Abstract
This short review takes into consideration the status of lipidomics as issued from almost a decade of development. Because of the huge number of molecular species analyzed, there is a trend in subdividing lipidomics according to subdomains, in particular relating to the function of molecules. It is also pointed out that lipid imaging without the use of exogenous probes will help making relationships between molecular structures and the topography of lipid assemblies, especially in cellular compartments. Finally, a fluxomics approach is proposed for lipid molecular species, both in terms of compartments and biochemical metabolism. The example of fluxolipidomics of essential fatty acids toward their enzyme-dependent oxygenated metabolites and further toward their degradation products is developed.
Introduction
Lipidomics is a term that has appeared in publication titles from the year 2003. Since that time, more than 200 articles including ‘lipidomics’ in their title have been published. Lipidomics can be considered as part of metabolomics, if we consider that membrane lipid components are metabolites although they are first structural entities, just as proteins of which the exhaustive study called proteomics is not classically included in metabolomics.
Over this almost decade, the definition of lipidomics has evolved, depending on the actors in the field. It could be considered as the full characterization of lipid species of a biological system. One may however include studies on proteins involved in lipid metabolism and function, and even functionally-associated genes. This enlarged concept of lipidomics is indeed quite ambitious because of the huge diversity of lipid molecular species in life. We then see the use of some sub-domains allowing deeper studies of a specific field, such as structural lipidomics, mediator lipidomics, etc. Also, lipidomics of some biological systems such as the brain, plants and prokaryotes would merit further investigation. Imaging lipids is also an approach which is mostly underdeveloped. Finally, we should realize that most of the analyses done, whatever their extent, concern restricted compartments at a given time. This is not enough to be fully relevant to metabolism that is always dynamic, and fluxes of lipid entities in function of time, between biological compartments and in the precursor-product relationships, must be taken into consideration in the future. This brief review intends to cover these issues to envision what could be the future of lipidomics.
Splitting lipidomics into sub-domains
The first publication stating lipidomics in its title (Han and Gross Citation2003) and two editorials published in the same year (Lagarde et al. Citation2003, Spener et al. Citation2003) considered a global and full characterization of lipids extracted from a biological system. This approach has been clearly used within the Lipid Maps initiative (Schmelzer et al. Citation2007). This has been applied with success to macrophages and their different membrane systems (Andreyev et al. Citation2010), and human plasma (Quehenberger et al. Citation2010). However the ten thousands of lipid molecules already known have pushed investigators to address their queries to more restricted fields in order to be more specific and allow discovering new molecular entities. Then, articles have been published on structural lipidomics that deals essentially with membrane lipids (Balogh et al. Citation2010, Gross and Han Citation2011). This approach reveals many differences in cells belonging to the same biological system as observed with blood cells (Leidl et al. Citation2008). Others are focused on storage lipids, e.g., triacylglycerols in hepatocytes (Hu et al. Citation2010) and adipocytes (Caesar et al. Citation2010).
More targeted lipidomics has been used toward a restricted class of molecules such as lipid mediators issued from polyunsaturated fatty acids (Serhan et al. Citation2007, Nicolaou et al. Citation2009). This targeted lipidomics can easily be addressed to a functional domain (Gross et al. Citation2005, Vellani et al. Citation2008) or metabolism (Guichardant et al. Citation2011) and called functional lipidomics.
We may guess however that classical lipidomics will be more and more attached to diverse applications with promising future such as in health and diseases (Shevchenko and Simons Citation2010), e.g., brain lipidomics that is strongly developing (Piomelli et al. Citation2007, Sparvero et al. Citation2010). Yet, some biological domains such as plants and prokaryotes have not much benefited from lipidomics approaches. Plant lipidomics has begun (Welti et al. Citation2007) but we hardly found lipidomics studies applied to prokaryotes, although the diversity is there as we may anticipate from the example of lipopolysaccharides (Wilkinson Citation1996).
Lipid imaging
An attractive in situ approach to complement lipidomics consists in imaging the various lipids of a biological system. A classical way is to develop fluorescent probes that are more or less specific of one class of structural lipids, especially membrane lipids. A well known probe is BODIPY, of which the core of ‘4,4-difluoro-4-bora-3a,4a-diaza-s-indacene’ is derivatized for different applications. A classical one is to covalently couple BODIPY with various lipids that could then be followed in cell compartments (Ariola et al. Citation2006, Khatchadourian et al. Citation2009, Wüstner et al. Citation2011). This approach has been improved with the use of a two-color fluorophore to image lipid droplets motility along microtubules (Spandl et al. Citation2009). One limitation of course is the structure alteration of such lipids by the probe. Alternatively, probes showing specific binding in situ to the target lipid could be used, e.g., lysenin, a worm toxin protein recently characterized as a specific ligand to sphingomyelin (Ishitsuka et al. Citation2004). Combined with cholera toxin that specifically binds ganglioside M1, this made possible to visualize the distribution of lipid rafts at the surface of living cells (Kiyokawa et al. Citation2005).
Investigators are now focusing on imaging lipids by direct observation in using improved microscopy methods or even mass spectroscopy on membranes. Among the various approaches, Brewster Angle Microscopy (BAM) allows to visualize clusters of phospholipids according to their composition and the interactions with proteins. As a matter of fact, BAM has been used with success to study the interaction of anti-apoptotic proteins with phospholipid Langmuir layers mimicking the mitochondrial inner membrane (Guillemin et al. Citation2010). This clearly showed that cardiolipin allows the clustering of phospholipids in the presence of the anti-apoptotic proteins.
New microscopy approaches allow visualizing lipid accumulation in space, without any dye or probe. The most promising is likely to be the Coherent Anti-Stokes Raman Scattering (CARS), that is a nonlinear optical version of Raman scattering, based on C-H bond stretches. Lipids being especially rich in those C–H bonds, the method is quite sensitive to dynamically track lipids in living systems, even in depth such as 0.1 mm. Vibrations of C–H bonds (2850 cm-1) being very different from those of C-D (2100 cm-1), deuterium-labeled lipids can easily be distinguished from endogenous ones in a pre-labeled system. An excellent review can be seen for the use of this technique for lipid imaging (Pezacki et al. Citation2011). It has been successfully used for quantitatively measuring the accumulation of lipid droplets (Hellerer et al. Citation2007), and the accumulation of lipids in atherosclerotic aorta (Lim et al. Citation2010).
Among the other non-invasive techniques to measure the accumulation of fat, magnetic resonance imaging (MRI) seems to be the most accurate as recently described to quantitate the liver fat (Reeder et al. Citation2011)
Mass spectrometric methods may also be used for lipid imaging at the surface of slices from living structures. Matrix-Assisted Laser Desorption Ionization – Imaging Mass Spectrometry (MALDI-IMS) seems to be the most popular approach to localize various lipids in two-dimensions. MALDI-IMS has been used with success for localizing phospholipids and glycolipids in the brain, and fatty acids in retinal sections (Murphy et al. Citation2009, Goto-Inoue et al. Citation2011). Other detection modes, such as the Time-Of-Flight Secondary Ion Mass Spectrometry, and others, can be applied as well to lipids. They have been compared in a recent review (Touboul et al. Citation2011) to point out its high resolution for imaging various lipids at the surface of tissue samples.
Fluxolipidomics
It is clear that the extensive analysis of metabolites in the metabolomics approach is not enough to understand the phenotype of a living system. Characterization of their metabolic network requires information on the fluxes of those metabolites. Studying fluxes associated to metabolomics has been considered as part of the systems biology and named fluxomics (Cascante and Marin Citation2008). Fluxolipidomics is then focusing to lipids, which is already a big task due to the huge number of lipid metabolites and compartments.
To illustrate this issue, we chose the field of lipid nutrition that is closely associated with part of mediator lipidomics, i.e., essential fatty acids and their various enzyme-dependent metabolites.
Linoleic and linolenic acids, essential polyunsaturated fatty acids (PUFA) precursors in mammals, may be converted in vivo by successive desaturation and elongation steps ().
Figure 1. Main desaturation and elongation pathways of essential polyunsaturated fatty acid precursors of the omega-6 and omega-3 series. Δ: desaturation; E: elongation; β-ox: β-oxidation.
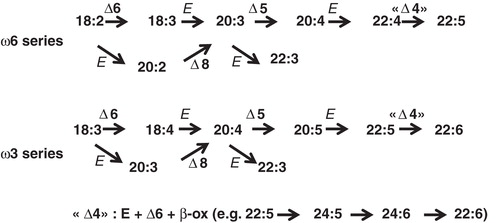
However, the rate of conversion is quite variable according to the PUFA considered. The use of 13C-labelled linolenic acid (18:3ω3) in humans allowed to evaluate its conversion into eicosapentaenoic acid (20:5ω3) and further to docosahexaenoic acid (22:6ω3). This conversion was in the order of 10% for each product, which means that approximately 1% of 22:6ω3 could be produced from 18:3ω3 (Burdge Citation2004). Then 20:5ω3 and especially 22:6ω3 must mainly be from exogenous sources, being notably consumed through marine lipids intake, to achieve the nutritional recommendations. Simultaneous studies on the conversion of 13C-linoleic acid (18:2ω6) into arachidonic (20:4ω6) and even adrenic (22:4ω6) acids have more recently been done, in presence of variable intakes of unlabelled precursors in flaxeed oil. The rate conversion of the precursors was affected by such intakes (Hussein et al. Citation2005). However, the overall metabolic fate of the two precursors, including the appearance of adrenic acid from arachidonic acid, remains to be evaluated. Beyond these well known members of the omega-3 and –6 families, very long chain PUFA have been reported in sperms (ω6) and the retina (ω3) (Poulos Citation1995), especially since new data have been collected on elongase isoforms (Agbaga et al. Citation2010).
In addition to the desaturation and elongation of essential C18 fatty acids, the downstream oxygenated metabolites known from 18:2, 18:3, 20:4, 20:5 and 22:6, have never been taken into consideration in these fluxomics studies ( summarizes the main enzyme-dependent oxygenated metabolites from those PUFA). Complete reviews on those metabolites can be found in several references (Samuelsson Citation1978, Citation1986, Lagarde Citation1988, Lagarde et al. Citation1989, Smith Citation2008, Spector Citation2009). The various oxygenated products of oxygenases can be further metabolized by different ways. A common fate of prostanoids is the NAD-dependent oxidation by 15-PG dehydrogenase (15-PGDH), expressed in large amount on the luminal side of pulmonary capillary endothelium (Ensor and Tai Citation1995, Tai et al. Citation2006), and now considered as a target in cancer therapy (Na et al. Citation2011). The corresponding oxo derivatives of PGs have reduced activities, but those from hydroxy-eicosatetratenoic acids (HETE), especially 5-oxo-ETE (Powell and Rokach Citation2005), have changed activity profiles. The dehydrogenation of hydroxylated products from PUFA () may be then considered as a way to generate new bioactive derivatives.
Figure 2. Known oxygenation pathways from the various polyunsaturated fatty acids. (A) Di-oxygenation of 18:2ω6/LA and 18:3ω3/ALA by 15-/ω6 lipoxygenase (Lox) followed by reduction with gluthatione peroxidase (GPx). Aborted oxygenation of LA and ALA by the inducible cyclooxygenase (Cox-2) is also mentioned. HODE: hydroxy-octadeca-dienoate; HOTE: hydroxy-octadeca-trienoate. (B) Di-oxygenation of 20:4ω6/ARA by cyclooxygenases (Cox-1/2), and isomerization of the intermediate PGH2 by prostaglandin synthases (PG-S) and thromboxane synthase (Tx-S), and by lipoxygenase (Lox) with further reduction of the products by GPx. PG: prostaglandin; Tx: thromboxane; HHT: hydroxy-heptadeca-trienoate; LT: leukotriene; HETE: hydroxy-eicosa-tetraenoate; GSH: reduced glutathione; Glu: glutamate. (C) Di-oxygenation of 20:5ω3/EPA. HEPE: hydroxy-eicosapenta-enoate. (D) Di-oxygenation of 22:6ω3/DHA. HDoHE: hydroxy-docosahexa-enoate. (E) Resolvins from 20:5ω3/EPA and 22:6ω3/DHA. (F) Cytochrome P450-dependent mono-oxygenation of 20:4ω6/ARA. EET: epoxy-eicosa-trienoate.
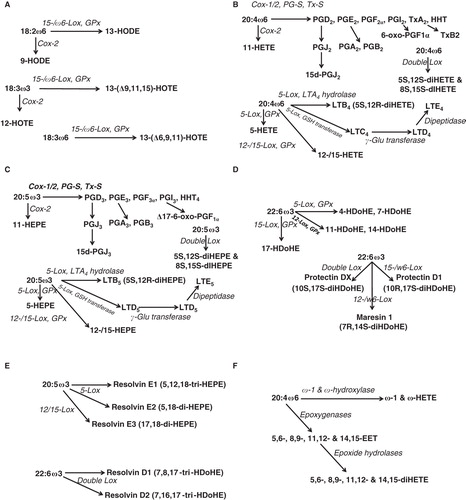
Figure 3. Main degradation pathways of oxygenated derivatives from PUFA. See previous legend in ; nor: minus one carbon compared to oxygenated products from PUFA, then beta-oxidation of eicosanoids converts these fatty acids into dinor and tetranor derivatives.
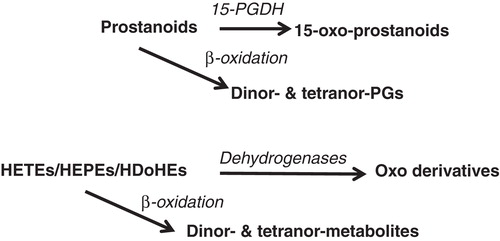
Another important metabolism of oxygenated products of PUFA is their partial beta-oxidation, leading for example to dinor- and tetranor-PGs (Diczfalusy Citation1994). Indeed, measuring those metabolites in urine is assumed to better reflect PGs level in circulation (Song et al. Citation2008).
A series of di- and tri-hydroxylated derivatives from PUFA have more recently been described with various potential anti-atherogenic properties through anti-inflammatory and anti-aggregatory effects. These products, from the long chain omega-3 PUFA 20:5ω3 and 22:6ω3, have been named protectins, resolvins and maresins (Serhan et al. Citation2007, Citation2008, Serhan Citation2009, Chen et al. Citation2011), a new resolvin E isomer (resolvin E3) having been just described from EPA (Isobe et al. Citation2012) (). The beta-oxidation of those products has not been reported yet, but it is likely that it is occurring in the animal body as it has been reported for HETEs (Spector et al. Citation1994).
In addition to all these dioxygenase (cyclooxygenases and lipoxygenases) products from PUFA, monoxygenation has been widely described with the production of a series of epoxy-eicosa-trienoic acids (EETs) as an example (Imig Citation2005, Spector and Norris Citation2007, Spector Citation2009), and their conversion by epoxide hydrolases (Fleming Citation2011, Pfister et al. Citation2010). The monooxygenases responsible for the production of epoxy derivatives are cytochrome P450 enzymes. Other enzymes of this type are omega and omega-1 hydroxylases reported as acting upon PUFA (Imai et al. Citation1989, Capdevila et al. Citation2000) (). It is likely that all the oxygenated products of dioxygenases, eicosanoids, docosanoids and octadecanoids are possibly metabolized by omega hydroxylases as it has been first described for leukotriene B4 (Hammarström et al. Citation1985).
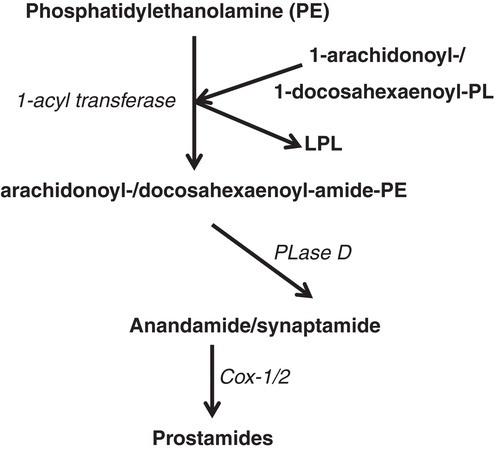
In addition to these various oxygenated derivatives of PUFA, anandamide, an endocannabinoid from 20:4ω6 has been described as an ethanolamide product (arachidonoyl-ethanolamide) issued from the phospholipase D cleavage of N-arachidonoyl-phosphatidylethanolamine (da Veiga et al. Citation2008). Anandamide exhibits cannabinoid effects in the brain and peripheral tissues through seven trans-membrane receptors (Sun et al. Citation2004). Anandamide can also be converted through the cyclooxygenase pathway into the so-called prostamide derivatives (Yang et al. Citation2005). 22:6ω3 has also been reported to be covalently bound to ethanolamine via the same biological process (Bisogno et al. Citation1999), and more recently named synaptamide because of its effect upon neurogenesis (Kim et al. Citation2011). shows the metabolism of 20:4ω6 and 22:6ω3 through the endocannabinoid pathway.
Figure 4. Anandamide and synaptamide are the N-acyl-ethanolamide derivatives from 20:4ω6/ARA and 22:6ω3/DHA, respectively. PE: phosphatidyl-ethanolamine; PL: phospholipid; LPL: lysophospholipid; PLase: phospholipase.
This quick overview clearly shows that a huge number of primary and secondary metabolites can be produced from essential fatty acids, the bulk being oxygenated derived compounds. Fluxolipidomics of this big family is hard to be completed, although the Lipid Maps consortium led by E.A. Dennis has developed this approach for eicosanoids (Gupta et al. Citation2009). However, a comprehensive view of metabolic fluxes regarding the products of 18:2ω6 or 18:3ω3 or both is desirable, although quite ambitious.
Conclusion
Enormous investigation remains to be done for mapping the lipid molecular species of the various biological systems. This lipidomics approach will certainly gain from targeting the biological systems analyzed on the basis of biological functions to make sense and avoid blind mapping that would inevitably and considerably increase the entropy in the data bank. Complementary studies on appropriate lipid imaging will allow organizing the numerous molecular species in space, and increase the meaningful of the lipidomics approach. Last but not least, the still life data gathered by these combined approaches will become lively by determining fluxes between lipid metabolites and lipid compartments. Such a fluxolipidomics will hopefully allow assessing the dynamics of lipids in the biological systems.
Acknowledgements
The authors thank the support from INSA-Lyon, INSERM, the LISA Carnot Institute, and the IBiSA GIS through the Functional Lipidomics platform.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Agbaga MP, Mandal MN, Anderson RE. 2010. Retinal very long-chain PUFAs: New insights from studies on ELOVL4 protein. J Lipid Res 51:1624–1642.
- Andreyev AY, Fahy E, Guan Z, Kelly S, Li X, McDonald JG, 2010. Subcellular organelle lipidomics in TLR-4-activated macrophages. J Lipid Res 51:2785–2797.
- Ariola FS, Mudaliar DJ, Walvick RP, Heikal AA. 2006. Dynamics imaging of lipid phases and lipid-marker interactions in model biomembranes. Phys Chem Chem Phys 8:4517–4529.
- Balogh G, Péter M, Liebisch G, Horváth I, Török Z, Nagy E, 2010. Lipidomics reveals membrane lipid remodelling and release of potential lipid mediators during early stress responses in a murine melanoma cell line. Biochim Biophys Acta 1801:1036–1047.
- Bisogno T, Delton-Vandenbroucke I, Milone A, Lagarde M, Di Marzo V. 1999. Biosynthesis and inactivation of N-arachidonoylethanolamine (anandamide) and N-docosahexaenoylethanolamine in bovine retina. Arch Biochem Biophys 370:300–307.
- Burdge G. 2004. Alpha-linolenic acid metabolism in men and women: Nutritional and biological implications. Curr Opin Clin Nutr Metab Care 7:137–144.
- Caesar R, Manieri M, Kelder T, Boekschoten M, Evelo C, Müller M, 2010. A combined transcriptomics and lipidomics analysis of subcutaneous, epididymal and mesenteric adipose tissue reveals marked functional differences. PLoS One 5:e11525.
- Capdevila JH, Falck JR, Harris RC. 2000. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J Lipid Res 41:163–181.
- Cascante M, Marin S. 2008. Metabolomics and fluxomics approaches. Essays Biochem 45:67–81.
- Chen P, Véricel E, Lagarde M, Guichardant M. 2011. Poxytrins, a class of oxygenated products from polyunsaturated fatty acids, potently inhibit blood platelet aggregation. FASEB J 25:382–388.
- da Veiga MA, Fonseca Bloise F, Costa-E-Sousa RH, Souza LL, Almeida NA, Oliveira KJ, 2008. Acute effects of endocannabinoid anandamide and CB1 receptor antagonist, AM251 in the regulation of thyrotropin secretion. J Endocrinol 199:235–242.
- Diczfalusy U. 1994. Beta-oxidation of eicosanoids. Prog Lipid Res 33:403–428.
- Ensor CM, Tai HH. 1995. 15-Hydroxyprostaglandin dehydrogenase. J Lipid Mediat Cell Signal 12:313–319.
- Fleming I. 2011. Cytochrome P450-dependent eicosanoid production and crosstalk. Curr Opin Lipidol 22:403–409.
- Goto-Inoue N, Hayasaka T, Zaima N, Setou M. 2011. Imaging mass spectrometry for lipidomics. Biochim Biophys Acta 1811:961–969.
- Gross RW, Han X. 2011. Lipidomics at the interface of structure and function in systems biology. Chem Biol 18:284–291.
- Gross RW, Jenkins CM, Yang J, Mancuso DJ, Han X. 2005. Functional lipidomics: The roles of specialized lipids and lipid-protein interactions in modulating neuronal function. Prostaglandins Other Lipid Mediat 77:52–64; Review.
- Guichardant M, Chen P, Liu M, Calzada C, Colas R, Véricel E, 2011. Functional lipidomics of oxidized products from polyunsaturated fatty acids. Chem Phys Lipids 164:544–548; Review.
- Guillemin Y, Lopez J, Gimenez D, Fuertes G, Valero JG, Blum L, 2010. Active fragments from pro- and antiapoptotic BCL-2 proteins have distinct membrane behavior reflecting their functional divergence. PLoS One 5:e9066.
- Gupta S, Maurya MR, Stephens DL, Dennis EA, Subramaniam S. 2009. An integrated model of eicosanoid metabolism and signaling based on lipidomics flux analysis. Biophys J 96:4542–4551.
- Hammarström S, Orning L, Bergström K. 1985. Metabolism of leukotrienes. Mol Cell Biochem 69:7–16.
- Han X, Gross RW. 2003. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: A bridge to lipidomics. J Lipid Res 44:1071–1109.
- Hellerer T, Axäng C, Brackmann C, Hillertz P, Pilon M, Enejder A. 2007. Monitoring of lipid storage in Caenorhabditis elegans using coherent anti-Stokes Raman scattering (CARS) microscopy. Proc Natl Acad Sci USA 104:14658–14663.
- Hu C, Hoene M, Zhao X, Häring HU, Schleicher E, Lehmann R, 2010. Lipidomics analysis reveals efficient storage of hepatic triacylglycerides enriched in unsaturated fatty acids after one bout of exercise in mice. PLos One 5:e13318.
- Hussein N, Ah-Sing E, Wilkinson P, Leach C, Griffin BA, Millward DJ. 2005. Long-chain conversion of [13C]linoleic acid and alpha-linolenic acid in response to marked changes in their dietary intake in men. J Lipid Res 46:269–280.
- Imai Y, Uno T, Nakamura M, Yokota H. 1989. Structure-function relationships of cytochrome P-450 laurate (omega-1)-hydroxylase. Drug Metab Rev 20:467–478.
- Imig JD. 2005. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol 289:F496–F503.
- Ishitsuka R, Yamaji-Hasegawa A, Makino A, Hirabayashi Y, Kobayashi T. 2004. A lipid-specific toxin reveals heterogeneity of sphingomyelin-containing membranes. Biophys J 86:296–307.
- Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, Nakanishi H, 2012. Identification and structure determination of novel anti-inflammatory mediator Resolvin E3, 17,18-Dihydroxyeicosapentaenoic acid. J Biol Chem 287:10525–10534.
- Khatchadourian A, Krumova K, Boridy S, Ngo AT, Maysinger D, Cosa G. 2009. Molecular imaging of lipid peroxyl radicals in living cells with a BODIPY-alpha-tocopherol adduct. Biochemistry 48:5658–5668.
- Kim HY, Spector AA, Xiong ZM. 2011. A synaptogenic amide N-docosahexaenoylethanolamide promotes hippocampal development. Prostaglandins Other Lipid Mediat 96:114–120.
- Kiyokawa E, Baba T, Otsuka N, Makino A, Ohno S, Kobayashi T. 2005. Spatial and functional heterogeneity of sphingolipid-rich membrane domains. J Biol Chem 280:24072–24084.
- Lagarde M. 1988. Metabolism of fatty acids by platelets and the functions of various metabolites in mediating platelet function. Prog Lipid Res 27:135–152.
- Lagarde M, Géloën A, Record M, Vance D, Spener F. 2003. Lipidomics is emerging. Biochim Biophys Acta 1634:61.
- Lagarde M, Gualde N, Rigaud M. 1989. Metabolic interactions between eicosanoids in blood and vascular cells. Biochem J 257:313–320.
- Leidl K, Liebisch G, Richter D, Schmitz G. 2008. Mass spectrometric analysis of lipid species of human circulating blood cells. Biochim Biophys Acta 1781:655–664.
- Lim RS, Kratzer A, Barry NP, Miyazaki-Anzai S, Miyazaki M, Mantulin WW, 2010. Multimodal CARS microscopy determination of the impact of diet on macrophage infiltration and lipid accumulation on plaque formation in ApoE-deficient mice. J Lipid Res 51:1729–1737.
- Murphy RC, Hankin JA, Barkley RM. 2009. Imaging of lipid species by MALDI mass spectrometry. J Lipid Res 50:S317–S322.
- Na HK, Park JM, Lee HG, Lee HN, Myung SJ, Surh YJ. 2011. 15-Hydroxyprostaglandin dehydrogenase as a novel molecular target for cancer chemoprevention and therapy. Biochem Pharmacol 82:1352–1360.
- Nicolaou A, Masoodi M, Mir A. 2009. Lipidomic analysis of prostanoids by liquid chromatography-electrospray tandem mass spectrometry. Methods Mol Biol 579:271–286.
- Pezacki JP, Blake JA, Danielson DC, Kennedy DC, Lyn RK, Singaravelu R. 2011. Chemical contrast for imaging living systems: Molecular vibrations drive CARS microscopy. Nat Chem Biol 7:137–145.
- Pfister SL, Gauthier KM, Campbell WB. 2010. Vascular pharmacology of epoxyeicosatrienoic acids. Adv Pharmacol 60:27–59.
- Piomelli D, Astarita G, Rapaka R. 2007. A neuroscientist's guide to lipidomics. Nat Rev Neurosci 8:743–754.
- Poulos A. 1995. Very long chain fatty acids in higher animals – a review. Lipids 30:1–14.
- Powell WS, Rokach J. 2005. Biochemistry, biology and chemistry of the 5-lipoxygenase product 5-oxo-ETE. Prog Lipid Res 44:154–183.
- Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, 2010. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res 51:3299–3305.
- Reeder SB, Cruite I, Hamilton G, Sirlin CB. 2011. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging 34:729–749.
- Samuelsson B. 1978. Prostaglandins and thromboxanes. Recent Prog Horm Res 34:239–258.
- Samuelsson B. 1986. Leukotrienes and other lipoxygenase products. Prog Lipid Res 25:13–18.
- Shevchenko A, Simons K. 2010. Lipidomics: Coming to grips with lipid diversity. Nat Rev Mol Cell Biol 11:593–598.
- Schmelzer K, Fahy E, Subramaniam S, Dennis EA. 2007. The lipid maps initiative in lipidomics. Methods Enzymol 432:171–183.
- Serhan CN. 2009. Systems approach to inflammation resolution: Identification of novel anti-inflammatory and pro-resolving mediators. J Thromb Haemost 7(Suppl 1):44–48.
- Serhan CN, Chiang N, Van Dyke TE. 2008. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 8:349–361.
- Serhan CN, Lu Y, Hong S, Yang R. 2007. Mediator lipidomics: Search algorithms for eicosanoids, resolvins, and protectins. Methods Enzymol 432:275–317.
- Smith WL. 2008. Nutritionally essential fatty acids and biologically indispensable cyclooxygenases. Trends Biochem Sci 33:27–37.
- Spandl J, White DJ, Peychl J, Thiele C. 2009. Live cell multicolor imaging of lipid droplets with a new dye, LD540. Traffic 10:1579–1584.
- Sparvero LJ, Amoscato AA, Kochanek PM, Pitt BR, Kagan VE, Bayir H. 2010. Mass-spectrometry based oxidative lipidomics and lipid imaging: Applications in traumatic brain injury. J Neurochem 115:1322–1336.
- Spector AA. 2009. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res 50:S52–S56.
- Spector AA, Gordon JA, Moore SA. 1994. Beta-oxidation of hydroxyeicosatetraenoic acids: A peroxisomal process. World Rev Nutr Diet 75:8–15.
- Spector AA, Norris AW. 2007. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol 292:C996–C1012.
- Spener F, Lagarde M, Geloen A, Record M. 2003. What is lipidomics? Eur J Lipid Sci Technol 105:481–482.
- Song WL, Wang M, Ricciotti E, Fries S, Yu Y, Grosser T, 2008. Tetranor PGDM, an abundant urinary metabolite reflects biosynthesis of prostaglandin D2 in mice and humans. J Biol Chem 283:1179–1188.
- Sun YX, Tsuboi K, Okamoto Y, Tonai T, Murakami M, Kudo I, 2004. Biosynthesis of anandamide and N-palmitoylethanolamine by sequential actions of phospholipase A2 and lysophospholipase D. Biochem J 380:749–756.
- Tai HH, Cho H, Tong M, Ding Y. 2006. NAD+-linked 15-hydroxyprostaglandin dehydrogenase: Structure and biological functions. Curr Pharm Des 12:955–962.
- Touboul D, Laprévote O, Brunelle A. 2011. Micrometric molecular histology of lipids by mass spectrometry imaging. Curr Opin Chem Biol;15;725–732.
- Vellani V, Petrosino S, De Petrocellis L, Valenti M, Prandini M, Magherini PC, 2008. Functional lipidomics. Calcium-independent activation of endocannabinoid/endovanilloid lipid signalling in sensory neurons by protein kinases C and A and thrombin. Neuropharmacology 55:1274–1279.
- Welti R, Shah J, Li W, Li M, Chen J, Burke JJ, 2007. Plant lipidomics: Discerning biological function by profiling plant complex lipids using mass spectrometry. Front Biosci 12:2494–2506.
- Wilkinson SG. 1996. Bacterial lipopolysaccharides – themes and variations. Prog Lipid Res 35:283–343.
- Wüstner D, Solanko L, Sokol E, Garvik O, Li Z, Bittman R, 2011. Quantitative assessment of sterol traffic in living cells by dual labeling with dehydroergosterol and BODIPY-cholesterol. Chem Phys Lipids 164:221–235.
- Yang W, Ni J, Woodward DF, Tang-Liu DD, Ling KH. 2005. Enzymatic formation of prostamide F2alpha from anandamide involves a newly identified intermediate metabolite, prostamide H2. J Lipid Res 46:2745–2751.