infiltrates, located close to the fovea (A) and in the retinal periphery (B). HD-OCT of a patient with active Behçet uveitis showing vitritis and cystoid macular edema with a small serous foveal detachment (C); (D) complete resolution of the vitritis and the macular edema 5 weeks after starting Golimumab therapy.
The case shows the efficacy of GLM in a patient with refractory Behçet uveitis. BD-associated uveitis is one of the most fulminant ocular inflammatory diseases, with a high risk of sight-threatening sequelae and affecting young people. Biological therapy with TNF antagonists has created new possibilities to control this condition, particularly in those patients refractory to conventional immunosuppressive agents.Citation8 GLM is a recent development in TNF antagonism and has been recently approved for the treatment of a variety of rheumatic inflammatory conditions,Citation3–5 with an acceptable safety profile, and remains the only TNF antagonist that has shown efficacy in patients refractory to other anti-TNF agents.Citation9 Due to GLM molecular structure, a fully human monoclonal antibody, GLM has a lower probability of developing neutralizing antibodies compared to other anti-TNF such as IFX, a chimeric monoclonal antibody, decreasing the risk of an allergic infusion reaction and any loss of efficacy. Although fully human, resistance to golimumab may potentially develop as well. Other advantages of GLM therapy over other TNF antagonists include the reduced dosing schedule, being a monthly subcutaneous self-administration, and avoidance of the time and costs related to the administration of intravenous treatment such as IFX.
In conclusion, in this case we show the benefits of GLM treatment for a patient with Behçet uveitis who developed resistance to infliximab after 14 months of treatment. To our knowledge, only two reports have been published related to GLM treatment in patients with ocular inflammation, one in idiopathic retinal vasculitisCitation9 and the other one in uveitis associated with juvenile idiopathic arthritis.Citation10 Although the follow-up after switch to golimumab was only 6 months, this case adds to the spectrum of uveitic conditions responsive to GLM. Further studies with longer follow-up are required to evaluate the long-term efficacy and safety of GLM in a larger number of patients with immune-mediated uveitis. Nevertheless, the case demonstrates the potent efficacy of GLM in inducing acute remission in otherwise IFX-refractory disease.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Perra D, Alba MA, Callejas JL, et al. Adalimumab for the treatment of Behcet’s disease: experience in 19 patients. Rheumatology (Oxford). 2012 [Epub ahead of print]
- Lee RW, Dick AD. Treat early and embrace the evidence in favour of anti-TNF-alpha therapy for Behçet’s uveitis. Br J Ophthalmol. 2010;94:269–270
- Keystone E, Genovese MC, Klareskog L, et al. Golimumab in patients with active rheumatoid arthritis despite methotrexate therapy: 52-week results of the GO-FORWARD study. Ann Rheum Dis. 2010;69:1129–1135
- Braun J, Deodhar A, Inman RD, et al. Golimumab administered subcutaneously every 4 weeks in ankylosing spondylitis: 104-week results of the GO-RAISE study. Ann Rheum Dis. 2012;71:661–667
- Kavanaugh A, van der Heijde D, McInnes IB, et al. Golimumab in psoriatic arthritis: one-year clinical efficacy, radiographic, and safety results from a phase III, randomized, placebo-controlled trial. Arthritis Rheum. 2012;64:2504–2517
- Jabs DA, Nussenblatt RB, Rosenbaum JT; Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–516
- Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354:1932–1939
- Okada AA, Goto H, Ohno S, et al. Ocular Behçet’s Disease Research Group of Japan. Multicenter study of infliximab for refractory uveoretinitis in Behçet disease. Arch Ophthalmol. 2012;130:592–598
- Smolen JS, Kay J, Landewé RB, et al. Golimumab in patients with active rheumatoid arthritis who have previous experience with tumour necrosis factor inhibitors: results of a long-term extension of the randomised, double-blind, placebo-controlled GO-AFTER study through week 160. Ann Rheum Dis. 2012 [Epub ahead of print]
- Cordero-Coma M, Salom D, Diaz-Llopis M, et al. Golimumab for uveitis. Ophthalmology. 2011;118:e3–e4
- William M, Faez S, Papaliodis GN, et al. Golimumab for the treatment of refractory juvenile idiopathic arthritis-associated uveitis. J Ophthalmic Inflamm Infect. 2012 [Epub ahead of print]