Abstract
Cys-loop receptors play important roles in signal transduction. The Gloeobacter ligand-gated ion channel (GLIC) pore binds similar compounds to Cys-loop receptor pores, but has the advantage of known structures in open and closed states. GLIC is activated by protons with a pEC50 of 5.4, and has a histidine residue (His 11’) in its pore-forming α-helix (M2) which is involved in gating. Here we explore the role of this His and other M2 residues using two-electrode voltage clamp of mutant receptors expressed in oocytes. We show that 11’His is very sensitive to substitution; replacement with a range of amino acids ablates function. Similarly altering its location in M2 to the 8’, 9’, 10’, 12’, 13’ or 14’ positions ablated function. Most substitutions of Ser6’ or Ile9’ were also non-functional, although not Ile9’Leu and Ile9’Val. Unexpectedly, an Ile9’His substitution was constitutively active at pH 7, but closed as [H+] increased, with a pIC50 of 5.8. Substitution at 2’, 5’ and 7’ had little effect on pEC50. Overall the data show Ser6’ and His11’ are critical for the function of the receptor, and thus distinguish the roles of these M2 residues from those of Cys-loop receptors, where substitutions are mostly well tolerated. These data suggest modellers should be aware of these atypical features when using the GLIC pore as a model for Cys-loop receptor pores.
Introduction
Cys-loop receptors are responsible for the transmission of fast synaptic impulses in the nervous systems and neuromuscular junctions, although homologous proteins exist in prokaryotes (Tasneem et al., Citation2005). One such homologue, which is activated by protons, is found in the cyanobacterium Gloeobacter violaceus and is known as GLIC (Gloeobacter ligand-gated ion channel). Crystal structures of GLIC reveal the presence of a largely β-sheet extracellular domain and a largely α-helical transmembrane domain as in eukaryotic Cys-loop receptors, but GLIC lacks a Cys-loop and an extended intracellular domain. High resolution structures of GLIC have been determined for both open and closed structures, the former coming from structures which have been crystallized at low pH, and the latter from mutant receptors which do not function (Bocquet et al., Citation2009; Hilf & Dutzler, Citation2009; Parikh et al., Citation2011; Gonzalez-Gutierrez et al., Citation2013; Sauguet et al., Citation2013).
GLIC has low sequence similarity to Cys-loop receptors, but many functionally important residues and structural features are conserved. Of particular interest is the region that traverses the membrane, which, like Cys-loop receptors, is composed of four transmembrane spanning α-helices (M1–M4) where M2 forms the pore. This region is primarily responsible for ion permeation and selectivity, and is also the binding site for a range of pore-blocking agents. There are number of similarities between the GLIC pore and the pores of the cation-selective nicotinic acetylcholine (nACh) and 5-HT3 receptors: They all have negatively charged residues at the intracellular side, hydrophilic residues at the pore lining 2′ and 6′ positions, and hydrophobic residues at the 9′, 13′ and 16′ positions (). Unsurprisingly, given these similarities, there is considerable overlap in the range of compounds that block the pores of these receptors (Alqazzaz et al., Citation2011). These data support the use of the GLIC pore as a model for vertebrate cation-selective Cys-loop receptor pores, although there are some notable differences in GLIC as the residue primarily responsible for ion selectivity (Glu) is located at the -2′ and not the -1′ position, there is no Leu at the 9′ position, and there is a charged residue (His) at the 11′ location ().
Figure 1. GLIC M2. (A) Alignment of the GLIC M2 region with those of related proteins showing the prime (′) notation used for comparison of this region. The alignment reveals conservation but also anomalies: Glu at -2′, Ile at 9′ and His at 11′. (B) Transmembrane helices M1, M2 and M3 of 2 of the 5 subunits of GLIC showing the location of His 11′ facing towards M3 and the pore lining residues.
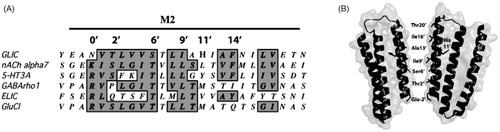
The 11′ His has previously been identified as a critical feature in the proton-gated opening by Wang et al. (Citation2012). These authors showed that substituting alternate residues here retained expression but ablated function, and their molecular dynamic simulations supported structural data in showing that His11′ forms an H-bond with the backbone oxygen of Ileu259 in M3 in the open state with no such bond in the locally closed structures (Prevost et al., Citation2012; Wang et al., Citation2012). The existence of an H-bond, generated following protonation of His11′, has subsequently been strongly supported by experiments using unnatural amino acids (Rienzo et al., Citation2014). Here we further explore the role of His11′, and in particular examine whether it can be located elsewhere in M2.
There have been fewer, or no, studies on other GLIC M2 residues, and so, in addition to His11′, in this study we probe a range of these residues, using site-directed mutagenesis combined with electrophysiological characterisation. The data reveal some similarities but also some differences in the roles of these residue in GLIC and Cys-loop receptors.
Materials and methods
Cell culture and oocyte maintenance
Xenopus laevis oocyte-positive females were purchased from NASCO (Fort Atkinson, WI) and maintained according to standard methods. Harvested stage V–VI Xenopus oocytes were washed in four changes of Ca2+-free ND96 (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES, pH 7.5), de-folliculated in 1.5 mg ml−1 collagenase Type 1A for approximately 2 h, washed again in four changes of ND96 (as above + 1.8 mM CaCl2) and stored in ND96 containing 2.5 mM sodium pyruvate, 0.7 mM theophylline and 50 mM gentamicin.
Receptor expression
A codon optimized version of GLIC, fused to the signal sequence of the α7 nACh receptor subunit and kindly gifted from P.J. Corringer, was cloned into pGEMHE for oocyte expression. Mutant receptors were created using QuikChange mutagenesis (Agilent, Santa Clara, CA). cRNA was transcribed in vitro from linearized pGEMHE cDNA template using the mMessage mMachine T7 kit (Ambion, Austin, TX). Stage V and VI oocytes were injected with 50 nl of ∼400 ng μl−1 cRNA, and currents were recorded 1–4 days post-injection.
Electrophysiology
Using two electrode voltage clamp, Xenopus oocytes were clamped at −60 mV using an OC-725 amplifier (Warner Instruments, Hamden, CT), Digidata 1322A and the Strathclyde Electrophysiology Software Package (Department of Physiology and Pharmacology, University of Strathclyde, UK; http://www.strath.ac.uk/Departments/PhysPharm/). Currents were filtered at a frequency of 1 kHz. Micro-electrodes were fabricated from borosilicate glass (GC120TF-10, Harvard Apparatus, Kent, UK) using a one-stage horizontal pull (P-87, Sutter Instrument Company, Novato, CA) and filled with 3 M KCl. Pipette resistances ranged from 1.0–2.0 MΩ. Oocytes were perfused with saline containing (in mM) NaCl (96), KCl (2), MgCl2 (1) and MES or HEPES (10), pH 3.5–8, at a constant rate of 12–15 ml min−1. Drug application was via a simple gravity-fed system calibrated to run at the same rate as the saline perfusion.
Analysis and curve fitting was performed using the 4 parameter equation in Prism (GraphPad Software, La Jolla, CA). Concentration-response data for each oocyte was normalised to the maximum current for that oocyte.
Immunofluorescence
Oocytes injected with GLIC mRNA were fixed 2–3 days post injection using 4% paraformaldehyde in PBS (150 mM NaCl, 10 mM phosphate buffer pH 7.4) for 2 h at room temperature. The oocytes were then rinsed 3× using TBS (150 mM NaCl, 50 mM Tris pH 7.4) at room temperature. Then oocytes were incubated overnight at 4 °C with a GLIC polyclonal antisera (rained against the sequence KDRRLAFDPVRSGVRVKTYE; Thermo Fisher Scientific) diluted 1:1000 in TBS containing 0.1% Tween 20 (TTBS) and 1% BSA. Oocytes were then washed 3× in TTBS at room temperature and incubated for 1 h in 1:1000 anti rabbit Alexa Fluor 594. Oocytes were then washed 3× in TTBS at room temperature and visualized using a Floid® Cell Imaging System (Life Technologies).
Sequence conservation
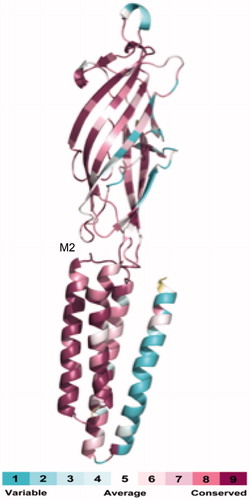
To estimate the degree of conservation of sequence conservation of the GLIC transmembrane region we used the Consurf server, which is a bioinformatics tool that can estimate the conservation of amino acid positions in a protein, based on phylogenetic relations between homologous sequences (Glaser et al., Citation2003). We used the closest 150 homologues of GLIC in this procedure, which were identified using BLAST (Altschul et al., Citation2005). Calculated conservation scores were divided into a colour scale for visualization, from turquoise (most variable) to maroon (most conserved).
Results
GLIC pore residue conservation
Analysis of the residues that form the transmembrane domain reveals that residues in M2 have considerable similarity to those in Cys-loop receptors. Comparing the sequence of GLIC to the nACh receptor α1 receptor subunit reveals a sequence similarity of 28%, while the sequence similarity in the M2 domain is 55%, increasing to 80% for the pore-lining residues. Further assessing the level of conservation of residues and mutational variability of GLIC relative to vertebrate Cys-loop receptors by analyzing the phylogenetic relationship of 150 sequences homologous to GLIC using Consurf (Glaser et al., Citation2003) shows low conservation in M4, with higher levels in M1–M3 and the highest level in M2 region ().
Figure 2. Similarity between GLIC and Cys-loop receptor subunits. This image, produced by the Consurf server from 150 homologous sequences, shows the level of conservation between GLIC and Cys-loop receptor subunits. Calculated conservation scores have been divided into a colour scale for visualization, from turquoise (most variable) to maroon (most conserved).
GLIC activation
Current amplitude was measured at a range of external H+ concentrations. Concentration response curves revealed a pEC50 of 5.4 (), similar to values previously reported, e.g. (Alqazzaz et al., Citation2011; Gonzalez-Gutierrez et al., Citation2013; Zimmermann & Dutzler, Citation2011).
Table 1. pEC50s for GLIC 9′ mutants.
His 11′ mutant receptors
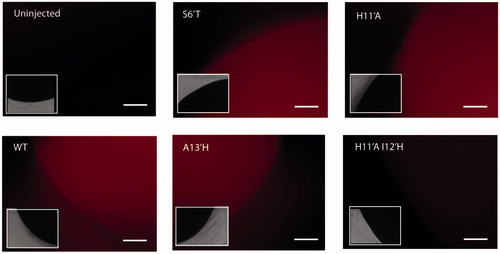
Substitution of His 11′ with hydrophobic (Ala, Cys, Val), hydrophilic (Lys, Arg, Glu) or aromatic (Phe, Tyr) residues resulted in receptors that did not respond when challenged with low pH. This could be due to receptors not being expressed or being expressed but not functional. Our immunofluorescence data support the latter explanation as this clearly demonstrated receptor expression (), as has been observed in previous studies which used an α-bungarotoxin binding tag inserted into GLIC to monitor cell surface expression (Wang et al., Citation2012).
Figure 3. GLIC oocytes expression. Representative example images of oocytes injected with WT and mutant GLIC mRNA and probed with a GLIC specific antisera indicate single GLIC substitutions at the 2′, 6′, 9 and 11′ positions do not ablate expression as they show red fluorescence, even though many of these do not function (not all data is shown). However there is little expression in double mutants with H11′A and a His at an alternative location in M2. Insets show the same image in a smaller size under white light. Scale bar = 50 μm. Data is representative of at least 4 oocytes.
Relocating the His 11′ residue
Previous studies in the α7 nACh receptor have shown that altering the location of an inserted Pro residue at the intracellular-end of M2 has similar effects if inserted in four adjacent positions (Corringer et al., 1999), and so we wished to test if we could similarly relocate His 11′. Thus we inserted a His at the 8′, 9′, 10′, 12′, 13′ and 14′ positions, in combination with a His11′Ala substitution. None of the resulting double mutant receptors responded when challenged with low pH (data not shown). Immunofluorescent data from these mutants () showed poor expression compared to the single mutants; thus it maybe that two substitutions in M2 have a deleterious effect on expression.
Single His mutations in M2
We further explored the role of single histidine mutations in the M2 channel. Introduction of single His mutations at intracellular pore lining residues (-2′, 2′, or 6′) and at residues close to His11′ (8′, 9′, 10′, 12′, 13′ or 14′) resulted in non-functional channels (data not shown) except Ile9′His which resulted in a constitutively open channel (described below). Immunofluorescent data indicated that all these single mutants were expressed ().
Substitutions at the 9′ residue
Substitution of the Ile9′ with Leu or Val resulted in receptors with pEC50s similar to WT (). However substituting Ile9′ with Asp, Phe, Lys, Asn, Ser or Trp resulted in non-functional but expressing receptors (, ).
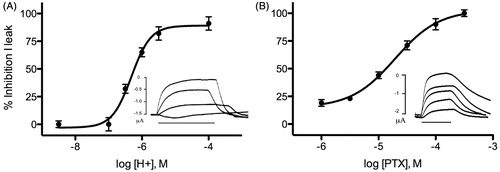
Introduction of His at the 9′ position yielded functional receptors which were constitutively open at pH 7, with leak currents of 0.5–6 μA, and closed at pH 4, where leak currents were minimal; a concentration-response curve revealed a pIC50 of 6.2 (). The constitutively open receptors were also blocked by picrotoxin, with an IC50 of 18 ± 0.1 μM (), not dissimilar to the picrotoxin IC50 in wild-type GLIC (3 μM) (Alqazzaz et al., Citation2011).
Figure 4. GLIC I9′H characteristics. (A) Effect of pH in closing the open channel. Insets show example traces which are constitutively open at pH 7 (1.5 μA leak current), while 30 s applications of decreasing pH (here 6, 5.5 and 4) show the current (Ileak) reverts towards 0, which it comes close to at pH 4. The IC50 derived from the curve was pH 6.2. (B) PTX blocks the open channel. Inset shows example responses at pH 7 (2 μA leak current) in the presence of 30 s applications of pH 7 buffer containing 0.3, 1, 10, 30 and 100 μM PTX. The IC50 derived from the curve was 18 μM. Data = mean ± SEM, n = 3–6.
Other M2 residues
Ser 6′ also proved sensitive to change; all the substitutions we examined rendered the receptor non-functional () although were expressed (), as was previously observed when this residue was substituted with Cys, Gly, Val and Thr (Parikh et al., Citation2011). Substitution of Glu-2′ to Ala, Lys or His also resulted in non-functional receptors. However altering residues 5′ and 7′ to Ala had no significant effect on receptor function, and similarly mutation of Thr2′ to Ala or Ser resulted in receptors with a pEC50 similar to wild-type receptors ().
Table 2. Other pore lining mutants.
Discussion
Our data reveal some novel features of the amino acids in the GLIC pore: The 9′ residue when replaced by His results in a constitutively open channel that closes with an IC50 not dissimilar to that of the WT EC50, Ser is obligatory at the 6′ location, and the 9′ and 11′ residues are very sensitive to substitution. These data demonstrate the importance of these residues in the M2 of GLIC, and also suggest differences in the roles of these and equivalent residues in Cys-loop receptors. This does not necessarily detract from the usefulness of GLIC as a structural model for these proteins. Recent studies, for example, have revealed an anaesthetic binding site in a cavity between M1, M2 and M3 (Nury et al., Citation2010), and there is evidence that binding to a similar location may be responsible for the actions of these compounds in vertebrate Cys-loop receptors. Functional extrapolation, however, may be less accurate as the molecular mechanism of action of GLIC is not yet clear. Nevertheless our data provides new information of the roles of a range of M2 residues, which are discussed in more detail below.
His 11′ in the transmembrane domain has been shown by multiple research groups to play a major role in GLIC channel opening (Bocquet et al., Citation2009; Prevost et al., Citation2012; Wang et al., Citation2012; Rienzo et al., Citation2014) and our data support this, although it may not be the primary proton binding site (Duret et al., Citation2011). Altering the location of His in M2 does not allow the receptor to retain function, in contrast to the location of Pro in the M1–M2 loop in anionic receptors, where the receptors maintain functionality with multiple Pro positions (Corringer et al., 1999). His is also not tolerated in other positions in M2 when His11′ is retained, except at 9′. The 9′ location has been well studied in Cys-loop receptors as it contributes to the “hydrophobic girdle” which inhibits ion flow in the resting receptor (Unwin, Citation2005). Hydrophilic residues here often facilitate channel opening, although were not tolerated in GLIC: Leu, Val, Cys and Ala were the only residues which allowed pH-gated channel opening (current work, Gonzalez-Gutierrez et al., Citation2013) suggesting that there are specific requirements for amino acids at the GLIC 9′ position. However introduction of His resulted in a constitutively open channel, which closed in a pH-dependent manner, with a pIC50 similar to that of the pEC50 in WT receptors. Constitutively open Cys-loop receptors caused by mutations of the 9′ residue have been previously described, for example a L9′T mutation results in constitutive activity in GABAA receptors, but these cannot then be activated (or inhibited) by GABA. They are, however, blocked by the pore blocker penicillin, indicating that open state of the pore in mutant receptors is similar to that in native receptors (Tierney et al., Citation1996). Similarly we observed that current through the I9′H mutant receptors could be blocked by the pore-blocker picrotoxin, with an IC50 similar to that of WT GLIC receptors. This shows that the structure of this pore was also not much altered in this mutant. We suggest that protonation of His residues at the 9′ location (which would occur as the pH decreases) would result in a positively charged “girdle” in the centre of the pore, inhibiting the flow of positively charged ions. This is reminiscent of the mechanism of action of the M2 proton channel, which has a centrally located pore lining His residue (Stouffer et al., Citation2008).
Previous work has shown that a L8′C mutation is also constitutively active, although the characteristics of the responses from this mutant were different: The holding current (1–5 μA) was not significantly reduced by 100 μM QX222 or by application of buffers at pH 9, 5 or 4.5 (Parikh et al., Citation2011). Thus the L8′C mutation may hold the pore open in a non-native configuration.
Another pore lining residue that was very sensitive to substitution was the 6′ residue; we did not obtain functional receptors if this was mutated to any other amino acid type (Thr, Val, Lys, Tyr, Ala, Trp). These data are consistent with previous studies which reported non-functional, but expressed, receptors with S6′G, S6′V and S6′T mutations (Sauguet et al., Citation2013). The 6′ residue is important in Cys-loop receptors as a binding residue for many pore-blocking compounds, and also has a role in gating, e.g. in 5-HT3 receptors a T6′S substitution alters the relative efficacy of a series of agonists, changing some (e.g. quipazine) from apparent antagonists to potent and efficacious agonists (Thompson et al., Citation2011). Our data suggest that this residue is critical for gating in GLIC.
The -2′ residue is also important: Substitution with Ala, His or Lys resulted in non-functional receptors. The side chain of Glu-2′ is at the intracellular entrance of the pore and the structure reveals narrowing to <0.5 A under the conditions used by Hilf and Dutzler (Citation2009). However in the structure solved by Bocquet et al. (Citation2009), the side chain of Glu-2′ is in a different location, rendering the pore much wider (2.3 Å). Thus the sensitivity to substitution we observed maybe because this residue controls both the size and the ion selectivity of the pore.
In conclusion, our data show that the 6′Ser and 11′His residues in the GLIC pore are especially critical for receptor function, and the receptor is sensitive to the type of residue at 9′. These data contrast with the wide variety of residues that can be accommodated without ablating function in these locations in many Cys-loop receptors. We speculate that this may be because His 11′ plays a major role in proton activation of the GLIC channel, and any changes to the chemical environment in this region prevents the specific interactions required for pore opening. We note that residues some distance from His11′ do appear to behave similarly to those in Cys-loop receptors: The 2′ residue, for example, has been explored in many Cys-loop receptors, where it has a role in binding pore blockers, and it has been shown to also have this role in GLIC (Alqazzaz et al., Citation2011). Our 2′ substitutions resulted in receptors with pEC50 values not dissimilar to WT, and therefore similar to data from Cys-loop receptors. Thus overall we conclude that information from GLIC could be useful for understanding Cys-loop receptors, but the unusual mechanism of action of GLIC means that caution must be applied when using the pore structure as a template for probing the structure and function of Cys-loop receptor pores.
| Abbreviations | ||
| nACh | = | nicotinic acetylcholine |
| AChBP | = | acetylcholine binding protein |
| GABA | = | γ-aminobutyric acid |
| GLIC | = | Gloeobacter ligand-gated ion channel |
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
SCRL held a Wellcome Trust Senior Research Fellowship. MA was funded by a Yousef Jameel Scholarship.
References
- Altschul SF, Wootton JC, Gertz EM, Agarwala R, Morgulis A, Schäffer AA, Yu YK. 2005. Protein database searches using compositionally adjusted substitution matrices. FEBS J 272:5101–5109
- Alqazzaz M, Thompson AJ, Price KL, Breitinger HG, Lummis SC. 2011. Cys-loop receptor channel blockers also block GLIC. Biophys J 101:2912–2918
- Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux JP, Delarue M, Corringer PJ. 2009. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature 457:111–114
- Corringer PJ, Bertrand S, Galzi JL, Devillers-Thiéry A, Changeux JP, Bertrand D. 1999. Mutational analysis of the charge selectivity filter of the alpha7 nicotinic acetylcholine receptor. Neuron 22:831–843
- Duret G, Van Renterghem C, Weng Y, Prevost M, Moraga-Cid G, Huon C, et al. 2011. Functional prokaryotic-eukaryotic chimera from the pentameric ligand-gated ion channel family. Proc Natl Acad Sci USA 108:12143–12148
- Glaser F, Pupko T, Paz I, Bell RE, Bechor-Shental D, Martz E, Ben-Tal N. 2003. ConSurf: Identification of functional regions in proteins by surface-mapping of phylogenetic information. Bioinformatics 19:163–164
- Gonzalez-Gutierrez G, Cuello LG, Nair SK, Grossman C. 2013. Gating of the proton-gated ion channel from Gloeobacter violaceus at pH 4 as revealed by X-ray crystallography. Proc Natl Acad Sci USA 110:18716–18721
- Hilf RJ, Dutzler R. 2009. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature 457:115–118
- Nury H, Van Renterghem C, Weng Y, Tran A, Baaden M, Dufresne V, et al. 2010. X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature 469:428–431
- Parikh RB, Bali M, Akabas MH. 2011. Structure of the M2 transmembrane segment of GLIC, a prokaryotic Cys loop receptor homologue from Gloeobacter violaceus, probed by substituted cysteine accessibility. J Biol Chem 286:14098–14109
- Prevost MS, Sauguet L, Nury H, Van Renterghem C, Huon C, Poitevin F, et al. 2012. A locally closed conformation of a bacterial pentameric proton-gated ion channel. Nat Struct Mol Biol 19:642–649
- Rienzo M, Lummis SCR, Dougherty DA. 2014. Structural requirements in the transmembrane domain of GLIC revealed by incorporation of noncanonical histidine analogs. Chem Biol 21:1700–1706
- Sauguet L, Poitevin F, Murail S, Van Renterghem C, Moraga-Cid G, Malherbe L. 2013. Structural basis for ion permeation mechanism in pentameric ligand-gated ion channels. EMBO J 32:728–741
- Stouffer AL, Acharya R, Salom D, Levine AS, Di Costanzo L, Soto CS, et al. 2008. Structural basis for the function and inhibition of an influenza virus proton channel. Nature 451:596–599
- Tasneem A, Iyer LM, Jakobsson E, Aravind L. 2005. Identification of the prokaryotic ligand-gated ion channels and their implications for the mechanisms and origins of animal Cys-loop ion channels. Genome Biol 6:R4
- Thompson AJ, Duke RK, Lummis SC. 2011. Binding sites for bilobalide, diltiazem, ginkgolide, and picrotoxinin at the 5-HT3 receptor. Mol Pharmacol 80:183–190
- Tierney ML, Birnir B, Pillai NP, Clements JD, Howitt SM, Cox GB, Gage PW. 1996. Effects of mutating leucine to threonine in the M2 segment of alpha1 and beta1 subunits of GABAA alpha1beta1 receptors. J Membr Biol 154:11–21
- Unwin N. 2005. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol 346:967–989
- Wang HL, Cheng X, Sine SM. 2012. Intramembrane proton binding site linked to activation of bacterial pentameric ion channel. J Biol Chem 287:6482–6489
- Zimmermann I, Dutzler R. 2011. Ligand activation of the prokaryotic pentameric ligand-gated ion channel ELIC. PLoS Biol 9:e1001101
