Abstract
Vascular-targeted drug delivery systems could provide more efficient and effective pharmaceutical interventions for treating a variety of diseases including cardiovascular, pulmonary, inflammatory, and malignant disorders. However, several factors must be taken into account when designing these systems. The diverse blood hemodynamics and rheology, and the natural clearance process that tend to decrease the circulation time of foreign particles all lessen the probability of successful carrier interaction with the vascular wall. An effective vascular-targeted drug delivery system must be able to navigate through the bloodstream while avoiding immune clearance, attach to the vascular wall, and release its therapeutic cargo at the intended location. This review will summarize and analyze current literature reporting on (1) nanocarrier fabrication methods and materials that allow for optimum therapeutic encapsulation, protection, and release; (2) localization and binding dynamics of nanocarriers as influenced by hemodynamics and blood rheology in medium-to-large vessels; (3) blood cells' responses to various types of nanocarrier compositions and its effects on particle circulation time; and (4) properties that affect nanocarrier internalization at the target site.
Introduction
The endothelium, a monolayer of cells lining the lumen of blood vessels, serves as a natural barrier in controlling the passage of material from blood into surrounding tissues. As such, the endothelium plays a functional role in the regulation of many bodily processes including blood pressure, clotting, angiogenesis, and inflammation. In recent years, the endothelium has become an attractive target for pharmaceutical intervention due to its prevalence throughout the body, its known association with the pathogenesis of several diseases, and its accessibility to intravenous agents (Gimbrone et al. Citation2000, Ross et al. Citation2001, Einav & Bluestein Citation2004, Wagner & Frenette Citation2008). To date, targeting of drugs to/via the endothelium (vascular wall) has been explored in many cardiovascular (Muzykantov Citation2001, Sakhalkar et al. Citation2003, Winter et al. Citation2006, Kaufmann et al. Citation2007), pulmonary (Dziubla et al. Citation2008), inflammatory, and malignant diseases.
Nanoparticles, particularly spheres, are typically proposed as carriers for vascular-targeted drug delivery due to their relative ease of fabrication and their capacity to navigate the circulatory system with minimal risk of vessel occlusion. However, injected targeted therapeutics often have been characterized by suboptimal and adverse systemic effects due to several factors including low adherence to the endothelium (Charoenphol et al. Citation2010), high rate of clearance by the immune system (Alexis et al. Citation2008), and an overall inability to reach the designated target whether it be intracellular compartments within endothelial cells (ECs) or other subluminal targets (Chen et al. Citation2006). A drug delivery vehicle that can overcome these issues to effectively target the endothelium could lead to vastly improved therapeutic treatments.
Overall, vascular-targeted drug carriers need to be designed to properly navigate the complex and diverse flow patterns of the vascular network, avoid immune clearance, and safely deliver therapeutics to the targeted cells, tissue or disease at effective concentrations. In this review, we focus on several considerations essential for the effective design of nanocarriers for vascular-targeted drug delivery applications. We examine properties associated with different carrier fabrication methods and materials that allow for optimum drug encapsulation, protection, and controlled release. Significant consideration is also given to the role of hemodynamics in nanoparticle navigation in circulation, particle margination (localization) and adhesion to the endothelium, and how the physio-chemical characteristics/compositions of particles may elicit adverse and deleterious interactions with blood components. We also summarize recent works focused on elucidating the mechanisms of endothelial internalization of nanoparticles.
Nanoparticle categories
The primary concerns of any targeted drug delivery system are the abilities to successfully load and deliver a desired therapeutic cargo to its target, and drug loading can vary greatly with different carrier materials and fabrication methods. Appropriate drug loading goes hand-in-hand with drug release kinetics – the rate of release determines the final in vivo distribution of the loaded drug and, hence, the overall effectiveness of the targeted therapy. Extensive drug release prior to a carrier reaching its target can severely interfere with the desired therapeutic effect. Materials that have been described for fabricating nanoparticles for drug delivery applications range from completely nonbiodegradable inorganic metals to ones mimicking living tissues such as liposomes. Each of these materials, the methods through which nanoparticles are fabricated, and the final carrier shape and size have advantages and disadvantages in providing protected delivery of therapeutics to the targeted site.
Inorganic materials that are commonly described for fabricating nanoparticles include gold (Cheng et al. Citation2008, Cheng et al. Citation2009), silver (Gurunathan et al. Citation2009), silica (Cho et al. Citation2008, Liu et al. Citation2009), iron oxides (Smith et al. Citation2007, Wilhelm et al. Citation2007), calcium carbonate (He et al. Citation2009), carbon nanotubes as well as fullerenes (Dhar et al. Citation2008, Southworth et al. Citation2009), and quantum dots (Takeda et al. Citation2008). However, their proposed use for vascular drug-targeting/imaging has been limited – few reports describe the use of inorganic material-based nanoparticles for vascular targeting (Radomski et al. Citation2005, Reddy et al. Citation2006, Bogdanov et al. Citation2007, Jiang et al. Citation2009). This is likely due to the careful tuning of pore sizes required to achieve the desired drug release rates when using non-degrading materials; completely nonporous particles entrap drugs too strongly, while highly porous particles can leach drugs out prior to reaching the delivery site. However, Southworth and coworkers reported that surrounding inorganic nanoparticles with a liposome could prevent premature drug release (Southworth et al. Citation2009). Inorganic particles also often have issues with cytotoxicity as they do not degrade to natural byproducts, and therefore they must be cleared from circulation in some other fashion to avoid deleterious side effects (Brown et al. Citation2001, Peters et al. Citation2004, Kagan et al. Citation2005, Lewinski et al. Citation2008). One exception to the rule for inorganic materials may be silicon.
Silicon is naturally found in bone and is suggested to have a role in the synthesis and stabilization of collagen that is important for bone health (Jugdaohsingh Citation2007). Oxidized (SiO2) porous silicon rapidly dissolves in aqueous environments to form silicic acid compounds that are readily processed and eliminated from the body; thus nanoparticles made from porous silicon are biocompatible and biodegradable (Anglin et al. Citation2008). A few researchers have recently shown that porous silicon nanoparticles (Tasciotti et al. Citation2008, Park et al. Citation2009) can be loaded with therapeutics for a variety of drug delivery applications and are shown to exhibit minimal inflammatory effects (Kilpelainen et al. Citation2009). However, the use of porous silicon nanoparticles for drug delivery is likely limited by the methods utilized to achieve drug loading (Salonen et al. Citation2008). For one, drugs loaded onto nanoparticle surfaces via covalent linkage are likely released only after complete degradation of the silicon matrix due to the high stability of covalent bonds. ‘The spontaneous adsorption’ drug loading method requires precise solvent choice and drug concentration (Salonen et al. Citation2008). Also, characterization of the amount of drug loaded via this method is a challenge, and ‘endcapping’ of the nanoparticle surface is typically necessarily if extended release is desirable (Anglin et al. Citation2008).
Liposome (lipid bilayer vesicles)-based nanoparticles have been widely studied for drug delivery applications due to their relative ease of fabrication, high loading yields of both hydrophobic and hydrophilic drugs, and membrane flexibility (Diebold et al. Citation2007, Shi et al. Citation2007, Zhu et al. Citation2007, Murphy et al. Citation2008, Liu et al. Citation2009, Peters et al. Citation2009, Southworth et al. Citation2009, Srinivasan et al. Citation2009). Since liposomes are typically constructed from naturally occurring phospholipids, they tend to pose a lower risk of eliciting unwanted toxic or antigenic reactions when used as drug carriers (Schwendener Citation2007). However, there may be some limitations to their use for vascular-targeted delivery. For one, as with most drug carriers, liposomes are easily cleared from the blood stream via the mononuclear phagocytes. The grafting/incorporation of poly(ethylene glycol) (PEG) chains onto carrier surfaces is suggested not to be a viable option for prolonging the in vivo circulation of liposomes since even low-level PEGylation (5–7 mol% depending on PEG chain length) can destroy their bilayer structure (Photos et al. Citation2003, Dos Santos et al. Citation2007). However, Li and co-workers recently demonstrated successful incorporation of higher levels of PEG (up to 20 mol%) into nanoliposomes via a double bilayer method where the inner bilayer was stabilized with strong charged interactions (Li & Huang Citation2009). Moreover, a PEGylated liposomal formulation of the chemotherapy drug doxorubicin (Doxil) has found success in clinical trials and has been approved for the treatment of several malignant and solid tumors (Gordon et al. Citation2001, Uyar et al. Citation2004, Strother & Matei Citation2009). Another likely concern with the use of liposomes for vascular-targeted delivery is the potential for their membrane integrity to be easily compromised when exposed to blood flow-induced shear forces at the vascular wall, depending on their size and lipid composition/chain length (Ondaral et al. Citation2006). However, more studies are needed to fully assess the stability of various liposomal formulations for vascular-targeted applications.
Nanoparticles fabricated from various polymers have been well studied (Gratton et al. Citation2007, Sutton et al. Citation2007, Ljubimova et al. Citation2008, Vargas et al. Citation2008, Patil et al. Citation2009, Citation Simone et al. Citation2009). The versatility of polymer-based nanoparticles in terms of starting material as well as potential in sizes and shapes make them attractive for drug delivery applications. The most widely used polymers include poly(lactide), poly(lactide-co-glycolide), poly(ϵ-caprolactone), and poly(ethylene glycol) as well as their copolymer mixes. Polymeric nanocarriers are often of a solid matrix form; however, polymeric carriers with a construct similar to liposomes have also been described for drug delivery (Discher et al. Citation1999, Photos et al. Citation2003, Gaspard et al. Citation2009). These polymer-based vesicles (polymersomes) are often made from amphipathic block co-polymers, and they retain the membrane flexibility properties of liposomes while having increased membrane stability. The polymeric nature of polymersomes also permits much greater PEGylation, enabling these particles to persist in blood circulation (Photos et al. Citation2003). Dendrimers, which are highly branched polymers, have also been widely explored for vascular-targeted drug delivery. These globular macromolecules tend to be on the order of tens of nanometers in size and are typically easy to load (Gillies & Frechet Citation2005). Dendrimers are further attractive for vascular-targeted delivery due to their relative ease of crossing biological barriers such as the blood brain barrier (Dhanikula et al. Citation2008, Kannan et al. Citation2009); however, they have a tendency to aggregate (Hong et al. Citation2007). Additionally, many dendrimers are cytotoxic, requiring additional surface derivatization with compounds such as PEG, polylysine, PLGA, or even etherification of surface groups in order to make them more biocompatible (Shcharbin et al. Citation2009). Another major disadvantage to the use of dendrimers relative to liposomal and polymeric carriers is their byzantine synthesis owing to their complex architecture (Bermejo et al. Citation2007).
Viral nanoparticles (VNPs) have recently been suggested for use as vascular-targeted drug carriers such as the cowpea mosaic virus (CPMV) (Manchester & Singh Citation2006, Shriver et al. Citation2009). Koudelka and coworkers recently showed that EC-surface expressed vimentin could mediate the specific binding of CPMV to both normal and tumor vasculature, suggesting that CPMV can be used for vascular imaging and drug delivery (Koudelka et al. Citation2009). In general, viruses are attractive for drug targeting applications since they are naturally evolved to have efficient circulation and a high capacity to target (bind and enter) cells. Furthermore, VNPs can be genetically engineered to display a large number of other targeting molecules on their surfaces in addition to their naturally built-in targeting mechanisms. However, a couple of limitations exist for the use of VNPs for vascular-targeting in humans. For one, VNPs are typically produced in bioreactors with complex fabrication processes (Pedro et al. Citation2008). Also, though most VNPs in development for human application are not human pathogens, toxicity remains an issue since comprehensive studies to evaluate their safety in humans have largely not been conducted (Manchester & Singh Citation2006). Human pathogen-based VNPs are known to elicit unwanted immune responses such as secretion of pro-inflammatory molecules and activation of mononuclear cells (Higginbotham et al. Citation2002, Cotter & Muruve Citation2005).
Targeting the vascular wall
Two main mechanisms are typically proposed for guiding nanoparticles to the vascular wall. The first, passive targeting, relies on exploiting the enhanced permeability of the endothelium in certain disease states, most notably in cancer (Kaul & Amiji Citation2005, Li et al. Citation2008) where extravasation of particles with diameters up to 200 nm readily occurs due to leaky tumor vasculature (Yuan et al. Citation1995). The second mechanism, active targeting, is achieved by decorating carrier surfaces with ligands, peptides or counter-receptors specific to molecules expressed on the endothelium. This delivery method is characterized by well-regulated biodistribution and improved therapeutic efficacy. Many endothelium-expressed molecules have been proposed for targeting nanoparticles to the vascular wall, including vascular endothelial growth factor receptors (VEGF-R) and their complexes in cancer-associated angiogenesis (Backer et al. Citation2005, Byrne et al. Citation2008) and leukocyte adhesion molecules (LAMs) in disease-associated inflammation (Eniola et al. Citation2002, Eniola et al. Citation2005). Appropriate EC-expressed molecules for specific targeting of different diseases/tissues and their targeting advantages/disadvantages have been adequately reviewed elsewhere (Everts et al. Citation2001, Hajitou et al. Citation2006, Citation Simone et al. Citation2009) and will not be a focus in this review.
Regardless of the targeting mechanism employed to guide localization, nanoparticles targeted to the vascular wall must first successfully navigate the dense population of cells in the bloodstream before they can bind to the endothelium. A high efficiency of drug carrier interaction with/adhesion to the endothelium is critical for achieving effective localized drug delivery, particularly for highly potent therapeutics.
Margination of nanoparticles to the vascular wall
Blood rheology and hemodynamics are known to affect how leukocytes marginate (localize, roll and arrest) to the vascular wall in vivo. Specifically, red blood cells (RBCs) preferentially align in the center of the bloodstream in flow, forcing leukocytes and platelets into a RBC-free plasma layer adjacent to the wall. This allows for an increased probability of leukocyte interaction with the endothelium (Munn et al. Citation1996, Pearson & Lipowsky Citation2000, Migliorini et al. Citation2002, Abbitt & Nash Citation2003, Pappu & Bagchi Citation2007). While their effects may not be relevant in microcirculation where blood cells tend to line up in single file (), hemodynamics and blood rheology will likely prescribe binding dynamics of drug carriers targeted to the endothelium in medium-to-large-sized blood vessels relevant in several human diseases that include polyarteritis, Kawasaki disease, giant cell arteritis, and atherosclerosis (Hoffman & Weyand Citation2002, Coleman Citation2008).
Figure 1. Illustration of nanoparticle margination in (A) capillary-sized vessel and (B) medium-to-larger-sized vessel with bulk blood flow.
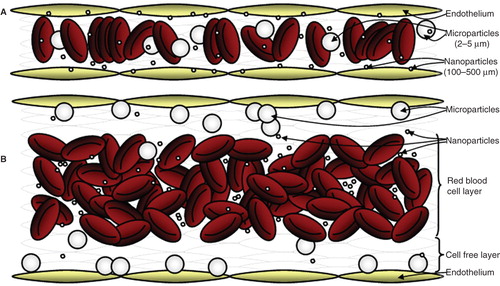
Though several researchers have attempted to understand how particles marginate to the wall from flow, little evidence has been presented in the past to suggest that nanoparticles can successfully marginate from the bloodstream to the vascular wall, particularly in medium-to-large blood vessels. Recently, Gentile and coworkers showed that nanoparticles in the 500–700 nm diameter range displayed low non-specific binding to the wall from low shear buffer flow (∼0.1 dynes/cm2) in a parallel plate flow chamber (PPFC) of 250 μm channel height (Gentile et al. Citation2008). Also, previous work by Eckstein and colleagues suggested that only spheres larger than 2 μm (up to 5 μm) exhibit large near-wall excess due to RBC-mediated localization to the wall (Aarts et al. Citation1988, Eckstein et al. Citation1988); however, this study did not extend to particles in the nanometer range. We recently reported that nanospheres (100–500 nm) displayed minimal margination from human blood flow to a monolayer of activated ECs in PPFCs of varying heights (125–700 μm) compared to significantly higher binding of intermediately-sized microspheres (2–5 μm) (Charoenphol et al. Citation2010). More importantly, our results suggested that introduction of pulsatility in blood flow (flow oscillating around 0 dynes/cm2 or between 3 and 30 dynes/cm2) did not improve nanoparticle margination but enhanced microparticle attachment to the endothelium two to four-fold compared to adhesion in laminar flow. While several works in the literature have shown successful targeting of nanospheres to the vascular wall in various in vivo animal models, these reports have typically focused on capillary-sized vessels (e.g., pulmonary vasculature) where nanosphere binding success is likely attributed to the smaller vessel-to-particle size ratio, i.e., increased probability of nanoparticle-wall collisions and adhesion (Kaul & Amiji Citation2005, Lu et al. Citation2006, Dziubla et al. Citation2008, Li et al. Citation2008, Zensi et al. Citation2009). Reports of nanospheres binding in large arteries in vivo have mostly been with particles introduced directly to the wall via cannulation of the arteries (Demos et al. Citation1999); thus this model does not adequately reflect margination through RBCs. While Chan et al. recently showed targeted nanoparticles can better bind to injured rat aorta via intravenous injection relative to non-targeted ones (Chan et al. Citation2010), this study did not report the binding efficiency of the targeted particles relative to the amount injected or particles of different sizes; thus it is not clear what percentage of injected particles were able to target the vessel wall.
Overall, the above-presented works suggest that spherical nanocarriers may not be adequate for vascular-targeted applications in medium-to-large size blood vessels due to RBC-hindered margination (). However, more in vivo work is still needed to fully characterize the ability of particles of different sizes to deliver therapeutics to walls in all vessels and over all physiological ranges of blood flow dynamics. Parallel efforts must also focus on identifying novel approaches that enhance the binding of nanoparticles in all vessels to fully develop vascular-targeting therapeutic capabilities. It may be that a departure from the spherical shape is necessary if a perfect balance of in vivo circulation time (Diebold et al. Citation2007), risk of vessel occlusion (low), and margination rate (high) is not achievable with spheres. Recent advances in methods for fabricating non-spherical polymeric particles have opened avenues for exploring the use of these particles as drug carriers (Rolland et al. Citation2005, Champion et al. Citation2007, Bhaskar et al. Citation2009, Heslinga et al. Citation2009, Acharya et al. Citation2010). A few works focused on vascular targeting have recently suggested that non-spherical particles display strong adhesion (and improved binding) in shear flow (Lee et al. Citation2009a, Citation2009b). However, these observations thus far have been theoretical or observed only in the absence of blood cells or in vivo in capillary-sized vessels; thus as of yet, it is not clear what the margination capacity of these particles would be in bulk blood flow in larger vessels.
Nanoparticle interaction with blood cells and longevity in circulation
Another major factor that is likely to impact the fate of vascular-targeted nanocarriers is their molecular and chemical interactions with blood cells. Nanocarriers show higher incidence of adverse interactions with blood cells than micro-sized ones. While many of the reported adverse effects are not a result of carrier size per se, nano-sized particles in blood have more intimate interaction with the cell membranes and thus have a better chance of eliciting cell response via other physical properties (such as material type, size, hydrophobicity, surface charge, and concentration). Adverse physiological outcomes that have been associated with vascular-targeted nanocarriers include hemolysis, platelet activation and aggregation, leukocyte activation and adhesion, and complement activation.
Hemolysis of red blood cells
Recent studies have shown that hemolysis (leakage of hemoglobin) can occur when nanoparticles interact with RBCs. Besides the increased risk of anemia, the debris released from damaged RBCs can initiate a phagocytic cascade that often leads to rapid clearance of nanoparticles and reduced targeting efficacy (Buehler et al. Citation2008). Many studies have attributed such induced hemolysis to nanoparticles with positively-charged surface characteristics (Domanski et al. Citation2004, Agashe et al. Citation2006, Bermejo et al. Citation2007). However, recent studies showed that nanoparticles with net negative charges also induced hemolysis (Mayer et al. Citation2009, He et al. Citation2010). Other studies examining the effects of nanoparticle surface properties have also found that nanoparticles fabricated in the presence of surface-active surfactants (e.g., polyoxyl-20-ether) induced hemolysis at high nanoparticle concentrations (Chouly et al. Citation1994, Koziara et al. Citation2005); however, the observed hemolytic activities were attributed to residual surfactant in the water phase of the nanoparticle formulations. Additionally, several authors have suggested that nanoparticle hemolytic activities can be reduced when PEG chains are grafted onto the nanoparticle surface (Kim et al. Citation2005, He et al. Citation2010). Size also plays a role in the induction of hemolysis by nanoparticles – Mayer and colleagues suggested that smaller-sized (20 nm) nanoparticles induced increased hemolytic behaviour in RBCs compared to larger (200 nm) particles, presumably a result of their higher probability of disrupting the cell membrane (Mayer et al. Citation2009).
Platelet aggregation and activation
Vascular-targeted nanoparticles are likely to interact with platelets that are highly concentrated in close proximity to the vascular wall due to their major role in thrombus formation necessary for endothelium repair. Movat and coworkers first suggested that nanoparticles influenced platelet activation in vivo (Movat et al. Citation1965). Activation of platelets outside of the normal physiological response can serve to initiate uncontrollable blood coagulation (Davi & Patrono Citation2007, Jennings Citation2009) and excessive clotting can lead to stroke or heart attack in some cases. Nanoparticles made from biocompatible inorganic materials such as gold and silver have been shown to affect platelet aggregation and activation (Hsu et al. Citation2006, Wiwanitkit et al. Citation2009). One in vitro study with human blood found that silver nanoparticles coated on a catheter induced platelet activation due to the presence of silver ions on the nanoparticle periphery (Stevens et al. Citation2009). However, another study suggested that silver nanoparticle treatment reversed platelet aggregation (Shrivastava et al. Citation2009). Carbon nanotubes have also been shown to induce platelet aggregation and enhance vascular thrombosis in rats (Radomski et al. Citation2005); in the same study, carbon nanospheres did not activate platelets, indicating particle shape may be a factor in influencing platelet behavior. Nanoparticle surface charge is also important; a few in vivo studies have shown that amine-coated polystyrene nanoparticles amplified thrombus formation whereas negatively-charged carboxylated-polystyrene particles inhibited activity (Nemmar et al. Citation2008, Bihari et al. Citation2009). Another study suggested that nanoparticle-induced platelet aggregation generally increased with a decrease in net negative particle surface charge (Miyamoto et al. Citation1989). However, Mayer and coworkers reported similar levels of thrombocyte activation by cationic and anionic polystyrene nanoparticles (Mayer et al. Citation2009). They also showed that 20 nm carboxylated-polystyrene nanoparticles exhibited a higher tendency to activate human platelets than 200 nm nanoparticles of similar surface characteristics (Mayer et al. Citation2009), suggesting that size may also play a role in platelet activation. Lundqvist and colleagues also reported that particles of different sizes bind different plasma proteins (Lundqvist et al. Citation2008) that could lead to different degrees of platelet activation. Overall, it appears that the capacity for nanoparticles to induce platelet activation and aggregation is not dependent on only one single factor. Instead, the thrombolytic activity induced by a nanoparticle formulation is a function of several factors that include nanoparticle size, shape, material characteristics, surface charge (type and extent), and hydrophobicity (Miyamoto et al. Citation1989).
Opsonization and reticuloendothelial system clearance
For many intravenously administered drug delivery systems, a high carrier blood circulation time (or low blood clearance rate) is desirable. It is known that various particle properties (size, surface charge, and hydrophobicity) may affect their opsonization – the process by which particles are marked for clearance by immune cells via serum protein absorbtion on their surface. Several works in the literature have suggested that nanoparticle opsonization (and hence clearance) rate decreases as their size decreases (Fang et al. Citation2006, Cedervall et al. Citation2007) – nanoparticles with sizes ≥100 nm are more rapidly cleared through phagocytic uptake. However, a lower size boundary of 10 nm exists where smaller nanoparticles are rapidly cleared via renal excretion. Overall, literature suggests nanoparticles in the 50–100 nm size range display the lowest blood clearance rates (Alexis et al. Citation2008, Sonavane et al. Citation2008). PEGylation of nanoparticles reduces their opsonization rate, allowing them to avoid recognition by macrophages in the RES and thus improve their circulation time (Alexis et al. Citation2008, Lim et al. Citation2008).
In addition to size, shape may also affect nanoparticle blood clearance rate. Geng and coworkers reported that worm-like micelles have a prolonged in vivo blood circulation (five day half-life) compared to spherical liposomes (Geng et al. Citation2007). Muro and coworkers recently found that anti-intercellular adhesion molecule (ICAM)-1 coated disks had longer circulation times in mice compared to spherical particles (Muro et al. Citation2008). Both of these works attributed the increased circulation time to the inability of blood phagocytes to effectively internalize these particles as previously reported by Champion and Mitragotri (Champion & Mitragotri Citation2006). It appears, however, that all non-spherical particles that have been reported to display longer in vivo circulation have had at least one of their axes in the micrometer size range. Thus, it is not clear at this point if shape alone will enhance the in vivo circulation of particles having all dimensions in the nanometer range.
Nanoparticle internalization at the vascular wall
Drug carriers that manage to avoid immune clearance and are able to overcome limitations imposed by blood rheology/dynamics to localize to the vascular wall must possess the appropriate moiety for target recognition. In the case of active targeting – this is typically by way of receptor-ligand-mediated adhesion via EC-expressed tissue/disease specific molecules as mentioned hereinbefore. Here, we focus on the fate of vascular-targeted nanoparticles beyond the initial EC target recognition.
Research on endothelial uptake of nanoparticles has focused on elucidating the mechanisms by which particles are internalized and what factors (i.e., particle geometry, surface modifications, and material) influence internalization rates. In the interest of designing an efficient drug delivery system, it is critical to identify and understand the uptake mechanisms that transport particles from the endothelial surface to intracellular sites (i.e., within the cytosol or the nucleus for genetic delivery) or subluminal targets (transcytosis); a nanocarrier's fate, and that of its contents, is ultimately determined by the endocytic pathway the endothelium utilizes to internalize the particle.
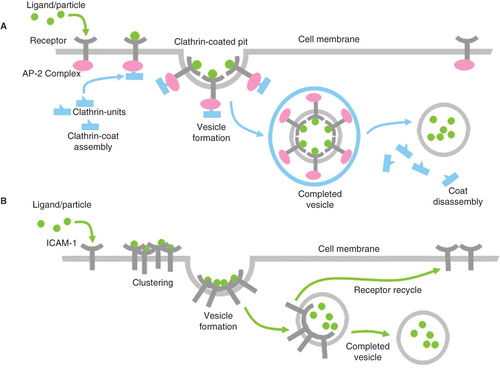
Endocytosis in ECs can occur via mechanisms that are characterized by either receptor-mediated or receptor-independent pathways. The two major receptor-mediated EC types of internalization include clathrin-mediated () (Traub Citation2009) and caveolae-mediated endocytosis. However, Muzykantov and coworkers recently described a unique receptor-mediated EC endocytosis modulated by cell (leukocyte) adhesion molecules (CAMs) as shown in Figure 2 (Muro et al. Citation2005, Muro et al. Citation2008, Citation Simone et al. Citation2009), and a few researchers have described EC internalization of large particles via receptor-mediated phagocytosis (Langeggen et al. Citation2003, Serda et al. Citation2009). Receptor-independent pathways that depend on membrane cholesterol and lipid raft domains have been identified in the uptake of larger-sized particles (Kirkham & Parton Citation2005, Stan Citation2006, Partlow et al. Citation2008), as well as macropinocytosis (Serda et al. Citation2009). To this end, a handful of studies have looked at how properties like particle size, shape, surface charge, surface ligand patterning, and other characteristics determine the mechanism by which ECs ingest nanoparticles.
Particle size and shape
There is a consensus in the literature that the size of nanoparticles affects not only the uptake rate but also the mechanism by which they are internalized. In general, larger particles take more time to ingest. Nanoparticles smaller than 500 nm to as tiny as dendrimers, quantum dots, and short carbon nanotubes are typically internalized via receptor-mediated endocytosis, particles less than 200 nm via clathrin-mediated and others mostly via caveolae-mediated (Rejman et al. Citation2004). Large particles (1–10 μm diameter) are often processed via macropinocytic/phagocytic pathways. Intermediate-sized carriers (0.5–1 μm) are internalized through a mix of both modes (studies across various animal models and complexities due to large variability in material properties make it difficult to conclude definitive size trends) (Decuzzi et al. Citation2009, Decuzzi et al. Citation2010).
Particle shape has also been found to affect uptake rate and pathway. Muro and coworkers found that in vitro endothelial ICAM-1-targeted 0.1 × 1 × 3 μm polystyrene disks exhibited four times slower uptake rates compared to 0.1 and 5 μm spheres (Muro et al. Citation2008). However, Gratton and colleagues found that cylindrical particles with large aspect ratios (150 nm diameter by 450 nm height) were internalized nearly four times faster by HeLa cells than less elongated particles; smaller cylinders were internalized to a lesser extent, but at the same rates as larger-volume spherical particles (Gratton et al. Citation2008). However, Chan and colleagues reported that HeLa cells internalized spherical gold nanoparticles at a higher rate than rod-shaped gold nanoparticles, and that the uptake of low aspect ratio rods was greater than that of ones with high aspect ratios (Chithrani et al. Citation2006). Overall, there is likely a coupled effect of shape and size in cellular internalization of particles; thus modification of particle shape may be another viable option to controlling intracellular drug targeting. However, additional studies specific to ECs are needed in order to fully define the effect of particle shape on the capacity for these cells to internalize nanoparticles.
Surface charge and other modifications
In addition to size and shape, a number of authors have shown that nanoparticle surface charge can also determine the eventual endocytic pathway elicited. Charged nanoparticles typically show higher uptake rates compared to neutrally-charged particles; however, cationic nanoparticles have been found to internalize more readily than anionic nanoparticles, likely due to their better interaction with the negatively-charged plasma membrane and clathrin-coated pits of the endothelium. A study by Serda and coworkers reported that negatively- and positively-charged silicon microparticles (1.6 μm) were equally internalized (45–50%) by human umbilical vein ECs after 1 h while internalization of neutrally-charged PEGylated silicon microparticles was lower (30%) (Serda et al. Citation2009). Interestingly, the study also found that serum-opsonized negatively-charged particles (oxidized silicon and negatively-charged polystyrene microparticles) had dramatically lowered uptake rates while opsonization failed to impact uptake rates for positively-charged microparticles and only slightly inhibited uptake of neutrally-charged PEGylated particles (Serda et al. Citation2009). Jallouli and coworkers found that porous 60 nm cationic and neutral particles readily transcytosed bovine brain capillary ECs and could show promise as candidates in crossing the blood brain barrier; their work also indicated that particle inner composition (in this case, a cationic nanoparticle with an anionic lipid core) could affect internalization and transport behavior (Jallouli et al. Citation2007). Harush-Frenkel studied the membrane transport fates of charged PEG-PLA nanoparticles through thin mucous-layer epithelial MDCK kidney cells and found that charged nanoparticles were mostly targeted through clathrin-mediated endocytosis (cationic particles having two-fold greater uptake than anionic particles), but also that a small fraction were mediated through a clathrin-independent, macropinocytic process. In addition to having an increased uptake rate, cationic particles have been found to avoid degradative lysosomal pathways that anionic particles seem to favor (Harush-Frenkel et al. Citation2008).
Nanoparticle surface conjugated targeting molecules
While traditional endocytosis usually refers to clathrin- and caveolae-mediated uptake, many researchers have focused on other receptor-mediated endocytic mechanisms, specifically studying inflammation-associated leukocyte adhesion molecules ICAM-1 and platelet-endothelial cellular adhesion molecule (PECAM)-1. Muro and coworkers, using pharmacological inhibitors, suggested that ICAM-targeted nanoparticles were ingested via internalization pathways distinct from more classical (clathrin-coated or caveoli pits) mechanisms (Muro et al. Citation2008). In a separate study, Muro et al. characterized the intracellular trafficking of ICAM-1 after CAM-mediated endocytosis of anti-ICAM-1-coated latex nanoparticles, specifically focusing on the effects of recurrent dosing. These authors found that subsequent targeting of ICAM-1 resulted in increasingly protective effects against lysosomal degradation as evidenced by prolonged protection against peroxidase injury when treating with consecutive doses of catalase-loaded anti-ICAM-1 nanocarriers (Muro et al. Citation2005).
A handful of groups have also looked at other CAM-specific targeting: Simionescu and colleagues found that when compared to non-labeled control particles, lipid-based nanoparticles coupled with an antibody against vascular cell adhesion molecule (VCAM)-1 were twice as likely to be internalized on tumor necrosis factor (TNF)-α activated endothelium (Voinea et al. Citation2005). Similar studies by Gosk and coworkers found that immunoliposomes targeted to VCAM-1 were more likely to bind and internalize in tumour cells than control immunoliposomes in their static and flow in vitro models; however, their in vivo mouse model studies were inconclusive, perhaps due to non-specific accumulation of the carriers (Gosk et al. Citation2008). Zhang and coworkers conjugated a peptide cLABL (known for its high avidity for domain-1 of ICAM-1 and has been shown to internalize readily to the cytoplasmic domain of ECs and T cells) to 250 nm diameter PEGylated PLGA particles; their subsequent study on particle uptake rates in IFN-γ activated HUVECs found that cLABL-conjugated nanoparticles showed rapid binding and internalization rates compared to blank PLGA-PEG nanoparticles (2–2.5 fold higher binding rates within 30 minutes and higher colocalization within lysosomes within 10–15 minutes of binding) (Zhang et al. Citation2008).
A general overview of typical fates of EC targeted nanoparticles as discussed in this section is summarized in .
Table I. Nanocarrier internalization pathways and fates.
Intracellular fate of vascular-targeted nanoparticles
In general, two main outcomes are desired for nanoparticles targeted to the vascular wall: (1) delivery of drug agents into EC cell nuclei or cytosols for therapeutic action or (2) transcytosis (transcellular delivery across the EC) when other cells are the target of drug actions. Regardless of which outcome is desired, the key challenge to intra/transcellular drug delivery is in avoiding natural cellular degradation processes (via the lysosome) that often interfere with the activity of loaded therapeutics.
Endosomal escape
To avoid acidic lysosomal degradation when drug delivery to the cytosol or nucleus is desired, nanocarriers must either (1) escape from the endosomes (intracellular vesicles formed from invagination of cell membrane) destined for lysosomal fusion or (2) utilize endocytic mechanisms that generate vesicles that do not traffic to the lysosome but deliver content directly to the cytosol. Clathrin-dependent endocytosis generally delivers nanoparticles to the lysosome where they and loaded therapeutics are subjected to acidic degradation (Bareford & Swaan Citation2007). Clathrin-independent endocytic pathways – caveolae-mediated (Nishikawa et al. Citation2009), lipid raft-mediated (Kaneda et al. Citation2010) and macropinocytosis – lead to less degradative fates, e.g., cytosol localization (Rejman et al. Citation2004, Rejman et al. Citation2005, Kaneda et al. Citation2010). However, these endocytic pathways tend to be much slower than clathrin-dependent pathways (Rejman et al. Citation2005). Thus a significant portion of research on intracellular drug delivery is focused on the design of carriers that can escape the endosomes in clathrin-mediated endocytosis. Recent works have focused on utilizing pH-sensitive carriers to mitigate endosomal escape. For example, nanoparticles with accessible amine groups can promote accumulation of chlorine ions when protonated at endosomal pH (Sonawane et al. Citation2003), and the accumulation of chlorine ions results in osmotic swelling and eventual endosome rupture – i.e., proton sponge effect (Boussif et al. Citation1995, Pack et al. Citation2000, Akinc et al. Citation2005). A similar approach is via pH-sensitive amphiphilic polymers that undergo a switch from a hydrophilic to a hydrophobic, ‘endosomal membrane-destabilizing’ state at endosomal pH values and thus mediate carrier escape to the cytosol (Kyriakides et al. Citation2002, Lackey et al. Citation2002).
Transcytosis
The majority of nanoparticle formulations developed for trans-endothelial drug delivery are modeled after macromolecules that are naturally transported across the endothelium via transcytosis. Macromolecules such as low-density lipoprotein (LDL), transferrin, albumin, and lactoferrin naturally undergo transcytosis through the brain capillary endothelium mainly via caveolae-mediated endocytosis (Fenart et al. Citation1999, Wang et al. Citation2009). Ke and coworkers reported that polyamidoamine dendrimers targeted to lipoprotein receptor-related protein-1 on brain capillary ECs showed successful transcytosis in both in vitro and in vivo mouse models (Ke et al. Citation2009). Likewise, Lu and coworkers showed that cationic albumin-coated nanoparticles were successfully transcytosed by rat brain capillary ECs via absorptive-mediated transcytosis (Lu et al. Citation2005). Several authors have also reported the effective EC transcytosis of surfactant-coated nanoparticles. These nanoparticles easily absorb apolipoproteins present in serum and utilize the LDL-receptor transport pathway (Alyaudtin et al. Citation2001, Kreuter et al. Citation2002, Kratzer et al. Citation2007).
Conclusions
The design goal of all targeted drug delivery systems is to have such systems arrive at the intended treatment site functionally intact. There are several facets to this in the particular case of vascular-targeted drug delivery. Efficient delivery to the target location requires maneuvering from the injection site through the bloodstream – while avoiding natural clearance processes – moving toward the vascular wall from blood flow, attaching to the intended site on the blood vessel wall, and releasing its cargo to the target. One huge obstacle with such delivery systems is that they are rapidly cleared from circulation depending on their size and shape. Nanoparticles of 50–100 nm have prolonged circulation, and PEGylation of these particles can further increase circulation time.
Particle stability is also critical to drug carrier in vivo functionality. Some materials, such as liposomes, are often not robust enough to withstand the shear flows of the bloodstream. Additionally, many types of nanoparticles tend to aggregate when injected into the body, skewing the performance of such particles from their intended behavior. Other systems have problems with cytotoxicity when exposed to living tissues or, as with some nonbiodegradable particles, may not be readily cleared from the body, causing bioaccumulation to occur. These challenges must be overcome through careful selection of materials and fabrication methods. Overall, nanoparticles in the 50 nm range are good for prolonged circulation time, but can induce hemolysis, platelet aggregation, and inflammation. On the other hand, nanoparticles in the 250–500 nm range have a low risk of hemolysis and inflammation, but are easily cleared from circulation.
While nanoparticles can successfully marginate to the wall for both active and passive targeting of the endothelium in capillary-sized vessels, they may not effectively marginate to the vascular wall in medium-to-large blood vessels. Instead, microspheres in the 2–5 μm range are suggested to be optimal for use as targeted carriers in these vessels; however, microspheres of these sizes are readily cleared and pose a high risk to capillary occlusion. Overall, detailed studies of particle margination to the vascular wall are still needed to elucidate the interplay between blood dynamics/rheology and blood clearance rate – as it relates to their size, shape and surface characteristics – in prescribing their efficiency in targeting the vascular wall. It may be that particles with high margination efficiency need not have prolonged blood circulation times for optimum effect.
Functionality of attached ligands is necessary and permits for the active targeting of the vasculature through adhesive interaction with endothelium-expressed molecules such as the selectins, ICAM-1, and PECAM-1. Once attached on the vascular wall, one must consider the ultimate fate of such particles. Endothelial cells readily internalize particles in the nanometer size range, and size, along with surface charge, dictates the endocytic pathway, internalization rate, and eventual delivery fate of these particles. Nanoparticle internalization occurs rapidly (within minutes) via receptor-mediated processes such as clathrin-, caveolae- and endothelial cell adhesion molecule-mediated endocytosis. Larger-sized particles (towards the micron range) are internalized to a lesser extent and at slower rates by receptor-independent mechanisms. Non-neutrally charged particles have been found to consistently exhibit higher internalization rates than neutral carriers. When internalized, cationic particles are readily transcytosed or localize to inner cell compartments, but avoid lysosomal degradative pathways that anionic particles tend to favour. Overall, most targeted interactions of nanocarriers with the endothelium of the vascular wall result in internalization of such particles. Thus, if other cells of the vascular wall (e.g., smooth muscle cells) or underlying tissue are the intended focus of drug action, consideration of methods that avoid EC uptake or promote transcytosis is necessary.
Figure 2. Types of receptor-mediated endocytosis include (A) clathrin-mediated endocytosis is a major receptor-mediated mechanism by which endothelial cells internalize particles involving the formation of a clathrin-coated vesicle; (B) cell adhesion molecule (CAM)-mediated endocytosis is clathrin-independent, using common adhesion receptors such as intercellular CAM (ICAM)-1 and vascular CAM (VCAM)-1.
Declaration of interest: The authors acknowledge funding from the American Heart Association (0735043N) and the National Science Foundation (EEC 0824182). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aarts PA, van den Broek SA, Prins GW, Kuiken GD, Sixma JJ, Heethaar RM. 1988. Blood platelets are concentrated near the wall, red blood cells, in the center in flowing blood. Arteriosclerosis 8:819–824.
- Abbitt KB, Nash GB. 2003. Rheological properties of the blood influencing selectin-mediated adhesion of flowing leukocytes. Am J Physiol Heart Circ Physiol 285:H229–240.
- Acharya G, Shin CS, McDermott M, Mishra H, Park H, Kwon IC, Park K. 2010. The hydrogel template method for fabrication of homogeneous nano/microparticles. J Control Release 141:314–319.
- Agashe HB, Dutta T, Garg M, Jain NK. 2006. Investigations on the toxicological profile of functionalized fifth-generation poly (propylene imine) dendrimer. J Pharm Pharmacol 58:1491–1498.
- Akinc A, Thomas M, Klibanov AM, Langer R. 2005. Exploring polyethylenimine-mediated DNA transfection, the proton sponge hypothesis. J Gene Med 7:657–663.
- Alexis F, Pridgen E, Molnar LK, Farokhzad OC. 2008. Factors affecting the clearance, biodistribution of polymeric nanoparticles. Mol Pharm 5:505–15.
- Alyaudtin RN, Reichel A, Lobenberg R, Ramge P, Kreuter J, Begley DJ. 2001. Interaction of poly(butylcyanoacrylate) nanoparticles with the blood-brain barrier in vivo, in vitro. J Drug Target 9:209–221.
- Anglin EJ, Cheng L, Freeman WR, Sailor MJ. 2008. Porous silicon in drug delivery devices, materials. Adv Drug Deliv Rev 60:1266–1277.
- Backer MV, Gaynutdinov TI, Patel V, Bandyopadhyaya AK, Thirumamagal BT, Tjarks W, Barth RF, Claffey K, Backer JM. 2005. Vascular endothelial growth factor selectively targets boronated dendrimers to tumor vasculature. Mol Cancer Ther 4:1423–1429.
- Bareford LM, Swaan PW. 2007. Endocytic mechanisms for targeted drug delivery. Adv Drug Deliv Rev 59:748–758.
- Bermejo JF, Ortega P, Chonco L, Eritja R, Samaniego R, Mullner M, de Jesus E, de la Mata FJ, Flores JC, Gomez R, Munoz-Fernandez A. 2007. Water-soluble carbosilane dendrimers: synthesis biocompatibility, complexation with oligonucleotides; evaluation for medical applications. Chemistry 13:483–495.
- Bhaskar S, Hitt J, Chang SW, Lahann J. 2009. Multicompartmental microcylinders. Angew Chem Int Ed Engl 48:4589–4593.
- Bihari P, Holzer M, Praetner M, Fent J, Lerchenberger M, Reichel CA, Rehberg M, Lakatos S, Krombach F. 2009. Single-walled carbon nanotubes activate platelets, accelerate thrombus formation in the microcirculation. Toxicology 269:148–154.
- Bogdanov AA, Jr., Lin CP, Kang HW. 2007. Optical imaging of the adoptive transfer of human endothelial cells in mice using anti-human CD31 monoclonal antibody. Pharm Res 24:1186–1192.
- Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. 1995. A versatile vector for gene, oligonucleotide transfer into cells in culture, in vivo: polyethylenimine. Proc Natl Acad Sci USA 92:7297–7301.
- Brown DM, Wilson MR, MacNee W, Stone V, Donaldson K. 2001. Size-dependent proinflammatory effects of ultrafine polystyrene particles: a role for surface area, oxidative stress in the enhanced activity of ultrafines. Toxicol Appl Pharmacol 175:191–199.
- Buehler PW, Vallelian F, Mikolajczyk MG, Schoedon G, Schweizer T, Alayash AI, Schaer DJ. 2008. Structural stabilization in tetrameric or polymeric hemoglobin determines its interaction with endogenous antioxidant scavenger pathways. Antioxid Redox Signal 10:1449–1462.
- Byrne JD, Betancourt T, Brannon-Peppas L. 2008. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev 60:1615–1626.
- Cedervall T, Lynch I, Lindman S, Berggard T, Thulin E, Nilsson H, Dawson KA, Linse S. 2007. Understanding the nanoparticle-protein corona using methods to quantify exchange rates, affinities of proteins for nanoparticles. Proc Natl Acad Sci USA 104:2050–2055.
- Champion JA, Katare YK, Mitragotri S. 2007. Making polymeric micro-, nanoparticles of complex shapes. Proc Natl Acad Sci USA 104:11901–11904.
- Champion JA, Mitragotri S. 2006. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA 103:4930–4934.
- Chan JM, Zhang L, Tong R, Ghosh D, Gao W, Liao G, Yuet KP, Gray D, Rhee JW, Cheng J, Golomb G, Libby P, Langer R, Farokhzad OC. 2010. Spatiotemporal controlled delivery of nanoparticles to injured vasculature. Proc Natl Acad Sci USA 107:2213–2218.
- Charoenphol P, Huang RB, Eniola-Adefeso O. 2010. Potential role of size, hemodynamics in the efficacy of vascular-targeted spherical drug carriers. Biomaterials 31:1392–1402.
- Chen B, Pogue BW, Hoopes PJ, Hasan T. 2006. Vascular, cellular targeting for photodynamic therapy. Crit Rev Eukaryot Gene Expr 16:279–305.
- Cheng Y, A CS, Meyers JD, Panagopoulos I, Fei B, Burda C. 2008. Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer. J Am Chem Soc 130:10643–10647.
- Cheng Y, Samia AC, Li J, Kenney ME, Resnick A, Burda C. 2009. Delivery, efficacy of a cancer drug as a function of the bond to the gold nanoparticle surface. Langmuir 26:2248–2255.
- Chithrani BD, Ghazani AA, Chan WC. 2006. Determining the size, shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett 6:662–668.
- Cho Y, Shi R, Borgens RB, Ivanisevic A. 2008. Functionalized mesoporous silica nanoparticle-based drug delivery system to rescue acrolein-mediated cell death. Nanomedicine 3:507–519.
- Chouly C, Bordenave L, Bareille R, Guerin V, Baquey A, Pouliquen D, Baquey C, Jallet P. 1994. In vitro study of the hemocompatibility of superparamagnetic contrast agent for magnetic resonance imaging. Clin Mater 15:293–301.
- Coleman L. 2008. Nutrition, rheumatic disease (nutrition, health). Totowa, NJ: Humana Press.
- Cotter MJ, Muruve DA. 2005. The induction of inflammation by adenovirus vectors used for gene therapy. Front Biosci 10:1098–1105.
- Davi G, Patrono C. 2007. Platelet activation, atherothrombosis. N Engl J Med 357:2482–2494.
- Decuzzi P, Godin B, Tanaka T, Lee SY, Chiappini C, Liu X, Ferrari M. 2010. Size, shape effects in the biodistribution of intravascularly injected particles. J Control Release 141:320–327.
- Decuzzi P, Pasqualini R, Arap W, Ferrari M. 2009. Intravascular delivery of particulate systems: does geometry really matter? Pharm Res 26:235–243.
- Demos SM, Alkan-Onyuksel H, Kane BJ, Ramani K, Nagaraj A, Greene R, Klegerman M, McPherson DD. 1999. In vivo targeting of acoustically reflective liposomes for intravascular, transvascular ultrasonic enhancement. J Am Coll Cardiol 33:867–875.
- Dhanikula RS, Argaw A, Bouchard JF, Hildgen P. 2008. Methotrexate loaded polyether-copolyester dendrimers for the treatment of gliomas: enhanced efficacy, intratumoral transport capability. Mol Pharm 5:105–116.
- Dhar S, Liu Z, Thomale J, Dai H, Lippard SJ. 2008. Targeted single-wall carbon nanotube-mediated Pt(IV) prodrug delivery using folate as a homing device. J Am Chem Soc 130:11467–11476.
- Diebold Y, Jarrin M, Saez V, Carvalho EL, Orea M, Calonge M, Seijo B, Alonso MJ. 2007. Ocular drug delivery by liposome-chitosan nanoparticle complexes (LCS-NP. Biomaterials 28:1553–1564.
- Discher BM, Won YY, Ege DS, Lee JC, Bates FS, Discher DE, Hammer DA. 1999. Polymersomes: tough vesicles made from diblock copolymers. Science 284:1143–1146.
- Domanski DM, Klajnert B, Bryszewska M. 2004. Influence of PAMAM dendrimers on human red blood cells. Bioelectrochemistry 63:189–191.
- Dos Santos N, Allen C, Doppen AM, Anantha M, Cox KA, Gallagher RC, Karlsson G, Edwards K, Kenner G, Samuels L, Webb MS, Bally MB. 2007. Influence of poly(ethylene glycol) grafting density, polymer length on liposomes: relating plasma circulation lifetimes to protein binding. Biochim Biophys Acta 1768:1367–1377.
- Dziubla TD, Shuvaev VV, Hong NK, Hawkins BJ, Madesh M, Takano H, Simone E, Nakada MT, Fisher A, Albelda SM, Muzykantov VR. 2008. Endothelial targeting of semi-permeable polymer nanocarriers for enzyme therapies. Biomaterials 29:215–227.
- Eckstein EC, Tilles AW, Millero FJ, 3rd. 1988. Conditions for the occurrence of large near-wall excesses of small particles during blood flow. Microvasc Res 36:31–39.
- Einav S, Bluestein D. 2004. Dynamics of blood flow, platelet transport in pathological vessels. Ann N Y Acad Sci 1015:351–366.
- Eniola AO, Krasik EF, Smith LA, Song G, Hammer DA. 2005. I-domain of lymphocyte function-associated antigen-1 mediates rolling of polystyrene particles on ICAM-1 under flow. Biophys J 89:3577–3588.
- Eniola AO, Rodgers SD, Hammer DA. 2002. Characterization of biodegradable drug delivery vehicles with the adhesive properties of leukocytes. Biomaterials 23:2167–2177.
- Everts M, Schraa A, de Leij L, Meijer D, Molema G. 2001. Vascular endothelium in inflamed tissue as a target for site selective delivery of drugs. In: Molema G (ed.) Drug targeting: organ-specific strategies. Germany: Wiley-VCH.
- Fang C, Shi B, Pei YY, Hong MH, Wu J, Chen HZ. 2006. In vivo tumor targeting of tumor necrosis factor-alpha-loaded stealth nanoparticles: effect of MePEG molecular weight, particle size. Eur J Pharm Sci 27:27–36.
- Fenart L, Casanova A, Dehouck B, Duhem C, Slupek S, Cecchelli R, Betbeder D. 1999. Evaluation of effect of charge, lipid coating on ability of 60-nm nanoparticles to cross an in vitro model of the blood-brain barrier. J Pharmacol Exp Ther 291:1017–1022.
- Gaspard J, Hahn MS, Silas JA. 2009. Polymerization of hydrogels inside self-assembled block copolymer vesicles. Langmuir 25:12878–12884.
- Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. 2007. Shape effects of filaments versus spherical particles in flow, drug delivery. Nat Nanotechnol 2:249–255.
- Gentile F, Curcio A, Indolfi C, Ferrari M, Decuzzi P. 2008. The margination propensity of spherical particles for vascular targeting in the microcirculation. J Nanobiotechnology 6:9.
- Gillies ER, Frechet JM. 2005. Dendrimers, dendritic polymers in drug delivery. Drug Discov Today 10:35–43.
- Gimbrone MA, Jr., Topper JN, Nagel T, erson KR, Garcia-Cardena G. 2000. Endothelial dysfunction, hemodynamic forces, atherogenesis. Ann NY Acad Sci 902:230–239; discussion 9–40.
- Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. 2001. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol 19:3312–3322.
- Gosk S, Moos T, Gottstein C, Bendas G. 2008. VCAM-1 directed immunoliposomes selectively target tumor vasculature in vivo. Biochim Biophys Acta 1778:854–863.
- Gratton SE, Pohlhaus PD, Lee J, Guo J, Cho MJ, Desimone JM. 2007. Nanofabricated particles for engineered drug therapies: a preliminary biodistribution study of PRINT nanoparticles. J Control Release 121:10–18.
- Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. 2008. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci USA 105:11613–11618.
- Gurunathan S, Lee KJ, Kalishwaralal K, Sheikpranbabu S, Vaidyanathan R, Eom SH. 2009. Antiangiogenic properties of silver nanoparticles. Biomaterials 30:6341–6350.
- Hajitou A, Pasqualini R, Arap W. 2006. Vascular targeting: recent advances, therapeutic perspectives. Trends Cardiovasc Med 16:80–88.
- Harush-Frenkel O, Rozentur E, Benita S, Altschuler Y. 2008. Surface charge of nanoparticles determines their endocytic, transcytotic pathway in polarized MDCK cells. Biomacromolecules 9:435–443.
- He Q, Zhang J, Shi J, Zhu Z, Zhang L, Bu W, Guo L, Chen Y. 2010. The effect of PEGylation of mesoporous silica nanoparticles on nonspecific binding of serum proteins, cellular responses. Biomaterials 31:1085–1092.
- He XW, Liu T, Xiao Y, Feng YL, Cheng DJ, Tingting G, Zhang L, Zhang Y, Chen YX. 2009. Vascular endothelial growth factor-C siRNA delivered via calcium carbonate nanoparticle effectively inhibits lymphangiogenesis, growth of colorectal cancer in vivo. Cancer Biother Radiopharm 24:249–259.
- Heslinga MJ, Mastria EM, Eniola-Adefeso O. 2009. Fabrication of biodegradable spheroidal microparticles for drug delivery applications. J Control Release 138:235–242.
- Higginbotham JN, Seth P, Blaese RM, Ramsey WJ. 2002. The release of inflammatory cytokines from human peripheral blood mononuclear cells in vitro following exposure to adenovirus variants, capsid. Hum Gene Ther 13:129–141.
- Hoffman GS, Weyand CM. 2002. Inflammatory disease of blood vessels New York, NY: Marcel Dekker.
- Hong S, Leroueil PR, Majoros IJ, Orr BG, Baker JR, Jr., Banaszak Holl MM. 2007. The binding avidity of a nanoparticle-based multivalent targeted drug delivery platform. Chem Biol 14:107–115.
- Hsu SH, Tang CM, Tseng HJ. 2006. Biocompatibility of poly(ether)urethane-gold nanocomposites. J Biomed Mater Res A 79:759–770.
- Jallouli Y, Paillard A, Chang J, Sevin E, Betbeder D. 2007. Influence of surface charge, inner composition of porous nanoparticles to cross blood-brain barrier in vitro. Int J Pharm 344:103–109.
- Jennings LK. 2009. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb Haemost 102:248–257.
- Jiang G, Park K, Kim J, Kim KS, Hahn SK. 2009. Target specific intracellular delivery of siRNA/PEI-HA complex by receptor mediated endocytosis. Mol Pharm 6:727–737.
- Jugdaohsingh R. 2007. Silicon, bone health. J Nutr Health Aging 11:99–110.
- Kagan VE, Bayir H, Shvedova AA. 2005. Nanomedicine, nanotoxicology: two sides of the same coin. Nanomedicine 1:313–316.
- Kaneda MM, Sasaki Y, Lanza GM, Milbrandt J, Wickline SA. 2010. Mechanisms of nucleotide trafficking during siRNA delivery to endothelial cells using perfluorocarbon nanoemulsions. Biomaterials 31:3079–3086.
- Kannan R, Navath R, Kurtoglu Y, Dai H, Wang B, Kannan S, Romero R. 2009. PAMAM dendrimers for brain delivery of therapeutics for the treatment of cerebral palsy: chemistry, in vivo efficacy, imaging. In: 61st AIChE Annual Meeting, 2009, Nashville, TN.
- Kaufmann BA, Sanders JM, Davis C, Xie A, Aldred P, Sarembock IJ, Lindner JR. 2007. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation 116:276–284.
- Kaul G, Amiji M. 2005. Tumor-targeted gene delivery using poly(ethylene glycol)-modified gelatin nanoparticles: in vitro, in vivo studies. Pharm Res 22:951–961.
- Ke W, Shao K, Huang R, Han L, Liu Y, Li J, Kuang Y, Ye L, Lou J, Jiang C. 2009. Gene delivery targeted to the brain using an Angiopep-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. Biomaterials 30:6976–6985.
- Kilpelainen M, Riikonen J, Vlasova MA, Huotari A, Lehto VP, Salonen J, Herzig KH, Jarvinen K. 2009. In vivo delivery of a peptide, ghrelin antagonist, with mesoporous silicon microparticles. J Control Release 137:166–170.
- Kim D, El-Shall H, Dennis D, Morey T. 2005. Interaction of PLGA nanoparticles with human blood constituents. Colloids Surf B Biointerfaces 40:83–91.
- Kirkham M, Parton RG. 2005. Clathrin-independent endocytosis: new insights into caveolae, non-caveolar lipid raft carriers. Biochim Biophys Acta 1745:273–286.
- Koudelka KJ, Destito G, Plummer EM, Trauger SA, Siuzdak G, Manchester M. 2009. Endothelial targeting of cowpea mosaic virus (CPMV) via surface vimentin. PLoS Pathog 5:e1000417.
- Koziara JM, Oh JJ, Akers WS, Ferraris SP, Mumper RJ. 2005. Blood compatibility of cetyl alcohol/polysorbate-based nanoparticles. Pharm Res 22:1821–1828.
- Kratzer I, Wernig K, Panzenboeck U, Bernhart E, Reicher H, Wronski R, Windisch M, Hammer A, Malle E, Zimmer A, Sattler W. 2007. Apolipoprotein A-I coating of protamine-oligonucleotide nanoparticles increases particle uptake, transcytosis in an in vitro model of the blood-brain barrier. J Control Release 117:301–311.
- Kreuter J, Shamenkov D, Petrov V, Ramge P, Cychutek K, Koch-Brandt C, Alyautdin R. 2002. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J Drug Target 10:317–325.
- Kyriakides TR, Cheung CY, Murthy N, Bornstein P, Stayton PS, Hoffman AS. 2002. pH-sensitive polymers that enhance intracellular drug delivery in vivo. J Control Release 78:295–303.
- Lackey CA, Press OW, Hoffman AS, Stayton PS. 2002. A biomimetic pH-responsive polymer directs endosomal release, intracellular delivery of an endocytosed antibody complex. Bioconjug Chem 13:996–1001.
- Langeggen H, Namork E, Johnson E, Hetland G. 2003. HUVEC take up opsonized zymosan particles, secrete cytokines IL-6, IL-8 in vitro. FEMS Immunol Med Microbiol 36:55–61.
- Lee SY, Ferrari M, Decuzzi P. 2009. Design of bio-mimetic particles with enhanced vascular interaction. J Biomech 42:1885–1890.
- Lee SY, Ferrari M, Decuzzi P. 2009. Shaping nano-/micro-particles for enhanced vascular interaction in laminar flows. Nanotechnology 20:495101.
- Lewinski N, Colvin V, Drezek R. 2008. Cytotoxicity of nanoparticles. Small 4:26–49.
- Li SD, Chen YC, Hackett MJ, Huang L. 2008. Tumor-targeted delivery of siRNA by self-assembled nanoparticles. Mol Ther 16:163–169.
- Li SD, Huang L. 2009. Nanoparticles evading the reticuloendothelial system: role of the supported bilayer. Biochim Biophys Acta 1788:2259–2266.
- Lim J, Guo Y, Rostollan CL, Stanfield J, Hsieh JT, Sun X, Simanek EE. 2008. The role of the size, number of polyethylene glycol chains in the biodistribution, tumor localization of triazine dendrimers. Mol Pharm 5:540–547.
- Liu J, Jiang X, Ashley C, Brinker CJ. 2009. Electrostatically mediated liposome fusion, lipid exchange with a nanoparticle-supported bilayer for control of surface charge, drug containment, delivery. J Am Chem Soc 131:7567–7569.
- Ljubimova JY, Fujita M, Khazenzon NM, Lee BS, Wachsmann-Hogiu S, Farkas DL, Black KL, Holler E. 2008. Nanoconjugate based on polymalic acid for tumor targeting. Chem Biol Interact 171:195–203.
- Lu W, Sun Q, Wan J, She Z, Jiang XG. 2006. Cationic albumin-conjugated pegylated nanoparticles allow gene delivery into brain tumors via intravenous administration. Cancer Res 66:11878–11887.
- Lu W, Tan YZ, Hu KL, Jiang XG. 2005. Cationic albumin conjugated pegylated nanoparticle with its transcytosis ability, little toxicity against blood-brain barrier. Int J Pharm 295:247–260.
- Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. 2008. Nanoparticle size, surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci USA 105:14265–14270.
- Manchester M, Singh P. 2006. Virus-based nanoparticles (VNPs): platform technologies for diagnostic imaging. Adv Drug Deliv Rev 58:1505–1522.
- Mayer A, Vadon M, Rinner B, Novak A, Wintersteiger R, Frohlich E. 2009. The role of nanoparticle size in hemocompatibility. Toxicology 258:139–147.
- Migliorini C, Qian Y, Chen H, Brown EB, Jain RK, Munn LL. 2002. Red blood cells augment leukocyte rolling in a virtual blood vessel. Biophys J 83:1834–1841.
- Miyamoto M, Sasakawa S, Ozawa T, Kawaguchi H, Ohtsuka Y. 1989. Platelet aggregation induced by latex particles. I. Effects of size, surface potential, hydrophobicity of particles. Biomaterials 10:251–257.
- Movat HZ, Weiser WJ, Glynn MF, Mustard JF. 1965. Platelet phagocytosis, aggregation. J Cell Biol 27:531–543.
- Munn LL, Melder RJ, Jain RK. 1996. Role of erythrocytes in leukocyte-endothelial interactions: mathematical model, experimental validation. Biophys J 71:466–478.
- Muro S, Gajewski C, Koval M, Muzykantov VR. 2005. ICAM-1 recycling in endothelial cells: a novel pathway for sustained intracellular delivery, prolonged effects of drugs. Blood 105:650–658.
- Muro S, Garnacho C, Champion JA, Leferovich J, Gajewski C, Schuchman EH, Mitragotri S, Muzykantov VR. 2008. Control of endothelial targeting, intracellular delivery of therapeutic enzymes by modulating the size, shape of ICAM-1-targeted carriers. Mol Ther 16:1450–1458.
- Murphy EA, Majeti BK, Barnes LA, Makale M, Weis SM, Lutu-Fuga K, Wrasidlo W, Cheresh DA. 2008. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proc Natl Acad Sci USA 105:9343–9348.
- Muzykantov VR. 2001. Targeting of superoxide dismutase, catalase to vascular endothelium. J Control Release 71:1–21.
- Nemmar A, Melghit K, Ali BH. 2008. The acute proinflammatory, prothrombotic effects of pulmonary exposure to rutile TiO2 nanorods in rats. Exp Biol Med (Maywood) 233:610–619.
- Nishikawa T, Iwakiri N, Kaneko Y, Taguchi A, Fukushima K, Mori H, Morone N, Kadokawa J. 2009. Nitric oxide release in human aortic endothelial cells mediated by delivery of amphiphilic polysiloxane nanoparticles to caveolae. Biomacromolecules 10:2074–2085.
- Ondaral S, Wagberg L, Enarsson LE. 2006. The adsorption of hyperbranched polymers on silicon oxide surfaces. J Colloid Interface Sci 301:32–39.
- Pack DW, Putnam D, Langer R. 2000. Design of imidazole-containing endosomolytic biopolymers for gene delivery. Biotechnol Bioeng 67:217–223.
- Pappu V, Bagchi P. 2007. Hydrodynamic interaction between erythrocytes, leukocytes affects rheology of blood in microvessels. Biorheology 44:191–215.
- Park JH, Gu L, von Maltzahn G, Ruoslahti E, Bhatia SN, Sailor MJ. 2009. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat Mater 8:331–336.
- Partlow KC, Lanza GM, Wickline SA. 2008. Exploiting lipid raft transport with membrane targeted nanoparticles: a strategy for cytosolic drug delivery. Biomaterials 29:3367–3375.
- Patil Y, Sadhukha T, Ma L, Panyam J. 2009. Nanoparticle-mediated simultaneous, targeted delivery of paclitaxel, tariquidar overcomes tumor drug resistance. J Control Release 136:21–29.
- Pearson MJ, Lipowsky HH. 2000. Influence of erythrocyte aggregation on leukocyte margination in postcapillary venules of rat mesentery. Am J Physiol Heart Circ Physiol 279:H1460–471.
- Pedro L, Soares SS, Ferreira GNM. 2008. Purification of bionanoparticles. Chem Eng Technol 31:815–825.
- Peters D, Kastantin M, Kotamraju VR, Karmali PP, Gujraty K, Tirrell M, Ruoslahti E. 2009. Targeting atherosclerosis by using modular, multifunctional micelles. Proc Natl Acad Sci USA 106:9815–9819.
- Peters K, Unger RE, Kirkpatrick CJ, Gatti AM, Monari E. 2004. Effects of nano-scaled particles on endothelial cell function in vitro: studies on viability, proliferation, inflammation. J Mater Sci Mater Med 15:321–325.
- Photos PJ, Bacakova L, Discher B, Bates FS, Discher DE. 2003. Polymer vesicles in vivo: correlations with PEG molecular weight. J Control Release 90:323–334.
- Radomski A, Jurasz P, Alonso-Escolano D, Drews M, Morandi M, Malinski T, Radomski MW. 2005. Nanoparticle-induced platelet aggregation, vascular thrombosis. Br J Pharmacol 146:882–893.
- Reddy GR, Bhojani MS, McConville P, Moody J, Moffat BA, Hall DE, Kim G, Koo YE, Woolliscroft MJ, Sugai JV, Johnson TD, Philbert MA, Kopelman R, Rehemtulla A, Ross BD. 2006. Vascular targeted nanoparticles for imaging, treatment of brain tumors. Clin Cancer Res 12:6677–6686.
- Rejman J, Bragonzi A, Conese M. 2005. Role of clathrin-, caveolae-mediated endocytosis in gene transfer mediated by lipo-, polyplexes. Mol Ther 12:468–474.
- Rejman J, Oberle V, Zuhorn IS, Hoekstra D. 2004. Size-dependent internalization of particles via the pathways of clathrin-, caveolae-mediated endocytosis. Biochem J 377:159–169.
- Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. 2005. Direct fabrication, harvesting of monodisperse, shape-specific nanobiomaterials. J Am Chem Soc 127:10096–10100.
- Ross JS, Stagliano NE, Donovan MJ, Breitbart RE, Ginsburg GS. 2001. Atherosclerosis, cancer: common molecular pathways of disease development, progression. Ann NY Acad Sci 947:271–292; discussion 292–3.
- Sakhalkar HS, Dalal MK, Salem AK, Ansari R, Fu J, Kiani MF, Kurjiaka DT, Hanes J, Shakesheff KM, Goetz DJ. 2003. Leukocyte-inspired biodegradable particles that selectively, avidly adhere to inflamed endothelium in vitro, in vivo. Proc Natl Acad Sci USA 100:15895–15900.
- Salonen J, Kaukonen AM, Hirvonen J, Lehto VP. 2008. Mesoporous silicon in drug delivery applications. J Pharm Sci 97:632–653.
- Schwendener RA. 2007. Liposomes in biology, medicine. Adv Exp Med Biol 620:117–128.
- Serda RE, Gu J, Bhavane RC, Liu X, Chiappini C, Decuzzi P, Ferrari M. 2009. The association of silicon microparticles with endothelial cells in drug delivery to the vasculature. Biomaterials 30:2440–2448.
- Shcharbin D, Pedziwiatr E, Blasiak J, Bryszewska M. 2009. How to study dendriplexes II: transfection, cytotoxicity. J Control Release 141:110–127.
- Shi X, Wang S, Meshinchi S, Van Antwerp ME, Bi X, Lee I, Baker JR, Jr. 2007. Dendrimer-entrapped gold nanoparticles as a platform for cancer-cell targeting, imaging. Small 3:1245–1252.
- Shrivastava S, Bera T, Singh SK, Singh G, Ramachandrarao P, Dash D. 2009. Characterization of antiplatelet properties of silver nanoparticles. ACS Nano 3:1357–1364.
- Shriver LP, Koudelka KJ, Manchester M. 2009. Viral nanoparticles associate with regions of inflammation, blood brain barrier disruption during CNS infection. J Neuroimmunol 211:66–72.
- Simone E, Ding BS, Muzykantov V. 2009. Targeted delivery of therapeutics to endothelium. Cell Tissue Res 335:283–300.
- Simone EA, Dziubla TD, Discher DE, Muzykantov VR. 2009. Filamentous polymer nanocarriers of tunable stiffness that encapsulate the therapeutic enzyme catalase. Biomacromolecules 10:1324–1330.
- Smith BR, Heverhagen J, Knopp M, Schmalbrock P, Shapiro J, Shiomi M, Moldovan NI, Ferrari M, Lee SC. 2007. Localization to atherosclerotic plaque, biodistribution of biochemically derivatized superparamagnetic iron oxide nanoparticles (SPIONs) contrast particles for magnetic resonance imaging (MRI). Biomed Microdevices 9:719–727.
- Sonavane G, Tomoda K, Makino K. 2008. Biodistribution of colloidal gold nanoparticles after intravenous administration: effect of particle size. Colloids Surf B Biointerfaces 66:274–280.
- Sonawane ND, Szoka FC, Jr., Verkman AS. 2003. Chloride accumulation, swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem 278:4826–4831.
- Southworth R, Kaneda M, Chen J, Zhang L, Zhang H, Yang X, Razavi R, Lanza G, Wickline SA. 2009. Renal vascular inflammation induced by Western diet in ApoE-null mice quantified by (19)F NMR of VCAM-1 targeted nanobeacons. Nanomedicine 5:359–367.
- Srinivasan R, Marchant RE, Gupta AS. 2009. In vitro, in vivo platelet targeting by cyclic RGD-modified liposomes. J Biomed Mater Res A 93:1004–1015.
- Stan RV. 2006. Endocytosis pathways in endothelium: how many? Am J Physiol Lung Cell Mol Physiol 290:L806–808.
- Stevens KN, Crespo-Biel O, van den Bosch EE, Dias AA, Knetsch ML, Aldenhoff YB, van der Veen FH, Maessen JG, Stobberingh EE, Koole LH. 2009. The relationship between the antimicrobial effect of catheter coatings containing silver nanoparticles, the coagulation of contacting blood. Biomaterials 30:3682–3690.
- Strother R, Matei D. 2009. Pegylated liposomal doxorubicin in ovarian cancer. Ther Clin Risk Manag 5:639–650.
- Sutton D, Nasongkla N, Blanco E, Gao J. 2007. Functionalized micellar systems for cancer targeted drug delivery. Pharm Res 24:1029–1046.
- Takeda M, Tada H, Higuchi H, Kobayashi Y, Kobayashi M, Sakurai Y, Ishida T, Ohuchi N. 2008. In vivo single molecular imaging, sentinel node navigation by nanotechnology for molecular targeting drug-delivery systems, tailor-made medicine. Breast Cancer 15:145–52.
- Tasciotti E, Liu X, Bhavane R, Plant K, Leonard AD, Price BK, Cheng MM, Decuzzi P, Tour JM, Robertson F, Ferrari M. 2008. Mesoporous silicon particles as a multistage delivery system for imaging, therapeutic applications. Nat Nanotechnol 3:151–157.
- Traub LM. 2009. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol 10:583–596.
- Uyar D, Kulp B, Peterson G, Zanotti K, Markman M, Belinson J. 2004. Cardiac safety profile of prolonged (≥6 cycles) pegylated liposomal doxorubicin administration in patients with gynecologic malignancies. Gynecol Oncol 94:147–151.
- Vargas A, Eid M, Fanchaouy M, Gurny R, Delie F. 2008. In vivo photodynamic activity of photosensitizer-loaded nanoparticles: formulation properties, administration parameters, biological issues involved in PDT outcome. Eur J Pharm Biopharm 69:43–53.
- Voinea M, Manduteanu I, Dragomir E, Capraru M, Simionescu M. 2005. Immunoliposomes directed toward VCAM-1 interact specifically with activated endothelial cells – a potential tool for specific drug delivery. Pharm Res 22:1906–1917.
- Wagner DD, Frenette PS. 2008. The vessel wall, its interactions. Blood 111:5271–5281.
- Wang P, Xue Y, Shang X, Liu Y. 2009. Diphtheria toxin mutant CRM197-mediated transcytosis across blood-brain barrier in vitro. Cell Mol Neurobiol 30:717–725.
- Wilhelm C, Bal L, Smirnov P, Galy-Fauroux I, Clement O, Gazeau F, Emmerich J. 2007. Magnetic control of vascular network formation with magnetically labeled endothelial progenitor cells. Biomaterials 28:3797–3806.
- Winter PM, Neubauer AM, Caruthers SD, Harris TD, Robertson JD, Williams TA, Schmieder AH, Hu G, Allen JS, Lacy EK, Zhang H, Wickline SA, Lanza GM. 2006. Endothelial alpha(v)beta3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler Thromb Vasc Biol 26:2103–2109.
- Wiwanitkit V, Sereemaspun A, Rojanathanes R. 2009. Gold nanoparticles, a microscopic view of platelets: a preliminary observation. Cardiovasc J Afr 20:141–142.
- Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK. 1995. Vascular permeability in a human tumor xenograft: molecular size dependence, cutoff size. Cancer Res 55:3752–3756.
- Zensi A, Begley D, Pontikis C, Legros C, Mihoreanu L, Wagner S, Buchel C, von Briesen H, Kreuter J. 2009. Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis, are delivered to neurones. J Control Release 137:78–86.
- Zhang N, Chittasupho C, Duangrat C, Siahaan TJ, Berkland C. 2008. PLGA nanoparticle–peptide conjugate effectively targets intercellular cell-adhesion molecule-1. Bioconjug Chem 19:145–152.
- Zhu J, Xue J, Guo Z, Zhang L, Marchant RE. 2007. Biomimetic glycoliposomes as nanocarriers for targeting P-selectin on activated platelets. Bioconjug Chem 18:1366–1369.