Abstract
The high antioxidant capacity of chlorogenic acid (CGA) in respect to biological systems is commonly known, though the molecular mechanism underlying that activity is not known. The aim of the study was to determine that mechanism at the molecular and cell level, in particular with regard to the erythrocyte and the lipid phase of its membrane. The effect of CGA on erythrocytes and lipid membranes was studied using microscopic, spectrophotometric and electric methods. The biological activity of the acid was determined on the basis of changes in the physical parameters of the membrane, in particular its osmotic resistance and shapes of erythrocytes, polar head packing order and fluidity of erythrocyte membrane as well as capacity and resistivity of black lipid membrane (BLM). The study showed that CGA becomes localized mainly in the outer part of membrane, does not induce hemolysis or change the osmotic resistance of erythrocytes, and induces formation of echinocytes. The values of generalized polarization and fluorescence anisotropy indicate that CGA alters the hydrophilic region of the membrane, practically without changing the fluidity in the hydrophobic region. The assay of electric parameters showed that CGA causes decreased capacity and resistivity of black lipid membranes. The overall result is that CGA takes position mainly in the hydrophilic region of the membrane, modifying its properties. Such localization allows the acid to reduce free radicals in the immediate vicinity of the cell and hinders their diffusion into the membrane interior.
Introduction
Among the polyphenolic compounds that occur in plants, chlorogenic acid, an ester of caffeic and quinic acid (3-caffeoyl-D-quinic acid), is an important natural antioxidant. It is present in various parts of the plant, in large quantities especially in dry plums, green leaves and fruit of the coffee tree, leaves and fruit of the apple tree, hawthorn, artichoke, nettle, beans, potato tubers, bilberries and sunflower seeds (Daayf & Lattanzio, Citation2008; Pchelkin, Citation2003; Zang et al., Citation2003). The amounts of CGA supplied to an organism depend on the diet, e.g. a cup of instant coffee (ca. 200 ml) contains 50–150 mg of chlorogenic acid (Clifford, Citation1999). Scientific studies have shown a positive impact of CGA on the organism. With its strong antioxidant and antibacterial properties it reduces the risk of diabetes, fungal infection and cancer, and also protects the liver and other organs (Benzie, Citation2003, Marques & Farah, Citation2009, Santos-Buelga et al., Citation2010).
Due to its properties, chlorogenic acid is a polyphenolic compound that is classified as a component of functional food, which is believed to prevent many diseases. For this reason, research is conducted on extraction of this acid from various plants, formation of its conjugates with other compounds, as well as microcapsulation, to improve its stability and storage capability (Shi et al., 2007).
Oxidation by free radicals in biological membranes, in particular membrane lipids, causes disturbance in membrane structure and distorts its functions, with consequent pathological changes in the organism. For this reason there is growing interest in natural antioxidants which, by scavenging the excess of free radicals, may prevent many serious diseases associated directly with the peroxidation of lipids (Arbos et al., Citation2008; Bukowska et al., Citation2007; Marinova et al., Citation2009; Rice-Evans et al., Citation1996, Citation1997; Sato et al., Citation2011; Suwalsky et al., Citation2007, Citation2008).
Scientific works published so far have been concerned mainly with the protective and therapeutic action of different plant extracts on the organism, as well as that of individual compounds within these extracts. However, there have been few basic biophysical studies concerning the structural and functional impact of polyphenols on biological membranes. It is important, therefore, to clarify the mechanism of the interaction between phenolic compounds and biological membranes, in particular with lipids.
In our preliminary research we observed a very high antioxidant activity of chlorogenic acid in respect to the membrane of erythrocytes, in comparison with other substances of plant origin and with Trolox® (Bonarska-Kujawa et al., Citation2011a). However the mechanism responsible for such very good antioxidant capacity of CGA has not yet been explained. Therefore, we performed a biophysical investigation on the effect of CGA on the physical properties of the erythrocyte and model membrane in order to explain the molecular mechanism of that capacity.
The research presented in this paper aimed to examine the impact of CGA on the properties of the membrane using various research methods. Erythrocytes were treated as a model of the cell, and their membrane as a model of the biological membrane (Bonarska-Kujawa et al., Citation2011a, Citation2011b, Citation2012, Citation2014a; Chaudhuri et al., Citation2007, Citation2009; Cyboran et al., Citation2011; Lifen et al., Citation2004; Perez-Fons et al., Citation2010). In order to determine the impact of CGA on the lipid phase of biological membranes, lipid membrane models were used in the investigation. Liposomes and black lipid membranes (BLM) were formed of lipids extracted from the membrane of erythrocytes called red blood cell lipids (RBCL).
The study of the influence of CGA on the erythrocyte membrane and lipid membrane models was performed by microscopic, spectrophotometric and electric methods. The effects of the interaction of CGA with the membranes were determined on the basis of hemolysis, osmotic resistance and shape of erythrocytes, packing arrangement of the lipid polar heads, fluidity of the membranes in their hydrophobic regions, and resistance and capacity of black lipid membranes. The results of biophysical studies on the effect of CGA on lipid model membranes and biological ones are expected to explain the acid’s very good antioxidant properties documented in our earlier paper (Bonarska-Kujawa et al., Citation2011a).
Materials and methods
Materials
Chlorogenic acid (3-caffeoyl-D-quinic acid), Pub Chem. CID 1794427 was purchased from Sigma Aldrich, Steinheim, Germany. The fluorescent probe 6-dodecanoyl-2-dimethylaminonaphthalene (Laurdan); 1,6-diphenyl-1,3,5-hexatriene (DPH) and 1-(4-trimethylammoniumphenyl)-6-phenyl-1,3,5-hexatriene p-toluenesulfonate (TMA-DPH) were purchased from Molecular Probes, Eugene, OR. The antioxidant standard (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox®) was purchased from Sigma-Aldrich Inc., Steinheim, Germany.
Model membrane
Red blood cells
The hemolytic and microscopic studies were conducted on fresh, heparinized pig blood. The choice of pig erythrocytes was dictated by the fact that this cell’s lipid composition is closest to that of human erythrocytes (Deuticke, Citation1977), and the blood was readily available. For washing the erythrocytes, and in the experiments, an isotonic phosphate solution of pH 7.4 (131 mM NaCl, 1.79 mM KCl, 0.86 mM MgCl2, 11.79 mM Na2HPO4·2H2O, 1.80 mM Na2H2PO4·H2O) was used. Full blood was centrifuged at 1350 g for 3 min (4 °C) to remove the plasma and leucocytes. Upon removal from plasma, the erythrocytes were washed four times in 0.9% NaCl for microscopic experiments and in phosphate solution for hemolytic studies and then incubated in the same solution but containing appropriate amounts of CGA.
Erythrocyte membranes
The fluorimetric studies were conducted on isolated erythrocyte membranes (ghosts), which were obtained from fresh blood using the Dodge method (Dodge et al., Citation1963). The content of erythrocyte membranes in the samples was determined on the basis of protein concentration, which was assayed using the Bradford method (Bradford, Citation1976). To obtain an isolate membrane, 30 ml of fresh heparinized blood of 80% hematocrit was taken. The erythrocyte membranes were suspended in an isotonic phosphate solution of pH 7.4, in a quantity such that the protein concentration in the samples was approximately 100 µg/ml.
Lipid membranes
Red blood cell lipids were extracted from erythrocyte membranes according to the method described by Maddy et al. (Citation1972). Briefly, erythrocyte membranes were washed three times in 2.5 mM phosphate buffer (pH = 8), containing 0.5 mM EDTA (final concentration: 8–10 mg/ml). To one volume of the ice-cold membrane suspension, 0.75 volume of ice-cold n-butanol was added, and the mixture was shaken. After 20 min at 0 °C, the two phases were separated by centrifugation (5 min, 22 000 g). Subsequently, the fraction of lipids dissolved in butanol was transferred to weighed flask and butanol was evaporated for approx. 1 h. After evaporation the flask was weighed in order to determine the quantitative presence of lipids. Then, to the dry lipid film was added a mixture of chloroform and methanol v.s 1:1. The final concentration of lipids was 10 mg/ml. The resulting lipid solution was stored in a freezer at −30 °C.
Small unilamellar liposomes (SUV) for fluorimetric experiments were composed of RBCL. The lipids were dissolved in a chloroform-methanol solvent and evaporated to dryness under nitrogen. Subsequently, a phosphate buffer of pH 7.4 was added to the obtained film and liposomes were formed by mechanical shaking. Then SUV were formed using a sonicator in the presence of fluorescent probes. Control samples contained only lipid suspension with fluorescent probes at 1000:1 (lipids:fluorescent molar ratio), CGA at a concentration of 0.005–0.05 mg/ml being added to the remaining samples.
For resistance and capacitance measurements BLMs were formed, using the Mueller-Rudin method (Mueller et al., Citation1962), from a solution of RBCL dissolved in n-decane, the lipid concentration being equal to 20 mg/ml.
Methods
Amphiphilicity of CGA
The partition coefficient (P) between octanol and phosphate buffer (pH 7.4) for CGA was determined by the spectrophotometric method described by Nenadis et al. (Citation2003). Briefly, the partition coefficient P was calculated based on the formula (Equation1(1) ):
(1)
where Ao = absorbance corresponding to the maximum concentration of CGA in the organic phase, represented by octanol, Ax = absorbance corresponding to the concentration of CGA that remained in the organic phase. The spectra were recorded using a spectrophotometer (Cary 300 Bio, Varian) in the range 200–380 nm (UV). The experiment was done in triplicate, with three identical samples in each run.
The partition coefficient of CGA between octanol and phosphate buffer was expressed as log P. With an increasingly negative value of log P the hydrophilic nature of the compounds, and thus their affinity to aqueous media compared with lipid media, increases.
Hemolysis and osmotic resistance of erythrocytes
The effect of CGA on hemolysis and osmotic resistance of erythrocytes was investigated using the spectrophotometric method described previously by Włoch et al. (Citation2013), with minor modifications.
Erythrocyte modification was conducted at 37 °C for 1 h, each sample containing 1 ml of erythrocyte suspension of 2% hematocrit and a varying concentration of CGA (in the range 0.01–0.10 mg/ml). After modification, 1 ml samples were taken, centrifuged, and the supernatant was assayed for hemoglobin content using a UV-Vis spectrophotometer (Cary 300 Bio, Varian) at 540 nm wavelength. Hemoglobin concentration in the supernatant, expressed as percentage of hemoglobin concentration of totally hemolyzed cells, was assumed as a measure of the extent of hemolysis.
For osmotic resistance, upon removal from plasma the erythrocytes were washed three times with a cool 310 mOsm PBS isotonic solution. To determine the effect of CGA on osmotic resistance of erythrocytes, the lowest and highest non-lytic concentrations were chosen. Next, 2% red cell suspensions containing CGA at 0.01 and 0.05 mg/ml were prepared and left for 1 h at 37 °C. After modification the erythrocytes were centrifuged and suspended in test tubes containing 0.5–0.86% NaCl solution and in isotonic (0.9%) NaCl solution. Also in solutions of the same concentrations unmodified red blood cells were suspended, constituting the control for osmotic resistance determinations. Then the suspension was stirred and centrifuged at 1350 g and 4 °C. After that the percentage of hemolysis was measured with a spectrophotometer at λ = 540 nm. On the basis of the results obtained, the NaCl percentage concentrations that caused 50% hemolysis (C50) were found. The C50 values were taken as a measure of osmotic resistance.
Erythrocyte shapes
The influence of CGA on erythrocyte shapes was investigated using the microscopic method described earlier by Bonarska-Kujawa et al. (Citation2014a), with minor modifications.
For investigation with the optical and electron microscope, the red cells separated from plasma were washed four times in a saline solution and suspended in the same solution but containing 0.01 and 0.05 mg/ml of CGA. Hematocrit in the modification solution was 2%, the modification lasting 1 h at 37 °C.
After modification the erythrocytes were prepared for the optical microscope and fixed with a 2.5% solution of glutaraldehyde. After that the red cells were observed under a biological optical microscope (Nikon Eclipse E200) equipped with a digital camera. The photographs obtained made it possible to count erythrocytes of various shapes, and then the percentage of the two basic forms (echinocytes and stomatocytes) in a population of ca. 800 cells was determined. The individual forms of erythrocyte cells were assigned morphological indices according to the Bessis scale (Bessis, Citation1977), where various shapes are given morphological indices as follows: spherostomatocytes (−4), stomatocytes II (−3), stomatocytes I (−2), discostomatocytes (−1), discocytes (0), discoechinocytes (1), echinocytes (2), spheroechinocytes (3), spherocytes (4).
For the electron microscopy assay, the erythrocytes after modification with CGA (0.01 and 0.05 mg/ml) were fixed in a 2.5% solution of glutaraldehyde. The red cells were then dehydrated with solutions of acetone of increasing concentrations and dried for 12 h at room temperature. The preparations were viewed and photographed in a scanning electron microscope (Tesla BS 300) at 20 kV. Erythrocytes thus prepared were deposited on microscope stages and subjected to X-ray microanalysis by means of an X-ray analyzer, Bruker AXS Quantax, collaborating with the ESPRIT ver. 1.8.2. program. Next, the samples were coated with gold using the Scancoat 6 (Edwards, London, UK) sprinkler. The material ultrastructure was analyzed using a scanning microscope (EVO LS15 ZEISS) with SE1 detector, under high vacuum and accelerating voltage EHT = 20 kV.
Fluidity and packing arrangement of the membranes
The effect of CGA on packing arrangement of lipids in the erythrocyte membrane (ghosts) and liposomes formed from RBCL was investigated using the fluorimetric method described earlier by Bonarska-Kujawa et al. (Citation2011b), with minor modifications. Fluorescence intensity was measured using three fluorescent probes: Laurdan, DPH and TMA-DPH. These probes were chosen because each of them becomes incorporated in a different region of the lipid bilayer. The measurements were conducted with a fluorimeter (CARY Eclipse of VARIAN), equipped with a Peltier temperature controller DBS (temp. accuracy ± 0.1 °C). The excitation and emission wavelengths were as follows: For probe DPH, λex = 360 nm, λem = 425 nm; and for probe TMA-DPH, λex = 358 nm, λem = 428 nm. The excitation wavelength for Laurdan was 360 nm, and the emitted fluorescence was recorded at two wavelengths of 440 and 490 nm.
On the basis of the measured fluorescence intensity of probes, the values of fluorescence anisotropy (A) for DPH and TMA-DPH and generalized polarization (GP) for Laurdan were calculated using the formulae described by Lakowicz (Citation2006) and Parasassi et al. (Citation1998).
Fluorescence intensity was measured using the mentioned fluorescent probes (Laurdan, DPH and TMA-DPH), whose concentration in the samples was 10 μM, while concentration of CGA was within the range 0.005–0.05 mg/ml at a temperature of 37 °C.
Capacity and resistance of black lipid membranes
Electrical properties of black lipid membranes depend on the structure of the membranes and the developments in the surrounding environment. The BLM can be treated as a serial connection of three capacitors, whose capacity is determined by the capacity of the hydrophobic layer (CG) and capacities of two hydrophilic layers (double electric layers, CP) on both sides of the membrane, according to the formula (Everitt & Haydon, Citation1968):
(2)
Black lipid membranes were formed on a 1.05 mm hole in the partition of a two-compartment chamber filled with 0.9% NaCl solution. The formation of the membranes was monitored visually and electrically by measuring the membrane capacitance, using a four-electrode system and the capacitance-to-period conversion method (Kalinowski & Figaszewski, Citation1995a). It was assumed that the membrane formation process was completed when the capacitance drift did not exceed 10 pF/min. The resistance of the membranes (R = 1/G) was calculated using cyclic voltamperograms recorded in the range −50 to +50 mV with a sweep speed of 10 mV/s. Voltamperometric measurements were performed using a four-electrode potentiostat-galvanostat (Kalinowski & Figaszewski, Citation1995b). The membranes displayed linear dependence of current on potential. The slope of a voltamperogram is equal to conductance of the membrane (G = 1/R). The slope was calculated using the least square method. Selected points of a voltamperogram were fitted to a linear equation.
Only bilayers with electrical capacitance and conductance equal to C = 0.2 µF/cm2 and R = 5–50 nS/cm2 were accepted for experiments. CGA was pipetted into the solution after a bimolecular membrane was spontaneously formed. CGA concentration was 0.001, 0.005 and 0.010 mg/ml. CGA was added on both sides of the lipid membrane after checking its stability. The electrical capacity C and resistance R were measured every 5 min for 1.5 h after addition of CGA. For each measurement the final value of electrical parameters was the average of five recorded values, normalized to the values of capacity and resistance of the lipid bilayer, C0 and R0, recorded before addition of CGA. Measurements were performed at room temperature (23–25 °C) using Ag/AgCl electrodes of 0.5 cm2 average area, immersed directly into the electrolyte solutions. Membrane surface area was determined on the basis of membrane photographs recorded in transmitted light.
Statistical analysis
Statistical analysis was carried out using Statistica 10.0 (StatSoft Inc.). All the experiments were performed five times. Analysis of variance was carried out and significance between means was determined using Dunnett’s post-hoc test. Results are presented as mean ± SD. Significant levels were defined at α = 0.05 or at α = 0.01.
Results
Amphiphilicity of CGA
The log P parameter calculated using Formula (1) confirms the hydrophilic character of the compounds. Negative values of the log P parameter indicate the hydrophilic character of the compounds and greater affinity to the aqueous phase, represented by phosphate buffer. The value of the coefficient of amphiphilicity (log P ± SD) of CGA is log P = −0.897 ± 0.025. For the standard antioxidant Trolox log P is −0.805 ± 0.044 (Bonarska-Kujawa et al., Citation2014b).
Hemolysis and osmotic resistance of erythrocytes
The results on the effect of CGA on hemolysis of erythrocytes testify that in the presence of CGA red blood cells do not undergo increased hemolysis compared with unmodified cells, hemolysis with CGA at 0.1 mg/ml not exceeding 2% in relation to the control. The control probe contained a suspension of unmodified erythrocyte cells in phosphate buffer (pH 7.4).
In the studies of the effect of CGA on erythrocyte osmotic resistance no significant differences between the degree of hemolysis in the control cells and those modified with CGA were found, in aqueous solutions of various concentrations of sodium chloride. C50 values of sodium chloride, expressed as a percentage, determined for control blood cells and those modified with CGA are: Control C50 = 0.682 ± 0.006%; 0.01 mg/ml CGA C50 = 0.669 ± 0.033%; 0.05 mg/ml CGA C50 = 0.677 ± 0.034%.
Erythrocyte shapes
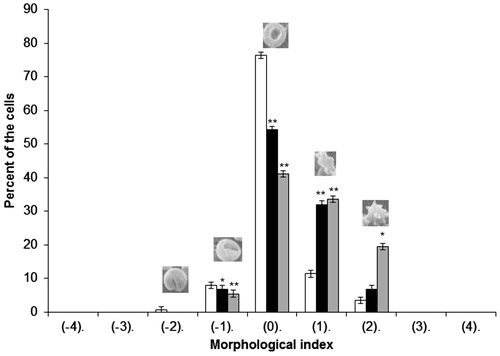
shows percentages of the various forms of cells in a population of erythrocytes modified with CGA at 0.01 and 0.05 mg/ml. As seen, CGA induces mostly various forms of echinocytes, primarily discoechinocytes.
Figure 1. Percentages of different shapes of erythrocytes induced by CGA at 0.05 mg/ml (grey bar) and 0.01 mg/ml (black bar) concentration, control (white bar). Results are expressed as average ± SD (n = 5). Various erythrocyte shapes are given morphological indices as follows: Spherostomatocytes (−4), stomatocytes II (−3), stomatocytes I (−2), discostomatocytes (−1), discocytes (0), discoechinocytes (1), echinocytes (2), spheroechinocytes (3), spherocytes (4). Statistical analysis was conducted using the Dunnett test. Statistically significant results are denoted by **α = 0.01, *α = 0.05.
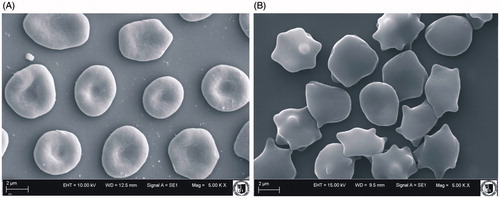
shows shapes of the erythrocytes modified by CGA at a concentration of 0.05 mg/ml as observed in a scanning electron microscope. CGA induced creation of echinocytes and discoechinocytes.
Fluidity and packing arrangement of the membranes
The effect of CGA on fluidity of the lipid phase of the erythrocyte membrane and on liposomes formed of RBCL was studied on the basis of fluorescence anisotropy measured with the fluorescent probes DPH and TMA-DPH. The results of DPH probe fluorescence anisotropy for such membranes are presented in . They indicate no changes caused by CGA in hydrocarbon chains of membrane lipids.
Table 1. Values of fluorescence anisotropy (A) of DPH and TMA-DPH probes for erythrocyte membranes and liposomes formed of erythrocyte lipids (RBCL) modified by CGA at 37 °C. Results are expressed as average ± SD (n = 5). The statistical significance of differences between average values of A and Acontrol was checked using the Dunnett test (<Acontrol).
As indicated by the values of fluorescence anisotropy at the level of the fourth carbon atom, the area where probe TMA-DPH is incorporated, CGA has practically no effect on fluidity of the RBCL and RBC membranes. A lack of significant changes for CGA is observed in the hydrophobic region where probe DPH is located.
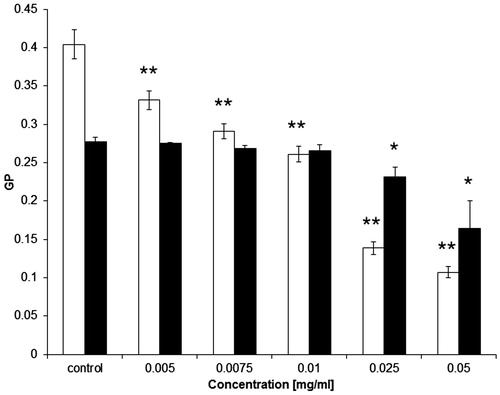
We also investigated, using the Laurdan probe, the packing arrangement in the hydrophilic part of erythrocyte ghosts and RBCL liposomes. The calculated values of general polarization (GP) decreased with increasing CGA concentration (), which is indicative of increasing disorder in the hydrophilic part of the lipid layer. However, the changes induced by CGA in RBCL liposome membranes are smaller than those in erythrocyte membranes.
Figure 3. Values of generalized polarization (GP) of Laurdan probe for erythrocyte membrane (□) and RBCL (▪) modified with CGA at 37 °C. Results are expressed as average ± SD (n = 5). Statistical analysis was conducted using Dunnett’s test. Statistically significant results at: *α = 0.05, **α = 0.01.
Capacity and resistance of black lipid membranes
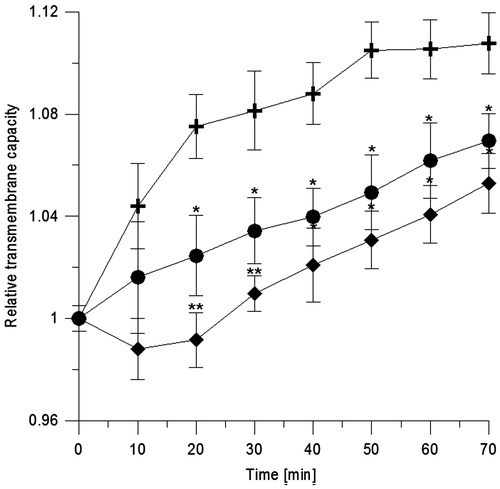
We studied the effect of CGA on the physical properties of black lipid membranes formed of RBCL, using electrical measurements. On addition of electrolyte, on both sides of the membrane, time changes in capacity and current were recorded. shows the kinetics of relative values of capacity (C/C0) of BLM in the presence of CGA at 0.001 and 0.01 mg/ml. The results show that CGA causes a concentration-dependent decrease in capacity of BLM.
Figure 4. Relative transmembrane capacity of BLM formed of erythrocyte lipids vs. time. Control symbol is (+) and in the presence of CGA at 0.001 mg/ml (•) and 0.01 mg/ml (♦). Statistical significance of differences between average values of capacitance for modified (C) and unmodified (C0) BLM was checked using the Dunnett test. Statistically significant results at *α = 0.05 and **α = 0.01.
specifies calculated values of relative resistivity (R/R0) of BLM, 45 min after modification with CGA. The results indicate that CGA causes a decrease in resistivity of black lipid membranes that amounts to about 40% of the control. The control value decreases only by about 3% after 45 min.
Table 2. Relative resistivity of black lipid membranes formed of erythrocyte membranes modified with CGA at different concentrations. Results are expressed as average ± SD (n = 5).
Discussion
In our previous studies (Bonarska-Kujawa et al., Citation2011a), CGA was found to possess high antioxidant capacity, though the mechanism of that activity was not known. In order to explain the molecular mechanism of the interaction between CGA and the biological membrane, we performed studies on the effect of the acid on the physical properties of the erythrocyte and lipid membrane. For better interpretation of the results for the erythrocyte membrane treated as a representative of the biological membrane, two membrane models were used in the experiment: liposomes, and black lipid membrane formed of RBCL. Such studies, performed in part on model membranes, have not been done before.
The results of the study show that CGA induces changes in biological membranes. CGA’s amphiphilicity was determined on the basis of its partition coefficient for the octanol/phosphate buffer system. The acid’s affinity to the aqueous phase (Nenadis et al., Citation2003), confirmed by our study, suggests that the compound will modify the hydrophilic region of the membrane. The partition coefficient was also determined for the standard hydrophilic antioxidant Trolox. The examination showed that both the compounds have hydrophilic character (similar negative value of log P), which explains their high, found by us earlier, capacity in protecting erythrocyte membranes against oxidation induced by free radicals in aqueous medium, e.g. organic radicals that develop in hemolytic cleavage of AAPH (Barclay et al., Citation1995; Bonarska-Kujawa et al., Citation2014b).
The hemolytic tests showed that CGA does not induce hemolysis, and therefore does not exert lytic action on red blood cells over a wide concentration range. The osmotic resistance assay showed no effect of CGA on osmotic resistance of the erythrocyte membrane, meaning that it does not act destructively on the membrane. This study shows that CGA, at concentrations markedly higher than physiological, does not disrupt the structure of the erythrocyte membrane. The maximum concentration that CGA can reach in blood plasma, e.g. after consumption of 170 mg of green tea, is 14.8 µmol/l (Farah et al., Citation2008), which falls well within the used range (2.82–282 µmol/l).
CGA induced modification in the shape of erythrocytes towards echinocytes. Formation of echinocytes is affected by substances located in the external monolayer, as evidenced by results published previously (Deuticke, Citation1968; Isomaa et al., Citation1987; Iglic et al., Citation1998; Sheetz and Singer, Citation1974). Moreover, the authors Pawlikowska-Pawlęga et al. (Citation2003) suggested that changes in erythrocyte shape could also be caused by interaction of some polyphenol molecules with the proteins of erythrocytes. These conjectures confirm results of other authors which indicate that CGA can form covalent bonds with proteins (Kang et al., Citation2004; Rawel et al., Citation2002; Sinisi et al., Citation2015; Wang et al., Citation2011). Based on the shapes induced by CGA, it is known that it becomes located in the outer monolayer of the lipid membrane. Such localization was found with the use of fluorescent probes.
CGA was shown to cause no changes in the properties of the hydrophobic part of the membranes. Such evidence is derived from the steady-state anisotropy measurements performed with the DPH and TMA-DPH fluorescent probes, which localize at different depths in the hydrophobic part of the membrane. The TMA-DPH probe is incorporated at the fourth carbon atom in the transient region between hydrophobic and hydrophilic parts of the bilayer. Practically no changes in the anisotropy value of DPH and TMA-DPH in erythrocyte and RBCL membranes occurred. A similar character of the interaction between polyphenolic compounds and membranes has been documented in numerous reports (Bonarska-Kujawa et al., Citation2011a, Citation2011b, Citation2012, Citation2014a, Citation2014b; Cyboran et al., Citation2012; Perez-Fons et al., Citation2010; Pruchnik et al., Citation2014). No change in the hydrophobic part implies that CGA is absent in that region of membrane. For this reason, using the Laurdan probe, the effect of CGA on the hydrophilic area of membrane was examined, expecting changes there because of the hydrophilic character of the molecule.
This supposition is confirmed by the fluorimetric studies performed with the Laurdan probe. This probe is particularly sensitive to the polarity of the environment, and monitors relevant differences in polarity of the different phase states of lipid bilayers (Harris et al., Citation2002; Parasassi et al., Citation1998). In this study a significant decrease in generalized polarization of Laurdan probe was observed with CGA at the highest concentrations used in erythrocytes and RBCL membranes. Such changes in polarization of Laurdan probe may result from different strength of binding between CGA and the respective membranes. As shown by the fluorimetric studies with the three probes, CGA binds to the surface polar head region of both the erythrocyte and model lipid membranes, affecting their packing arrangement, as evidenced by changes in GP, while barely penetrating the hydrophobic part, as evidenced by the lack of changes in fluorescence anisotropy (A).
In addition, the fluidity studies on erythrocyte membranes and liposomes formed of membrane lipids (RBCL) showed that the two membranes differ in their biophysical properties. The unmodified membranes, as evidenced in fluorimetric studies with the Laurdan, TMA-DPH and DPH probes, differ in their packing arrangement and lipid dynamics. The erythrocyte membrane, compared with the lipid membrane, has a more regular and denser packing of lipid molecules, which are thus less mobile. These differences must be connected with the presence of proteins in the erythrocyte membrane. Differences between the results for erythrocyte and lipid membranes may follow from different distribution of lipids in the liposome bilayer and erythrocyte membrane, in the latter the lipid asymmetry being maintained by appropriate mechanisms.
Monitoring the impact of CGA on the electrical properties of black lipid membranes may contribute to a better understanding of the molecular mechanism underlying its interaction with the membrane (Fettiplace et al., Citation1975). To this end, we examined the effect of CGA on capacity and resistance of BLM treated as a model lipid membrane in order to gain information on the physical properties of the hydrophilic and hydrophobic parts of the membrane caused by CGA. The results obtained in electric studies showed that in the presence of CGA there is a decrease in capacity and resistance of BLM, indicating that CGA binds to the black lipid membrane. The change in BLM capacity may be caused by CGA penetrating into the region of the membrane phospholipid polar heads. The presence of CGA in the hydrophilic area of membrane, treated as an electric double layer, causes changes in capacity, which may be due to both changes in electric charge distribution and change in orientation of the polar heads of lipids. Such a cause for capacity changes was proposed by Movileanu et al. (Citation2000) and Everitt & Haydon (Citation1968). CGA may therefore affect the magnitude of electrostatic interactions in the hydrophilic layer of the lipid bilayer, thus changing the dipole potential of the membrane. This is made possible by the presence of polar OH groups in the CGA molecule. The presence of this compound in the surface layer of the membrane also has an influence on the observed changes in BLM resistance. Hydrophilic compounds, including CGA, are believed to cause up to several-fold changes in resistance of black lipid membranes (Bender & Tien, Citation1987; Koronkiewicz & Kalinowski, Citation2004; Lauger et al., Citation1967). CGA in the range of concentrations used causes changes in the membrane potential, which is probably related to changes in the spatial orientation of the heads of phospholipids in the hydrophilic area of BLM.
The results of the study allow an interpretation of the antioxidant action of CGA reported by Bonarska-Kujawa et al. (Citation2011a). In that study it was shown that CGA takes position in the hydrophilic area of the membrane, and owing to that it effectively protects the erythrocyte membrane against oxidation. The protection is more effective than that provided by Trolox against free radicals induced by, e.g. AAPH (Trolox IC50 = 0.0039 ± 0.0003 mg/ml, CGA IC50 = 0.0009 ± 0.0001 mg/ml, Bonarska-Kujawa et al., Citation2011a). The higher antioxidant capacity of CGA is likely to be connected with different localization of the two compounds in the hydrophilic area of membrane. The studies on Trolox (results not included) conducted with the use of fluorescent probes indicate that the compound does not induce changes in the hydrophilic and hydrophobic regions of erythrocyte and lipid membranes. Unlike for CGA, the lack of changes in the polar part of the erythrocyte and lipid membranes evidenced in the presence of Trolox with the use of Laurdan probe can be explained both by the small size of its molecules and localization mainly in the aqueous phase adjacent to the membrane.
It is known that the partition and location of Trolox in the membrane surface are pH dependent and depend also on the type of lipids used (Barclay et al., Citation1995). In a phosphate solution of pH 7.4 (used in our experiments) Trolox has a negatively charged carbonyl group, which enables it to interact electrostatically with the choline group of membrane lipids (Lucio et al., Citation2009). Such interaction may result in changes caused by Trolox in areas situated above the place whence the Laurdan probe emits fluorescence, making its presence within membrane undetectable.
Conclusions
All the experimental results show that CGA induces changes in the hydrophilic part of both the erythrocyte membrane and the lipid membrane formed of red blood cells lipids. This compound becomes incorporated mainly into the outer hydrophilic part of the membranes. CGA had practically no influence on fluidity in the hydrophobic region of the membranes, i.e. in the area of their hydrocarbon chains.
A similar suggestion was made by Arrora & Strasbourg (Citation1997), by saying that polyphenols, including phenolic acids, due to numerous hydroxyl groups strongly interact electrostatically with the surface of the membrane.
The presence of CGA in the body is very important for protection against many serious diseases induced by oxidative stress, as it reduces the concentration of free radicals that cause oxidation of biological structures. Such protective effects take place at CGA concentrations which do not cause any destructive action on the membranes, evidenced by, e.g. our hemolytic and osmotic resistance assays.
To reach an appropriate concentration of CGA in the body, the acid can also be introduced into the organism by using nanotechnology that would enable liposome-encapsulated CGA to be distributed within the body.
| Abbreviations | ||
| DPH | = | 1,6-diphenyl-1,3,5-hexatriene |
| GP | = | generalized polarization |
| TMA-DPH | = | 1-(4-trimethylammoniumphenyl)-6-phenyl-1,3,5-hexatriene p-toluenesulfonate |
| Laurdan | = | 6-dodecanoyl-2-dimethylaminonaphthalene |
| BLM | = | black lipid membranes |
| CGA | = | chlorogenic acid |
| RBCL | = | red blood cell lipids |
| SUV | = | small unilamellar vesicles |
| Trolox® | = | 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid. |
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was financially supported from funds of the statutory activities of the Department of Physics and Biophysics, Wroclaw University of Environmental and Life Sciences.
References
- Arbos KA, Claro ML, Borges L, Santos CAM, Weffort-Santos AM. 2008. Human erythrocyte as a system for evaluating the antioxidant capacity of vegetable extracts. Nutr Res 28:457–463
- Arrora A, Strasbourg GM. 1997. Development and validation of fluorescence spectroscopic assays to calculate antioxidant efficacy. J Am Oil Chem Soc 74:1031–1040
- Barclay LR, Artz JD, Mowat JJ. 1995. Partitioning and antioxidant action of the water-soluble antioxidant, Trolox, between the aqueous and lipid phases of phosphatidylcholine membranes: 14C tracer and product studies. Biochim Biophys Acta 1237:77–85
- Bender CI, Tien HT. 1987. Electrical properties of bilayer lipid membranes containing iodine and iodide, investigated by cyclic voltammetry. Anal Chim Acta 201:51–58
- Benzie IFF. 2003. Evolution of dietary antioxidants. Comp Biochem Phys A 136:113–126
- Bessis M. 1977. La forme et la deformabilite des erythrocytes normaux et dans certaines anemies hemolytiques congenitales [Erythrocyte form and deformability for normal blood and some hereditary hemolytic anemias]. Nouvelle Revue Francaise d’Hematologie 18:75–94
- Bonarska-Kujawa D, Cyboran S, Oszmiański J, Kleszczyńska H. 2011a. Extracts from apple leaves and fruits as effective antioxidants. J Med Plants Res 5:2339–2347
- Bonarska-Kujawa D, Cyboran S, Żyłka R, Oszmiański J, Kleszczyńska H. 2014a. Biological activity of blackcurrant extracts (Ribes nigrum L.) in relation to erythrocyte membranes. Biomed Res Int 2014:783059. doi: 10.1155/2014/783059
- Bonarska-Kujawa D, Pruchnik H, Cyboran S, Żyłka R, Oszmiański J, Kleszczyńska H. 2014b. Biophysical mechanism of the protective effect of the polyphenols extracts from blue honeysuckle (Lonicera caerulea L. var. kamtschatica Sevast.) against lipid peroxidation of erythrocyte and lipid membranes. J Mem Biol 247:611–625
- Bonarska-Kujawa D, Pruchnik H, Kleszczyńska H. 2012. Interaction of selected anthocyanins with erythrocytes and liposome membranes. Cel Mol Biol Let 17:289–308
- Bonarska-Kujawa D, Pruchnik H, Oszmiański J, Sarapuk J, Kleszczyńska H. 2011b. Changes caused by fruit extracts in the lipid phase of biological and model membranes. Food Biophys 6:58–67
- Bradford M. 1976. Rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
- Bukowska B, Michałowicz J, Krokosz A, Sicińska P. 2007. Comparison of the effect of phenol and its derivatives on protein and free radical formation in human erythrocytes (in vitro). Blood Cells Mol Dis 39:238–244
- Chaudhuri S, Banerjee A, Basu K, Sengupta B, Sengupta PK. 2007. Interaction of flavonoids with red blood cell membrane lipids and proteins: antioxidant and antihemolytic effects. Int J Biol Macromol 41:42–48
- Chaudhuri S, Biswapathik P, Sengupta PK. 2009. Ground and excited state proton transfer and antioxidant activity of 7-hydroxyflavone in model membranes: absorption and fluorescence spectroscopic studies. Biophys Chem 139:29–36
- Clifford MN. 1999. Chlorogenic acid and other cinnamates: nature, occurrence and dietary burden. J Sci Food Agric 7:362–372
- Cyboran S, Bonarska-Kujawa D, Kapusta I, Oszmiański J, Kleszczyńska H. 2011. Antioxidant potentials of polyphenolic extracts from leaves of trees and fruit bushes. Curr Top Biophys 34:15–21
- Cyboran S, Oszmiański J, Kleszczyńska H. 2012. Interaction between plant polyphenols and the erythrocyte membrane. Cell Mol Biol Lett 17:77–88
- Daayf F, Lattanzio V. 2008. Recent Advances in Polyphenol Research, Vol. 1. Oxford, UK: Wiley-Blackwell
- Deuticke B. 1968. Transformation and restoration of biconcave shape of human erythrocytes induced by amphiphilic agents and changes of ionic environment. Biochim Biophys Acta 163:494–500
- Deuticke B. 1977. Properties and structural basis of simple diffusion pathways in the erythrocyte membrane. Rev Physiol Biochem Pharmacol 78:2–79
- Dodge JT, Mitchell C, Hanahan DJ. 1963. The preparation and chemical characteristics of hemoglobin-free ghosts of erythrocytes. Arch Biochem 100:119–130
- Everitt CH, Haydon DA. 1968. Electrical capacitance of a lipid membrane separating two aqueous phases. J Theor Biol 18:371–379
- Farah A, Monteiro M, Donangelo CM, Lafay S. 2008. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J Nutr 138:2309–2315
- Fettiplace R, Gordon LGM, Hladky SB, Requena J, Zingsheim HP, Hydon DA. 1975. Techniques in the formation and examination of “black” lipid bilayer membranes. In Korn ED, ed. Methods in Membrane Biology, vol. 4. New York: Plenum Press, 1–75
- Harris FM, Best KB, Bell JD. 2002. Use of laurdan fluorescence intensity and polarization to distinguish between changes in membrane fluidity and phospholipid order. Biochim Biophys Acta 1565:123–128
- Iglic A, Kralj-Ilgic V, Hagerstand VH. 1998. Amphiphile induced echinocyte-spheroechinocyte transformation of red blood cell shape. Eur Biophys J 27:335–339
- Isomaa B, Hagerstrand H, Paatero G. 1987. Shape transformations induced by amphiphiles in erythrocytes. Biochim Biophys Acta 899:93–103
- Kalinowski S, Figaszewski Z. 1995a. A four electrode potentiostat-galvanostat for measurements of bilayer lipid membrane capacitance. Meas Sci Technol 6:1043–1049
- Kalinowski S, Figaszewski Z. 1995b. A four electrode potentiostat-galvanostat for studies of bilayer lipid membranes. Meas Sci Technol 6:1050–1056
- Kang J, Liu Y, Xie MX, Li S, Jiang M, Wang YD. 2004. Interaction of human serum albumin with chlorogenic acid and ferulic acid. Biochim Biophys Acta 1674:205–214
- Koronkiewicz S, Kalinowski S. 2004. Influence of cholesterol on electroporation of bilayer lipid membranes: chronopotentiometric studies. Biochim Biophys Acta 1661:196–203
- Lakowicz JR. 2006. Fluorescence polarization. In: Principles of Fluorescence Spectroscopy. New York/London: Plenum Press, 353–382
- Lauger P, Lesslauer W, Marti E, Richter J. 1967. Electrical properties of bimolecular phospholipid membranes. Biochim Biophys Acta 135:20–32
- Lifen H, Zhou B, Yang L, Liu ZL. 2004. Inhibition of free radical initiated peroxidation of human erythrocyte ghosts by flavanols and their glycosides. Org Biomol Chem 2:1419–1423
- Lucio M, Nunes C, Gaspar D, Ferreira H, Lima JFC, Reis S. 2009. Antioxidant activity of vitamin E and Trolox: understanding of the factors that govern lipid peroxidation studies in vitro. Food Biophys 4:312–320
- Maddy AH, Dunn MJ, Kelly PG. 1972. The characterization of membrane proteins by centrifugation and gel electrophoresis. A comparison of proteins prepared by different methods. Biochim Biophys Acta 288:263–278
- Marinova EM, Toneva A, Yanishlieva N. 2009. Comparison of the antioxidative properties of caffeic and chlorogenic acids. Food Chem 114:1498–1502
- Marques V, Farah A. 2009. Chlorogenic acids and related compounds in medicinal plants and infusions. Food Chem 113:1370–1376
- Movileanu L, Neagoe I, Flonta ML. 2000. Interaction of the antioxidant flavonoid quercetin with planar lipid bilayers. Int J Pharm 205:135–146
- Mueller P, Rudin DO, Tien HT, Wescott WC. 1962. Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature 194:979–980
- Nenadis N, Boyle S, Bakalbassis EG, Tsimidou M. 2003. An experimental approach to structure-activity relationships of caffeic and dihydrocaffeic acids and related monophenols. J Am Oil Chem Soc 80:451–458
- Parasassi T, Krasnowska EK, Bagatolli L, Gratton E. 1998. Laurdan and prodan as polarity-sensitive fluorescent membrane probes. J Fluorescence 8:365–373
- Pawlikowska-Pawlęga B, Gruszecki WI, Misiak LE, Gawron A. 2003. The study of the quercetin action on human erythrocyte membranes. Biochem Pharmacol 66:605–612
- Pchelkin VP. 2003. Medical plants, natural phenolic and lipophilic complexes of chlorogenic acid. Pharma Chem J 37:27–29
- Perez-Fons L, Garzon MT, Micol V. 2010. Relationship between the antioxidant capacity and effect of rosemary (Rosmarinus officinalis L.) polyphenols on membrane phospholipid order. J Agric Food Chem 58:161–171
- Pruchnik H, Bonarska-Kujawa D, Kleszczyńska H. 2014. Effect of chlorogenic acid on the phase transition in phospholipid and phospholipid/cholesterol membranes. J Therm Anal Calorim 118:943–950
- Rawel HM, Rohn S, Krause HP, Kroll J. 2002. Structural changes induced in bovine serum albumin by covalent attachment of chlorogenic acid. Food Chem 78:443–455
- Rice-Evans CA, Miller NJ, Paganga G. 1996. Structure-antioxidant activity relationships of flavonoids and phenolic compounds. Free Rad Biol Med 20:933–956
- Rice-Evans CA, Miller NJ, Paganga G. 1997. Antioxidant properties of phenolic compounds. Trends Plant Sci 2:152–159
- Santos-Buelga C, Escribano-Bailon MT, Lattanzio V. 2010. Recent Advances in Polyphenol Research, Vol. 2. Oxford, UK: Wiley-Blackwell
- Sato Y, Itagaki S, Kurokawa T, Ogura J, Kobayashi M, Hirano T, et al. 2011. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int J Pharm 403:136–138
- Sheetz MP, Singer SJ. 1974. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Nat Acad Sci USA 71:4457–4461
- Shi G, Rao L, Yu H, Xiang H, Pen G, Long S, Yang C. 2007. Yeast-cell-based microencapsulation of chlorogenic acid as a water-soluble antioxidant. J Food Eng 80:1060–1067
- Sinisi V, Forzato C, Cefarin N, Navarini L, Berti F. 2015. Interaction of chlorogenic acids and quinides from coffee with human serum albumin. Food Chem 168:332–340
- Suwalsky M, Orellana P, Avello M, Villena F. 2007. Protective effect of Ugni molinae Turcz against oxidative damage of human erythrocytes. Food Chem Toxicol 45:130–135
- Suwalsky M, Vargas P, Avello M, Villena F, Sotomayor CP. 2008. Human erythrocytes are affected in vitro by flavonoids of Aristotelia chilensis (Maqui) leaves. Int J Pharm 363:85–90
- Wang Y, Zhang T, Xu J, Du W. 2011. Comparison of the binding affinity of chlorogenic acid with two serum albumins. Int J Biol Macromol 48:81–86
- Włoch A, Kapusta I, Bielecki K, Oszmiański J, Kleszczyńska H. 2013. Activity of hawthorn leaf and bark extracts in relations to biological membrane. J Membrane Biol 246:545–556
- Zang L, Cosma G, Garden H, Castranova V, Vallyathan V. (2003). Effect of chlorogenic acid on hydroxyl radical. Mol Cell Biochem 247:205–210