Abstract
Detergents are amphiphilic compounds that have crucial roles in the extraction, purification and stabilization of integral membrane proteins and in experimental studies of their structure and function. One technique that is highly dependent on detergents for solubilization of membrane proteins is solution-state NMR spectroscopy, where detergent micelles often serve as the best membrane mimetic for achieving particle sizes that tumble fast enough to produce high-resolution and high-sensitivity spectra, although not necessarily the best mimetic for a biomembrane. For achieving the best quality NMR spectra, detergents with partial or complete deuteration can be used, which eliminate interfering proton signals coming from the detergent itself and also eliminate potential proton relaxation pathways and strong dipole-dipole interactions that contribute line broadening effects. Deuterated detergents have also been used to solubilize membrane proteins for other experimental techniques including small angle neutron scattering and single-crystal neutron diffraction and for studying membrane proteins immobilized on gold electrodes. This is a review of the properties, chemical synthesis and applications of detergents that are currently commercially available and/or that have been synthesized with partial or complete deuteration. Specifically, the detergents are sodium dodecyl sulphate (SDS), lauryldimethylamine-oxide (LDAO), n-octyl-β-D-glucoside (β-OG), n-dodecyl-β-D-maltoside (DDM) and fos-cholines including dodecylphosphocholine (DPC). The review also considers effects of deuteration, detergent screening and guidelines for detergent selection. Although deuterated detergents are relatively expensive and not always commercially available due to challenges associated with their chemical synthesis, they will continue to play important roles in structural and functional studies of membrane proteins, especially using solution-state NMR.
Introduction
Detergents are amphiphilic compounds usually having a well-defined hydrophilic domain often referred to as the ‘hydrophilic head’ and a separate hydrophobic domain often referred to as the ‘hydrophobic tail’ (). The high aqueous solubility of detergent molecules, as monomers and as micelles or when associated with other molecules, has given them a crucial role in the extraction, purification and stabilization of integral membrane proteins and in experimental studies of their structure and function including crystallization and NMR spectroscopy (le Maire et al., Citation2000, Damberg et al., Citation2001, Garavito & Ferguson-Miller, Citation2001, Seddon et al., Citation2004, Privé, Citation2007, Kim et al., Citation2009, Arnold & Linke, Citation2008, Lin & Guidotti, Citation2009, Linke, Citation2009, Sonoda et al., Citation2011, Arachea et al., Citation2012). The detergent molecules act as a membrane mimetic by surrounding the membrane protein in a protein-detergent micelle complex () that solubilizes and/or stabilizes them in an aqueous environment, and therefore allows them to be used in a wide range of experimental techniques. Under such conditions, care has to be taken as to what extent the native structure and functional activity of the protein is retained. There are of course a number of other membrane mimetics used in the final stages of experimental studies with membrane proteins including organic solvents, lipids, bicelles, nanodiscs, fluorinated surfactants and amphipols (Bayburt & Sligar, Citation2010, Popot, Citation2010, Warschawski et al., Citation2011, Dürr et al., Citation2012,Citation2013, Inagaki et al., Citation2013, Zhou & Cross, Citation2013, Zoonens and Popot, Citation2014) which have different advantages and disadvantages, but this review is limited to detergent micelles. Even with these alternative membrane mimetics, detergents are often still used during the early stages of experimental procedures with membrane proteins, for example in initial solubilization from the native membrane.
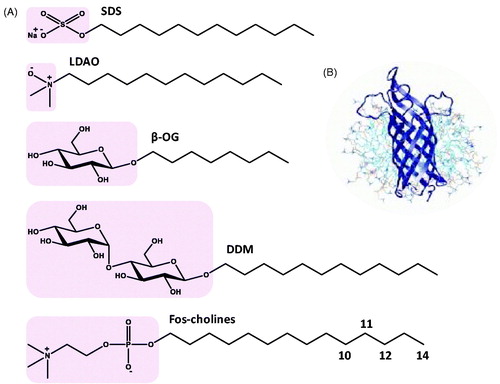
Figure 1. Structures of detergents and of a protein-micelle complex. (A) Chemical structures of the detergents sodium dodecyl sulphate (SDS), lauryldimethylamine-oxide (LDAO), n-octyl-β-D-glucoside (β-OG), n-dodecyl-β-D-maltoside (DDM) and fos-cholines -10, -11, -12 and -14. The pink areas indicate the hydrophilic head groups. (B) Illustration of a protein-detergent micelle complex: Molecular dynamics simulation of the outer membrane β-barrel protein OmpA from Escherichia coli in a dodecylphosphocholine micelle. The picture of the OmpA-micelle complex was reproduced with permission from Bond and Sansom (Citation2003), which was originally published in JMB (Bond PJ, Sansom MS. 2003. Membrane protein dynamics versus environment: Simulations of OmpA in a micelle and in a bilayer. J Mol Biol 329:1035–1053), copyright by Elsevier Science Ltd 2003. This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
One technique that has been highly dependent on detergents for structural and functional studies of integral membrane proteins is solution-state NMR spectroscopy. Detergents often provide the best solubility, stability, isotropic and homogenous samples, and particle sizes that tumble fast enough for achieving high-resolution and high-sensitivity NMR spectra. Detergent samples are also generally more straightforward to prepare than those using other membrane mimetics. Indeed, the large majority of integral membrane protein structures determined by solution-state NMR have used proteins solubilized in detergent micelles (Page et al., Citation2006, Kim et al., Citation2009, Nietlispach & Gautier, Citation2011, Patching, Citation2011, Klammt et al., Citation2012, Arora, Citation2013, Maslennikov & Choe, Citation2013, Reckel and Hiller, Citation2013). An earlier assessment of the prevalence of detergent types in membrane structural biology revealed that approximately 40% of 115 membrane protein structures determined by NMR were prepared in dodecylphosphocholine micelles, whilst nearly 40% of 1200 membrane protein structures determined by X-ray crystallography were in the sugar-based detergents n-octyl-β-D-glucoside, n-decyl-β-D-maltoside or n-dodecyl-β-D-maltoside (Oliver et al., Citation2013, Raman et al., Citation2006). The most prolific detergents used in experimental studies are not necessarily the best for retaining the native structure and functional activity of the protein, however. In many cases, detergents with partial deuteration or complete deuteration (perdeuteration) have been used for NMR. Deuterated detergents eliminate interfering proton signals in NMR spectra that come from the detergent itself, which can be very intense and not easily removed by the NMR pulse sequence. They also eliminate potential proton relaxation pathways and strong dipole-dipole interactions that would otherwise contribute to line broadening effects on the spectra. Using deuterated detergents therefore provides better resolution and sensitivity, access to overlapped areas of the protein spectrum and simplifies the application of more advanced pulse sequences. The potential benefits from using deuterated detergents for NMR studies of membrane proteins were first demonstrated with the 26-residue amphiphilic peptide melittin bound to fully deuterated dodecylphoshocholine micelles (Brown, Citation1979, Brown & Wüthrich, Citation1981). These conditions allowed an almost complete assignment of 1H NMR resonances and the measurement of 1H-1H nuclear Overhauser effects (NOEs) to reveal global features for the conformation of micelle-bound melittin. Proton detected NMR experiments usually require the use of deuterated detergents. Heteronuclear NMR experiments can help in eliminating the interference of detergent signals through the intrinsic filtering effects of the pulse sequence. It has been suggested that for large membrane protein-detergent complexes there is no significant difference in the quality of heteronuclear correlation spectra obtained using a non-deuterated or perdeuterated detergent (Arora and Tamm, Citation2001). For example, the quality of [15N,1H]-TROSY and TROSY-HNCA spectra of the 30 kDa human β-barrel outer membrane protein VDAC-1 was independent of the degree of detergent deuteration (Hiller & Wagner, Citation2009). The same study, however, also showed a sensitivity decrease of around 10–30% in 15N-resolved [1H,1H]-NOESY spectra when going from deuterated to protonated detergent and use of deuterated detergent was essential for the recording of 3D and 4D NOESY-type spectra of isoleucine, leucine and valine methyl groups (Hiller & Wagner, Citation2009). It therefore appears that while backbone directed experiments may be performed in a protonated detergent, NOE experiments and experiments involving side-chain signals have clear benefits from the use of deuterated detergents. Also, the studies described above that comment on the effects of a deuterated detergent on the quality of heteronuclear correlation spectra both refer to β-barrel membrane proteins. We suggest that the effects of a deuterated detergent are protein-specific since in our experience with a large α-helical membrane protein, a deuterated detergent was essential for achieving the best quality [15N,1H]-TROSY spectra (see later). The use of a deuterated detergent is generally beneficial to solution-state NMR structural studies of membrane proteins.
Despite the essential requirement of deuterated detergents for structural and functional studies of membrane proteins using solution-state NMR and other techniques, only a relatively few are commercially available or have been produced at all. Principal reasons for this are the challenges and costs associated with their chemical synthesis. The amphipathic nature of detergents immediately introduces extra challenges to the synthesis of these compounds and their precursors, especially in work-up and purification steps. Introduction of partial or complete deuteration into detergent compounds requires identification of suitable and commercially available deuterated starting compounds and/or reagents along with an appropriate synthetic route. Furthermore, the deuterated precursor compounds are themselves generally relatively expensive since they have to be produced by biological or chemical deuteration. Details for producing specific deuterated precursor compounds for synthesis of deuterated detergents are described in later sections of this review. The original source of deuterium nuclei for these deuterated precursor compounds is usually deuterium oxide (D2O). D2O is produced using the Girdler-Sulphide or Girdler-Spevack process that depends on exchange of deuterium atoms between molecules in a mixture of water and hydrogen sulphide (Andreeva, Citation2001); this is followed by final concentration to 99.8% heavy water by vacuum distillation or electrolysis based on Nobel prize winning work by Urey and co-workers (Urey et al., Citation1932, Washburn & Urey, Citation1932).
It is fortunate that, chemically, deuterium behaves similarly to ordinary hydrogen, but there are significant differences in bond energy and in bond length for compounds of heavy hydrogen isotopes that are larger than the isotopic differences in any other element. For example, the C-D bond is around 10 times stronger than the C-H bond and therefore more resistant to breakage; O-D, N-D and S-D bonds are also stronger than the corresponding protonated forms (Katz, Citation1965, Thomas Citation1971). Such large effects are seen because when hydrogen is replaced with deuterium the mass is doubled, whilst there is a much smaller change in mass for isotope substitutions of other elements such as 12C for 13C or 14N for 15N. Consequently, deuteration can have large effects on the rates of chemical and biochemical reactions, especially when the position of deuteration is directly involved in the breaking or formation of covalent bonds in the rate limiting step (primary kinetic isotope effect) (Wiberg Citation1955, Westheimer Citation1961). Indeed, experimental kinetic isotope effect values for deuterium (= rate of reaction with protium/rate of reaction with deuterium, kH/kD) can be as large as 10 or more with a theoretical maximum of 18 (Bigeleisen & Goeppert-Mayer, Citation1947, Saunders et al., Citation1960, Westheimer Citation1961, Pascal et al., Citation1986, Yu et al., Citation1987, Krumbiegel, Citation2011, Pedras et al., Citation2011), whilst experimental values for 13C or 15N are typically in the range 1.01–1.07 with a theoretical maximum of 1.25 (Bigeleisen & Goeppert-Mayer, Citation1947, Isaacs, Citation1987, Wade, Citation1999, Krumbiegel, Citation2011). The deuterium kinetic isotope effect has been used to study reaction mechanisms, enhance the stability of technical products against oxidative and hydrolytic degradation and to alter the rates of metabolism and the pharmacokinetic effects of drug compounds (Wiberg Citation1955, Hoffman et al., Citation1983, White et al., Citation1983, Baldwin et al., Citation2004, Cleland, Citation2005, He et al., Citation2006, Shao & Hewitt, Citation2010, Krumbiegel, Citation2011, Sharma et al., Citation2012, Simmons & Hartwig, Citation2012, Manley et al., Citation2013, Guengerich, Citation2013, Timmins, Citation2014). Deuterium isotope effects on non-covalent interactions between molecules are generally much less significant but they can be substantial (Wade, Citation1999). Based on some of the investigations performed so far, deuteration of proteins does not appear to have significant effects on protein structure, but it can affect protein function and substrate specificity through kinetic isotope effects (Murad et al., Citation1977, Yang & Ishizaki, Citation1992, Hochuli et al., Citation2000, Mittermaier & Kay, Citation2002, Fisher & Helliwell, Citation2008, de Ghellinck et al., Citation2014). No published work appears to be available on the effects of deuteration on detergent properties (e.g., shape, CMC, aggregation number, solubility, thermodynamic and volumetric parameters, phase behaviour) or the effects of deuterated detergents on the solubilization of proteins or on protein structure or function.
The following sections in this review consider detergent screening approaches, the properties and chemical synthesis of deuterated detergents and examples of their applications for structural and functional studies of membrane proteins using solution-state NMR spectroscopy and other techniques, and some guidelines for choosing an appropriate detergent.
Detergent screening
In choosing the most suitable detergent for NMR studies with a membrane protein a number of factors have to be considered. These include achievement of good quality spectra from a stable NMR sample under conditions that retain the native structure and activity of the protein. The detergent that provides the highest quality NMR spectra is not necessarily the best for retaining structure and activity, so a balanced view has to be taken. Due to curvature of the water-micelle interface detergents that form the largest micelles tend to have the least deleterious effects on membrane protein structure, but it is more challenging to achieve high resolution NMR spectra for larger complexes.
The screening of detergents for experimental studies with membrane proteins has some common attributes for using a wide range of techniques, including crystallography and NMR. An initial screen of multiple detergents can simply determine if the protein is soluble or if it precipitates. This can involve purification in a mild and stable detergent, binding to an affinity column, washing and elution with a buffer containing a new detergent and running on an SDS-PAGE gel. If the protein precipitates in the new detergent it will remain on the column or if the protein is solubilized by the new detergent it will be eluted and give a band on the gel. In this test the concentrations of both protein and detergent can be varied and it can be made high-throughput. Under conditions of successful solubilization, the protein can then be tested for structural and functional integrity, monodipersity and thermal stability using a range of enzymatic, ligand binding or spectroscopic assays of which some can be made high-throughput. Circular dichroism spectroscopy can be used to test secondary structure (far-UV) and tertiary structure (near-UV) integrity, thermal stability and ligand binding activity (Kelly & Price, Citation2000, Miles & Wallace, Citation2006, Patching et al., Citation2012, Bettaney et al., Citation2013, Matsuo & Gekko, Citation2013, Siligardi et al., Citation2014). Fluorescence spectroscopy can be used to test structural integrity (spectral shape), ligand binding activity and thermal stability (Kalverda et al., Citation2014) and a microscale fluorescent screen using the thiol-specific fluorochrome N-[4-(7-diethylamino-4-methyl-3-coumarinyl)phenyl]maleimide (CPM) has been developed for screening stability (Alexandrov et al., Citation2008). Light scattering and turbidity measurements can be used to test for aggregation and to measure particle size (Goñi and Alonso, Citation2000, Postis et al., Citation2008, Slotboom et al., Citation2008, Neale et al., Citation2013, Meyer et al., Citation2015). Differential filtration can be used to screen stability and particle size (Vergis et al., Citation2010). Electrophoresis (e.g., SDS-PAGE, native-PAGE) and chromatography (e.g., size exclusion) can be used alongside these techniques to test for structural degradation, aggregation, monodispersity and oligomerization and to measure the size of protein-detergent complexes. Analytical ultracentrifugation can be used to investigate the oligomeric state and the detergent-to-protein ratio in protein-detergent complexes and to evaluate sample homogeneity (Maslennikov et al., Citation2007). Cell-free expressed membrane proteins can be produced in the presence of a detergent, which can also serve as an initial test for solubilization or precipitation (Klammt et al., Citation2005). Because deuterated detergents are generally not used for crystallography, the use and screening of detergents for crystallography will not be considered further in this review and the reader is referred to other published work on this theme (Yeh et al., Citation2006, Privé, Citation2007, Tate, Citation2010, Sonoda et al., Citation2011, Kang et al., Citation2013).
Regardless of the techniques described above, the detergent solubilized protein will only be suitable for solution-state NMR studies if it produces sufficiently high quality multidimensional NMR spectra in terms of sensitivity and resolution, number and dispersion of peaks and lifetime of the sample. This can initially be assessed by obtaining 2D correlation 15N-1H HSQC or TROSY or 13C-1H methyl-TROSY NMR spectra for highly pure 15N- and/or 13C-labelled protein in homogenous samples with different detergents and with varying concentrations of protein and detergent and using different temperatures, pH values and salt concentrations (Krueger-Koplin et al., Citation2004, Page et al., Citation2006, Chen et al., Citation2011). Larger proteins will likely require deuteration of the protein and benefit from a deuterated detergent for the reasons already described. The area under the amide proton region in 1D 1H spectra as a function of time can be used as an indicator of NMR sample stability (Krueger-Koplin et al., Citation2004). The lifetime of peaks in the NMR spectra should correlate with measurements in the stability of protein structural integrity and activity obtained using other techniques under the same sample and temperature conditions. For some detergent-solubilized membrane proteins, such as receptors or those with an enzymatic function, an activity assay using the natural ligand or substrate may be feasible. For other membrane proteins, such as channels and transporters, an assay of their functional activity is not usually possible under detergent solubilized conditions, so an assay of inhibitor binding may be used. In some cases membrane protein activity may be demonstrated in an NMR experiment by observing the shift, appearance or disappearance of peaks following addition of an appropriate ligand. The size of membrane protein-detergent complexes in the NMR sample can be estimated from pulsed field gradient (PFG) translational diffusion measurements (Krueger-Koplin et al., Citation2004, Horst et al., Citation2011, Yao et al., Citation2014) or from rotational correlation times obtained using NMR relaxation measurements (Korchuganov et al., Citation2004, Krueger-Koplin et al., Citation2004, Lee et al., Citation2006). For membrane proteins available only in small quantities, microcoil NMR technology has been developed for screening the detergent solubilization, proper folding and translational diffusion of microscale quantities of membrane proteins destined for structural studies (Zhang et al., Citation2008, Stanczak et al., Citation2009, Horst et al., Citation2012, Stanczak et al., Citation2012). There have been some attempts to rationalize the predictive selection of detergents for optimal solubilization, sample homogeneity and native protein folding with specific membrane proteins in NMR structural studies. These include the design of mixed micelles based on matching of micelle dimensions to those of the hydrophobic surface of the protein to avoid exchange processes that reduce NMR observations (Columbus et al., Citation2009), correlation of micelle properties with ligand-binding activity (O’Malley et al., Citation2011), assessment of amino acid sequence hydropathy (Nadeau et al., Citation2012) and the effects of detergent concentration and changes in effective CMC values for specific detergents in the presence of a membrane protein (Horst et al., Citation2012). Clearly, more work on this theme has to be performed before robust generalized guidelines can be made for predictive detergent selection.
Deuterated detergents
The use of deuterated detergents in experimental studies with membrane proteins using solution-state NMR and other techniques introduces extra considerations, which include the pattern of deuteration in the detergent molecule, availability and cost. Deuterated detergents that are currently advertised as commercially available (up to the end of 2014) are listed as follows: Sodium dodecyl sulphate (SDS) in perdeuterated form (d25-SDS); lauryldimethylamine-oxide (LDAO) in perdeuterated form (d31-LDAO); n-octyl-β-D-glucoside (β-OG) with the aliphatic tail deuterated (d17-β-OG) and perdeuterated (d24-β-OG); n-dodecyl-β-D-maltoside (DDM) with the aliphatic tail deuterated (d25-DDM); fos-choline-10 (n-decylphosphocholine) with a semi-deuterated head (d9-fos-choline-10) and with a perdeuterated head (d13-fos-choline-10); fos-choline-11 (n-undecylphosphocholine) with a semi-deuterated head (d9-fos-choline-11) and with a perdeuterated head (d13-fos-choline-11); fos-choline-12 (dodecylphosphocholine, DPC) with a semi-deuterated head (d9-DPC), perdeuterated head (d13-DPC), tail deuterated (d25-DPC) and perdeuterated (d38-DPC); fos-choline-14 (n-tetradecylphosphocholine) with a semi-deuterated head (d9-fos-choline-14), perdeuterated head (d13-fos-choline-14) and perdeuterated (d42-fos-choline-14). The chemical structures of these detergents are shown in and some of their properties are given in .
Table I. Properties of detergents. These properties are for the undeuterated compounds and were obtained from catalogues of the product suppliers: Anatrace, Cambridge Isotope Laboratories, Cortecnet, Generon, Sigma-Aldrich.
Sodium dodecyl sulphate
Sodium dodecyl sulphate (SDS), also known as sodium lauryl sulphate, is an anionic detergent with an aliphatic 12-carbon chain and a small negatively charged head group ( and ). SDS is a harsh detergent often used as a protein denaturant, hence its use in polyacrylamide gel electrophoresis for the separation of proteins and for estimation of their molecular masses in a denatured state. Despite these properties, SDS has been used to solubilize a significant number of membrane proteins for investigations of their structure and function. In the case of solution-state NMR, the principal reason for this is its ability to form relatively small and uniform complexes with membrane proteins that tumble fast enough in solution to achieve high-resolution spectra. The large majority of NMR structures determined in SDS micelles are for membrane peptides or for relatively small α-helical membrane proteins. Structures of membrane proteins determined in SDS micelles include fd coat protein (Almeida & Opella, Citation1997), the mitochondrial membrane protein stannin (Buck-Koehntop et al., Citation2005), MerF of the mercury detoxification system from Morganella Morganii (Howell et al., Citation2005), the human zetazeta-transmembrane domain (Call et al., Citation2006), a thermostable mutant of the potassium ion channel KcsA (Chill et al., Citation2006), the human DAP12-NKG2C heterotrimeric immunoreceptor complex (Call et al., Citation2010), LC4 region of CC chemokine receptor 5 (Miyamoto & Togiya, Citation2011) and regulatory subunits of the of the Na,K-ATPase (Franzin et al., Citation2007, Teriete et al., Citation2007, Gong et al., Citation2015). The synthesis of SDS is most commonly achieved by sulphonation of n-dodecanol (Takei et al., Citation1985) using sulphur trioxide (Mitsuda & Kono, Citation2012), chlorosulphonic acid (Mészáros et al., Citation2005) or sulphuric acid (Yamamoto, Citation2000) followed by neutralization of the resultant sulphate using sodium hydroxide or sodium carbonate (). Uniformly deuterated SDS (d25-SDS) can be produced by using d25-n-dodecanol in this synthesis. d25-n-Dodecanol, which is commercially available, can be produced by reduction of the deuterated fatty acid (d23-n-dodecanoic acid) using lithium aluminium deuteride (LiAlD4). An early method for preparing saturated fatty acids with deuteration at all carbon positions was to heat fatty acids with D2O in the presence of alkali (KOH) and active platinum (van Heyningen et al., Citation1938), but this method gave only partial deuteration at each position. A simple and efficient method for preparing fully deuterated fatty acids was later developed by heating (195 °C) fatty acids and deuterium gas over a palladium on charcoal catalyst (Hsiao et al., Citation1974). High isotopic purity deuterium gas (>99.98%) can be produced by electrolysing high isotopic purity D2O (99.8%) down to around 30% of its original volume (Persky and Kuppermann, Citation1974). A similar approach has been used for deuteration of fatty acyl chains in synthetic phospholipid molecules (Török et al., Citation1993). There has also been a renewed and increased interest in using a variety of hydrogen/deuterium-exchange reactions at carbon centres for production of deuterium-labelled compounds rather than using classical synthesis with deuterated precursors (Atzrodt et al., Citation2007). Due to its relative ease of synthesis and longstanding commercial availability, d25-SDS has been one of the most commonly used deuterated detergents for solubilising membrane proteins, principally for solution-state NMR measurements of structure, dynamics and ligand binding interactions.
Figure 2. Synthesis of d25-SDS and its use in solubilizing helix A from brome mosaic virus protein 1a and the C-terminal region of the Frizzled receptor 1. (A) Synthesis of d25-SDS from d25-n-dodecanol. The grey area shows the region of deuteration. (B) (i) [15N-1H]HSQC spectrum with assignments of brome mosaic virus 1a helix A bound to 100 mM d25-SDS micelles and (ii) ensemble of 20 structures (backbone atoms only) determined for helix A bound to an SDS micelle where white = helix and grey = coil. This picture was modified from Liu et al. (Citation2009), which was originally published in PLoS Pathog (Liu L, Westler WM, den Boon JA, Wang X, Diaz A, Steinberg HA, Ahlquist P. 2009. An amphipathic alpha-helix controls multiple roles of brome mosaic virus protein 1a in RNA replication complex assembly and function. PLoS Pathog 5:e1000351), copyright by Liu et al., Citation2009. (C) (i) [15N-1H]HSQC spectrum with assignments of the C-terminal region of the Frizzled receptor 1 (residues 623-647) in d25-SDS micelles, (ii) NOESY spectrum with NOE interactions labelled and (iii) structure determined from 104 NOE restraints. This picture was modified from Gayen et al. (Citation2013) which was originally published in Molecules (Gayen S, Li Q, Kim YM, Kang C. 2013. Structure of the C-terminal region of the Frizzled receptor 1 in detergent micelles. Molecules 18:8579–8590), copyright by Gayen et al., Citation2013. This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
![Figure 2. Synthesis of d25-SDS and its use in solubilizing helix A from brome mosaic virus protein 1a and the C-terminal region of the Frizzled receptor 1. (A) Synthesis of d25-SDS from d25-n-dodecanol. The grey area shows the region of deuteration. (B) (i) [15N-1H]HSQC spectrum with assignments of brome mosaic virus 1a helix A bound to 100 mM d25-SDS micelles and (ii) ensemble of 20 structures (backbone atoms only) determined for helix A bound to an SDS micelle where white = helix and grey = coil. This picture was modified from Liu et al. (Citation2009), which was originally published in PLoS Pathog (Liu L, Westler WM, den Boon JA, Wang X, Diaz A, Steinberg HA, Ahlquist P. 2009. An amphipathic alpha-helix controls multiple roles of brome mosaic virus protein 1a in RNA replication complex assembly and function. PLoS Pathog 5:e1000351), copyright by Liu et al., Citation2009. (C) (i) [15N-1H]HSQC spectrum with assignments of the C-terminal region of the Frizzled receptor 1 (residues 623-647) in d25-SDS micelles, (ii) NOESY spectrum with NOE interactions labelled and (iii) structure determined from 104 NOE restraints. This picture was modified from Gayen et al. (Citation2013) which was originally published in Molecules (Gayen S, Li Q, Kim YM, Kang C. 2013. Structure of the C-terminal region of the Frizzled receptor 1 in detergent micelles. Molecules 18:8579–8590), copyright by Gayen et al., Citation2013. This Figure is reproduced in colour in the online version of Molecular Membrane Biology.](/cms/asset/b5b5f0a2-349a-4c4e-a00d-cb4e16e5c39a/imbc_a_1125536_f0002_c.jpg)
Early NMR studies with the ion channel-forming pentadecapeptide gramicidin A achieved high-resolution 2D spectra for the peptide solubilized in d25-SDS micelles. These were used to confirm that the ion-channel state of gramicidin A adopts an N-terminal to N-terminal (head-to-head) dimer formed by two right-handed, single-stranded helices with 6.3 residues per turn (Arseniev et al., Citation1985, Bystrov et al., Citation1986). This work also demonstrated the future potential for investigating the structure and function of ion channels solubilized in detergent micelles using solution-state NMR spectroscopy. Gramicidin A solubilized in d25-SDS micelles was later used as a simplified model for transmembrane ion channels to investigate their interactions with the anaesthetic and non-immobilizer compounds 1-chloro-1,2,2-trifluorocyclobutane and 1,2-dichlorohexafluoro-cyclobutane, respectively, by 2D 1H-1H NOESY measurements (Tang et al., Citation1999). The former compound significantly altered the chemical shifts of tryptophan indole N-H protons near the channel entrance, consistent with anaesthetic compounds causing a functional change of the channel by interacting with the amphipathic domains at the peptide-lipid water interface. Another model membrane protein, the 50-residue M13 coat protein, which becomes an integral membrane protein during the infection stage of the life cycle of the M13 phage, has been solubilized in d25-SDS micelles for measurement of side-chain dynamics by 1H NMR (O’Neil & Sykes, Citation1989). 1H-exchange rates for a primary amide in the side chain of glutamine-15 and for the indole amine of tryptophan-26 were measured. Whilst the glutamine-15 proton exchanged at a rate identical with that in glutamine model peptides, the tryptophan-26 indole amine proton exchange was biphasic, possibly reflecting protein dimerization or aggregation in the SDS micelles. 1H NMR measurements on a model transmembrane helix based on the GCN4 leucine zipper solubilized in d25-SDS micelles helped to demonstrate how inter-helical hydrogen bonding drives strong interactions in membrane proteins (Zhou et al., Citation2000). This was achieved by monitoring cross peaks in NOESY spectra that revealed information about an asparagine side chain and helical secondary structure near that position. A number of complementary NMR approaches have been used to investigate the structure and interaction of mastoparan, a 14-residue peptide toxin from wasp venom, with lipid membranes (Hori et al., Citation2001). These included determination of the 3D structure of mastoparan solubilized in d25-SDS using 1H NOE measurements and distance geometry calculation, which revealed a straight amphipathic α-helix. Combined with solid-state NMR experiments that described the interaction, orientation and insertion of mastoparan with lipid bilayers, the results were used to propose a pore-forming peptide that can undergo a flip-flop between monolayers and therefore movement of mastoparan across the membrane. The conformation of orexin-B, an orphan G-protein coupled receptor agonist and neuropeptide implicated in sleep-wakefulness and feeding regulation, in d25-SDS micelles was determined by 2D NMR and molecular modelling (Miskolzie et al., Citation2003). The 28-residue peptide had a secondary structure containing two α-helical segments (residues 7–18 and 22–26) believed to be involved in membrane binding and the unstructured C-terminus (residues 27 and 28) is proposed to have conformational freedom for binding to the receptor. Interaction of the neurotransmitters dopamine and acetylcholine with an amphiphilic resorcinarene receptor solubilized in d25-SDS micelles has also been investigated by 1H NMR measurements (Demura et al., Citation2005). Distances of these neurotransmitters from the hydrophilic cavity of the receptor were estimated based on calculation of the ring current shift using atomic coordinates obtained from molecular dynamics calculation. NMR analysis of an 18-residue amphipathic peptide (residues 392–409, also known as helix A) solubilized in d25-SDS has helped to demonstrate how it controls the multiple roles of the brome mosaic virus protein 1a in RNA replication complex assembly and function (Liu et al., Citation2009). This study included determination of the 3D structure of the peptide based on measurement of NOE restraints and additional dihedral angle constraints, which revealed an α-helical conformation for residues 397–406 (). Screening of detergents for NMR analysis of the catalytic C-terminal domain (residues 466–718) of the Stt3p subunit from yeast oligosaccharyl transferase identified d25-SDS to be the most suitable (over DDM, DPC, digitonin, LDAO and OG) since it produced a 2D [15N-1H]HSQC spectrum with good dispersion and narrow line widths providing a count of 245 resolved peaks out of 263 non-proline residues (Huang et al., Citation2010). Furthermore, CD spectra showed that the C-terminus of Stt3p is highly helical and has a stable tertiary structure in SDS micelles. Although this work did not yet determine the structure of the Stt3p C-terminal domain, peptide ligand binding was measured using NMR saturation transfer difference (STD) and titration experiments with Ile/Leu/Val-methyl-protonated samples and 2D [15N-1H]HSQC spectra, respectively. A structure of a 25-residue sequence (residues 623-647) from the C-terminal domain of the Frizzled receptor 1 in d25-SDS micelles was determined from 104 NOE restraints () (Gayen et al., Citation2013). This revealed that residues 627–639 formed an α-helix and that the C-terminus of the peptide was not structured. The NMR structure and an analysis of the helices hydrophobic properties indicated that it is an amphipathic α-helix, which may have similar function to the helix 8 of classical G protein-coupled receptors at the membrane interface.
In addition to NMR, d25-SDS has also found use in small angle neutron scattering (SANS) studies with membrane proteins as a contrasting reagent, especially for investigation of association states and conformational changes (Breyton et al., Citation2013). For example, SANS has contributed to a study of the multimeric forms of the small multidrug resistance protein EmrE solubilized in d25-SDS micelles revealing different shapes for EmrE at varying concentrations of detergent and in presence of the substrate tetraphenyl phosphonium (Bay et al., Citation2010). In these experiments the use of d25-SDS instead of hydrogenated SDS enhanced the contrast for EmrE within the detergent micelle. The water solvent was contrast-matched with d25-SDS so that the detergent became invisible due to the differences in deuterium/hydrogen scattering angles and resulting in the scattering pattern being that of EmrE alone.
Lauryldimethylamine-oxide
Lauryldimethylamine-oxide (LDAO), also known as N,N-dimethyldodecylamine-N-oxide (DDAO), is a non-denaturing zwitterionic detergent with an aliphatic 12-carbon chain ( and ). LDAO has been used to solubilize a small number of membrane proteins for determination of their 3D structure by solution-state NMR. These proteins include the autonomously folding Bacillus subtilis protein mistic that can be used for high-level production of other membrane proteins (Roosild et al., Citation2005), the human voltage-dependent anion channel (VDAC-1) (Bayrhuber et al., Citation2008, Hiller et al., Citation2008, Hiller & Wagner, Citation2009) and transmembrane domains of the α4 and β2 subunits of the nicotinic acetylcholine receptor (Bondarenko et al., Citation2012). Uniformly deuterated LDAO (d31-LDAO) is commercially available from a number of sources and a synthesis from d23-dodecanoic acid has been described (Orädd et al., Citation1995). d23-Dodecanoic acid was reacted with d6-dimethylamine to give d29-N,N-dimethyldodecanoylamide, which was reduced to the amine using d4-lithium aluminium hydride then oxidation with hydrogen peroxide gave d31-LDAO (). The same work also used the d31-LDAO and 2H solid-state NMR to investigate phase equilibria and molecular packing in a system of LDAO/peptide gramicidin D/water (Orädd et al., Citation1995). The deuterated precursor compound d23-dodecanoic acid is commercially available and its production by deuteration of the unlabelled fatty acid was described in the section for SDS. d6-Dimethylamine can be prepared from d9-trimethylamine via d9-trimethylamine-N-oxide (Renaud & Leitch, Citation1968) and the d9-trimethylamine can be prepared by heating deuterated methyl iodide (CD3I) and ammonium hydroxide (NH4OH) (Walther et al., Citation1981). The d4-lithium aluminium hydride (LiAlD4) can be prepared from lithium deuteride (LiD) and aluminium bromide (AlBr3) with prior preparation of lithium deuteride by the direct combination of lithium and deuterium at 700 °C with the deuterium having been obtained from D2O by the use of magnesium (Holding & Ross, Citation1958). The solution-state NMR structure determination of human VDAC-1 used d31-LDAO for solubilization revealing a novel β-barrel fold with 19 transmembrane strands and with the first and last strands parallel with each other giving a closed structure () (Hiller et al., Citation2008). The NMR structure of human VDAC-1 was in close agreement with a combined NMR/X-ray crystal structure of the same protein (Bayrhuber et al., Citation2008) and with an X-ray crystal structure of mouse VDAC-1 (Ujwal et al., Citation2008, Hiller & Wagner, Citation2009).
Figure 3. Synthesis of d31-LDAO and its use in solubilizing the human voltage-dependent anion channel (VDAC-1) and use of tail-deuterated decyl-N,N′-dimethyl amine oxide in solubilizing Escherichia coli outer membrane protein OmpF. (A) Synthesis of d31-LDAO from d23-dodecanoic acid. The grey areas show the regions of deuteration. (B) (i) [15N-1H]-TROSY spectrum of [U-2H,15N]VDAC-1 in d31-LDAO micelles, (ii) [13C-1H]HMQC spectrum of [U-2H,13C,15N; 1Hδ-IL; 1Hγ-V]VDAC-1 in d31-LDAO micelles highlighting the spectral regions for Ile residues (red box, 11/11 assigned) and Leu plus Val residues (blue box, 8/12 Val and 17/28 Leu assigned), (iii) structure of human VDAC-1 shown as a side view (top) and from above (bottom) with the N-terminus in blue and C-terminus in red, which were drawn using PDB file 2K4T and PDB Protein Workshop 3.9 (Moreland et al., Citation2005). Pictures of the spectra were reproduced with permission from Hiller et al. (Citation2008), which were originally published in Science (Hiller S, Garces RG, Malia TJ, Orekhov VY, Colombini M, Wagner G. 2008. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science 321:1206–1210, copyright by American Association for the Advancement of Science 2008. (C) (i) Crystal structure of the E. coli outer membrane protein OmpF in tetragonal crystal form as an above view with the N-terminus in blue and C-terminus in red, which was drawn using PDB file 1OPF and PDB Protein Workshop 3.9 (Moreland et al., Citation2005). (ii) Single-crystal neutron diffraction density map of OmpF in tail-deuterated decyl-N,N′-dimethyl amine oxide detergent contrast mapped parallel to the three-fold trimer axis where the porin trimer is represented by the Cα trace (pink) obtained from the X-ray crystal structure. This picture was reproduced with permission from Pebay-Peyroula et al. (Citation1995), which was originally published in Structure (Pebay-Peyroula E, Garavito RM, Rosenbusch JP, Zulauf M, Timmins PA. 1995. Detergent structure in tetragonal crystals of OmpF porin. Structure 3:1051–1059, copyright by Elsevier Inc. 1995. (iii) Structure of the detergent d21-decyl-N,N′-dimethyl amine oxide. The grey area shows the region of deuteration. This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
![Figure 3. Synthesis of d31-LDAO and its use in solubilizing the human voltage-dependent anion channel (VDAC-1) and use of tail-deuterated decyl-N,N′-dimethyl amine oxide in solubilizing Escherichia coli outer membrane protein OmpF. (A) Synthesis of d31-LDAO from d23-dodecanoic acid. The grey areas show the regions of deuteration. (B) (i) [15N-1H]-TROSY spectrum of [U-2H,15N]VDAC-1 in d31-LDAO micelles, (ii) [13C-1H]HMQC spectrum of [U-2H,13C,15N; 1Hδ-IL; 1Hγ-V]VDAC-1 in d31-LDAO micelles highlighting the spectral regions for Ile residues (red box, 11/11 assigned) and Leu plus Val residues (blue box, 8/12 Val and 17/28 Leu assigned), (iii) structure of human VDAC-1 shown as a side view (top) and from above (bottom) with the N-terminus in blue and C-terminus in red, which were drawn using PDB file 2K4T and PDB Protein Workshop 3.9 (Moreland et al., Citation2005). Pictures of the spectra were reproduced with permission from Hiller et al. (Citation2008), which were originally published in Science (Hiller S, Garces RG, Malia TJ, Orekhov VY, Colombini M, Wagner G. 2008. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science 321:1206–1210, copyright by American Association for the Advancement of Science 2008. (C) (i) Crystal structure of the E. coli outer membrane protein OmpF in tetragonal crystal form as an above view with the N-terminus in blue and C-terminus in red, which was drawn using PDB file 1OPF and PDB Protein Workshop 3.9 (Moreland et al., Citation2005). (ii) Single-crystal neutron diffraction density map of OmpF in tail-deuterated decyl-N,N′-dimethyl amine oxide detergent contrast mapped parallel to the three-fold trimer axis where the porin trimer is represented by the Cα trace (pink) obtained from the X-ray crystal structure. This picture was reproduced with permission from Pebay-Peyroula et al. (Citation1995), which was originally published in Structure (Pebay-Peyroula E, Garavito RM, Rosenbusch JP, Zulauf M, Timmins PA. 1995. Detergent structure in tetragonal crystals of OmpF porin. Structure 3:1051–1059, copyright by Elsevier Inc. 1995. (iii) Structure of the detergent d21-decyl-N,N′-dimethyl amine oxide. The grey area shows the region of deuteration. This Figure is reproduced in colour in the online version of Molecular Membrane Biology.](/cms/asset/523c487a-9d51-4978-b6f0-c3e6bb1f75c5/imbc_a_1125536_f0003_c.jpg)
A tail-deuterated form a of a related detergent decyl-N,N′-dimethyl amine oxide has been used in single-crystal neutron diffraction studies with the Escherichia coli outer membrane protein OmpF in its tetragonal crystal form (Pebay-Peyroula et al., Citation1995). In the tetragonal crystal form of OmpF the protein surface normally buried in the membrane is accessible to the detergent solution and therefore provides an opportunity for protein-detergent interactions to be studied. Using the X-ray crystal structure (Cowan et al. Citation1995) as a model, the neutron diffraction measurements revealed how detergent molecules bind to the hydrophobic region of the OmpF trimer that is exposed to lipid in its native environment (). These measurements used partially deuterated decyl-N,N′-dimethyl amine oxide in order to increase the contrast between protein and detergent (Pebay-Peyroula et al., Citation1995).
n-Octyl-β-D-glucoside
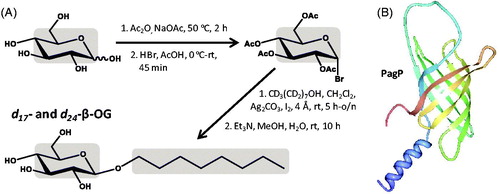
n-Octyl-β-D-glucoside (β-OG) is a non-ionic detergent with an aliphatic eight-carbon chain ( and ). The mild and non-denaturing properties of β-OG make it an attractive detergent to solubilize membrane proteins for studies of their structure and function, but its shorter chain length can contribute to protein deactivation. Only a few NMR structures of membrane proteins have been determined in β-OG micelles, presumably because the shorter aliphatic chain does not usually produce the most stable sample and/or best quality spectra compared with the longer chain detergents. β-OG is commercially available with just the aliphatic chain deuterated (d17-β-OG) and in perdeuterated form (d24-β-OG), which can be synthesized by coupling n-octanol with D-glucose using one or both of these starting compounds in their deuterated forms, respectively (Keana & Roman, Citation1978, Rosevear et al., Citation1980) (). The deuterated precursor compound d17-n-octanol is commercially available and can be produced by reduction of the deuterated fatty acid d15-n-octanoic acid with deuterated lithium aluminium hydride (LiAlD4); the deuteration of fatty acids was described in the section for SDS. The d7-D-glucose is commercially available and can be isolated from the hydrolysate of the carbohydrate fraction of algae grown in a deuterated medium or prepared using an isotopic hydrogen-exchange technique that introduces deuterium by catalytic exchange with carbon-bound hydrogen. The exchange reaction uses D2O and deuterated Raney nickel, which itself is produced using D2O (Koch & Stuart, Citation1978). A synthesis of β-OG, and of other sugar-containing detergents, using a microwave-assisted glycosylation reaction from methyl glycosides may be useful for improving the yields of the deuterated compounds (Yoshimura et al., Citation2005). The best known NMR structure for a membrane protein solubilized in d24-β-OG micelles is that for the bacterial outer membrane enzyme PagP, which transfers a palmitate chain from a phospholipid to lipid A (Hwang et al., Citation2002). The structure, which was also determined in d38-DPC micelles, consists of an eight-stranded anti-parallel β-barrel preceded by an N-terminal amphipathic α-helix (). Deuterated forms of β-OG have also been used for solubilization of membrane proteins in SANS experiments (Breyton et al., Citation2013), including d17-β-OG with the outer-membrane 16-stranded β-barrel transport protein FhaC of the Bordetella pertussis filamentous hemagglutinin adhesion (Gabel et al., Citation2014). SANS measurements were combined with molecular modelling to describe the solution structure of FhaC in which the N-terminal α-helix was inside the pore consistent with the crystal structure (Clantin et al., Citation2007).
Figure 4. Synthesis of d17- and d24-β-OG and use of d24-β-OG in solubilizing the bacterial outer membrane enzyme PagP. (A) Synthesis of d17- and d24-β-OG from d17-n-octanol and d7-D-glucose. The grey areas show the regions of deuteration. (B) Structure of PagP with the N-terminus in blue and C-terminus in red, which was drawn using PDB file 1MM5 and PDB Protein Workshop 3.9 (Moreland et al., Citation2005). This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
n-Dodecyl-β-D-maltoside
n-Dodecyl-β-D-maltoside (DDM) is a non-ionic detergent with an aliphatic 12-carbon chain ( and ), which tends to disrupt lipid-lipid and lipid-protein interactions but not protein-protein interactions. The mild and non-denaturing properties of DDM make it a commonly used detergent for the extraction and purification of membrane proteins and for solubilization in experimental studies of their structure, dynamics and function (Ward et al., Citation2000, Seddon et al., Citation2004, Arnold & Linke, Citation2008, Pagliano et al., Citation2012, Rouse et al., Citation2013). DDM tends to retain the native structure and functional activity of membrane proteins to a greater extent than any other detergents (Alexandrov et al., Citation2008). It can therefore serve as the control condition when screening a number of different detergents for structural and functional studies of membrane proteins and can be used to validate the use of other detergents. Despite being one of the most successful detergents for membrane protein crystallization (Newstead et al., Citation2008, Parker & Newstead, Citation2012, He et al., Citation2014), very few NMR studies of membrane proteins have been performed using DDM, however, since it tends to form relatively large protein-micelle complexes compared with those of other detergents. NMR studies using DDM include work with the potassium ion channel KcsA (Takeuchi et al., Citation2007) and with the E. coli sugar transport protein GalP (Kalverda et al., Citation2014). DDM with deuteration in just the aliphatic chain (d25-DDM) is commercially available, but a perdeuterated form (d39-DDM) is not. d25-DDM can be synthesized by coupling maltose with d25-n-dodecanol () (Hines et al., Citation1997, Vacklin et al., Citation2005a) and this has been used in solution-state NMR studies with bacteriorhodopsin (Patzelt et al., Citation2002, Schubert et al., Citation2002) and with the potassium ion channel KcsA (Imai et al., Citation2010). It has also been used in investigations of the composition of supported model membranes determined by neutron reflection (Vacklin et al., Citation2005a, 2005b), in studies of hydrogen oxidation by a membrane-bound hydrogenase immobilized on gold electrodes (Ciaccafava et al., Citation2012) and in SANS studies of solubilized membrane proteins (Breyton et al., Citation2013). The first synthesis of d39-DDM has recently been reported (Hiruma-Shimizu et al., Citation2014). This first required the coupling of two molecules of d7-D-glucose with an α(1→4) glycosidic bond to give d14-maltose followed by coupling with d25-n-dodecanol to give d39-DDM (). Methods for preparing the deuterated precursor compounds d25-n-dodecanol and d7-D-glucose were described in the sections for SDS and β-OG, respectively. The synthesized d39-DDM has been used to solubilize the 52 kDa E. coli sugar transport protein GalP to achieve the best resolution and highest sensitivity [15N-1H]TROSY spectra for amino acid selective labelled samples of a membrane protein with 12 unique transmembrane-spanning α-helices () (Kalverda et al., Citation2014). Based on the costs of the synthesis, the cost of d39-DDM per typical NMR sample was £500–£1000. This work also achieved high resolution [13C-1H]methyl-TROSY spectra for ILV-selective labelled samples of GalP and used the spectra to detect binding of a small-molecule inhibitor, which was possible using non-deuterated DDM for solubilization. For performing more advanced methyl-TROSY based experiments on the largest membrane protein-detergent complexes, use of a deuterated detergent is likely to be essential, however.
Figure 5. Synthesis of d25- and d39-DDM and use of d39-DDM in solubilizing the E. coli sugar transport protein GalP. (A) Synthesis of d25- and d39-DDM from d25-n-dodecanol and d7-D-glucose, see the work of Hiruma-Shimizu et al. (Citation2014) for the complete synthesis, reaction and experimental details. The grey areas show the regions of deuteration. (B) [15N-1H]TROSY spectrum at 900 MHz of [U-2H, 15N2-Trp]GalP in d39-DDM micelles reproduced from Kalverda et al. (Citation2014) in this journal. This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
![Figure 5. Synthesis of d25- and d39-DDM and use of d39-DDM in solubilizing the E. coli sugar transport protein GalP. (A) Synthesis of d25- and d39-DDM from d25-n-dodecanol and d7-D-glucose, see the work of Hiruma-Shimizu et al. (Citation2014) for the complete synthesis, reaction and experimental details. The grey areas show the regions of deuteration. (B) [15N-1H]TROSY spectrum at 900 MHz of [U-2H, 15N2-Trp]GalP in d39-DDM micelles reproduced from Kalverda et al. (Citation2014) in this journal. This Figure is reproduced in colour in the online version of Molecular Membrane Biology.](/cms/asset/a69eb0f4-41ea-4938-9dd5-02b4116d3677/imbc_a_1125536_f0005_c.jpg)
Fos-cholines
The fos-cholines are zwitterionic detergents with a range in lengths of their aliphatic carbon chain ( and ). By far the most commonly used of these is fos-choline-12, also known as dodecylphosphocholine or DPC, which has a 12-carbon aliphatic chain. DPC is also one of the most commonly used of all detergents, notably it has been used to solubilize a large number of membrane proteins for determination of their structures by NMR. An earlier assessment of the prevalence of detergent types in membrane structural biology revealed that approximately 40% of 115 membrane protein structures determined by NMR were prepared in DPC (Raman et al., Citation2006, Oliver et al., Citation2013). These structures include the bacterial outer membrane proteins OmpA (Arora et al., Citation2001, Cierpicki et al., Citation2006), PagP (Hwang et al., Citation2002), OmpG (Liang and Tamm, Citation2007) and OmpX (Hagn et al., Citation2013), the α-helical proteins human phospholamban (Oxenoid and Chou, Citation2005), KcsA-charybdotoxin complex (Yu et al., Citation2005), disulfide bond formation protein B (DsbB) (Zhou et al., Citation2008), diacylglycerol kinase (DAGK) (Van Horn et al., Citation2009), voltage sensor domain from KvaP (Shenkarev et al., Citation2010), p7 channel from hepatitis C virus (Montserret et al., Citation2010, OuYang et al., Citation2013), mitochondrial uncoupling protein 2 (Berardi et al., Citation2011) and others (Patching, Citation2011, http://www.drorlist.com/nmr/MPNMR.html). A mention of all structures is beyond the scope of this review, so a full list of NMR structures of membrane proteins determined in DPC micelles (up to the end of 2014) is given in Supplementary Table S1 (available online).
Approximately a half of the NMR membrane protein structures used perdeuterated DPC (d38-DPC), which is commercially available and can be synthesized from d25-n-dodecanol, d4-ethylene glycol and d9-trimethylamine () (Magolda & Johnson, Citation1985). The first reported synthesis of d38-DPC used a similar approach but began with the production of deuterated n-dodecanol from dodecanoic acid (Brown, Citation1979). DPC is also commercially available with a semi-deuterated head (d9-DPC), a perdeuterated head (d13-DPC) and with just the aliphatic chain deuterated (d25-DPC), which are obtainable by using just one or two of the deuterated starting compounds or reagents in the synthesis. Methods for preparing the deuterated precursor compounds d25-n-dodecanol and d9-trimethylamine were described in the sections for SDS and LDAO, respectively. d4-Ethylene glycol (HOCD2CD2OH) can be produced by catalytic oxidation of d4-ethylene (CD2CD2) by atmospheric oxygen at 200–300 °C over a catalyst containing metallic silver to give d4-ethylene oxide followed by hydration. The d4-ethylene can be produced by the action of zinc dust suspended in dioxane on d4-dibromoethane (BrCD2CD2Br), which is prepared from d2-acetylene (CDCD) and deuterium bromide (DBr) (Leitch & Morse, Citation1952). d2-Acetylene can be prepared from 1,4-dioxane and D2O and deuterium bromide prepared by the action of D2O on redistilled phosphorus tribromide (Leitch & Morse, Citation1952). The Fos-cholines with other chain lengths can be produced in deuterated forms by using the appropriate deuterated alcohol in the synthesis (). One of the first NMR structures of a β-barrel membrane protein, the transmembrane domain of OmpA, was determined with the protein solubilized in d38-DPC micelles to reveal an eight-stranded antiparallel β-barrel () (Arora et al., Citation2001) that was closely similar to a crystal structure of the same protein in n-octyltetraoxyethylene micelles (Pautsch and Schulz, Citation1998,Citation2000).
Figure 6. Synthesis of deuterated fos-cholines and use of d38-DPC in solubilizing the bacterial outer membrane protein OmpA and use of d42-fos-choline-14 in solubilizing the human DAP12-NKG2C complex. (A) Synthesis of deuterated fos-cholines. The grey areas show the regions of deuteration. (B) (i) [15N-1H]TROSY spectrum of OmpA in d38-DPC micelles. This picture was reproduced by permission from Macmillan Publishers Ltd: [Nature Structural Biology] (Arora A, Abildgaard F, Bushweller JH, Tamm LK. 2001. Structure of outer membrane protein A transmembrane domain by NMR spectroscopy. Nat Struct Biol 8:334–338), copyright (2001). (ii) Structure of OmpA with the N-terminus in blue and C-terminus in red, which was drawn using PDB file 1G90 and PDB Protein Workshop 3.9 (Moreland et al., Citation2005). (C) [15N-1H]HSQC spectra of trimer samples segmentally labeled with 15N-2H on the DAP12-only strand (i) or on the DAP12-NKG2C strand (ii) and of the DAP12 homodimer alone (iii) for samples in 250 mM d42-fos-choline-14 with 25 mM d25-SDS and (iv) structure of the DAP12-NKG2C complex with the N-terminus in blue and C-terminus in red, which was drawn using PDB file 2L35 and PDB Protein Workshop 3.9 (Moreland et al., Citation2005). Pictures of the spectra were reproduced by permission from Macmillan Publishers Ltd: [Nature Immunology] (Call ME, Wucherpfennig KW, Chou JJ. 2010. The structural basis for intramembrane assembly of an activating immunoreceptor complex. Nat Immunol 11:1023–1029), copyright (2010). This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
![Figure 6. Synthesis of deuterated fos-cholines and use of d38-DPC in solubilizing the bacterial outer membrane protein OmpA and use of d42-fos-choline-14 in solubilizing the human DAP12-NKG2C complex. (A) Synthesis of deuterated fos-cholines. The grey areas show the regions of deuteration. (B) (i) [15N-1H]TROSY spectrum of OmpA in d38-DPC micelles. This picture was reproduced by permission from Macmillan Publishers Ltd: [Nature Structural Biology] (Arora A, Abildgaard F, Bushweller JH, Tamm LK. 2001. Structure of outer membrane protein A transmembrane domain by NMR spectroscopy. Nat Struct Biol 8:334–338), copyright (2001). (ii) Structure of OmpA with the N-terminus in blue and C-terminus in red, which was drawn using PDB file 1G90 and PDB Protein Workshop 3.9 (Moreland et al., Citation2005). (C) [15N-1H]HSQC spectra of trimer samples segmentally labeled with 15N-2H on the DAP12-only strand (i) or on the DAP12-NKG2C strand (ii) and of the DAP12 homodimer alone (iii) for samples in 250 mM d42-fos-choline-14 with 25 mM d25-SDS and (iv) structure of the DAP12-NKG2C complex with the N-terminus in blue and C-terminus in red, which was drawn using PDB file 2L35 and PDB Protein Workshop 3.9 (Moreland et al., Citation2005). Pictures of the spectra were reproduced by permission from Macmillan Publishers Ltd: [Nature Immunology] (Call ME, Wucherpfennig KW, Chou JJ. 2010. The structural basis for intramembrane assembly of an activating immunoreceptor complex. Nat Immunol 11:1023–1029), copyright (2010). This Figure is reproduced in colour in the online version of Molecular Membrane Biology.](/cms/asset/70253013-ad9a-4721-af38-d53f462c0801/imbc_a_1125536_f0006_c.jpg)
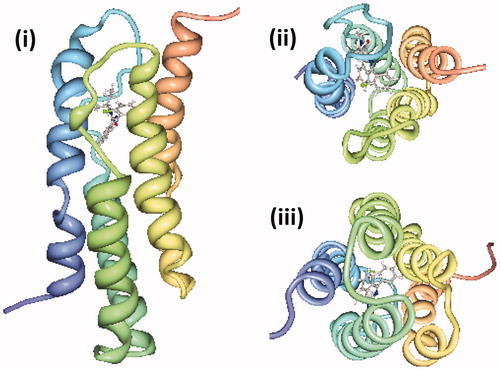
Recent NMR structures of α-helical membrane proteins determined in d38-DPC micelles include amyloid precursor protein transmembrane domains (Nadezhdin et al., Citation2011,Citation2012, Chen et al., Citation2014), phospholamban pentamer phosphorylated at serine 16 (Vostrikov et al., Citation2013), HIV-1 envelope glycoprotein gp41 ectodomain (Roche et al., Citation2014) and a gp41 envelope membrane proximal region trimer (Reardon et al., Citation2014) and the 18 kDa mitochondrial translocator protein with a high affinity ligand (Jaremko et al., Citation2014). The latter protein mediates the uptake of cholesterol and porphyrins into mitochondria and its expression is strongly up-regulated in areas of brain injury and in neuroinflammatory conditions. High quality NMR spectra of the protein that enabled structure determination were only achieved for the ligand-bound state. The structure had a tight bundle of five unique transmembrane-spanning α-helices with the ligand positioned towards the cytoplasmic side (). Ligand-induced stabilization of the structure led to a proposed molecular mechanism for the stimulation of cholesterol transport into mitochondria (Jaremko et al., Citation2014). The longer chain d42-Fos-choline-14 has been used with d25-SDS in a ratio of 10:1 to solubilise the human DAP12-NKG2C immunoreceptor complex for determination of its NMR structure (Call et al., Citation2010). The transmembrane domain of the DAP12 signalling molecule has two identical α-helices and the transmembrane domain of the natural killer cell activating receptor NKG2C has one α-helix that packs in an antiparallel orientation along the surface of the DAP12 dimer (). These recent structures demonstrate that deuterated forms of DPC and other fos-cholines are still the most successful membrane mimetic for determination of membrane protein structures by solution-state NMR, including those involved in human diseases.
Figure 7. NMR structure of the mitochondrial translocator protein with high affinity ligand determined in d38-DPC micelles. The NMR structure of the mitochondrial translocator protein with high affinity ligand 1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinoline-carboxamide (PK11195) determined in d38-DPC micelles (Jaremko et al., Citation2014) is shown as a side view (i), viewed from the cytoplasm (ii) and viewed from the intermembrane space (iii) with the N-terminus in blue and C-terminus in red, which were drawn using PDB file 2MGY and PDB Protein Workshop 3.9 (Moreland et al., Citation2005). This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
Guidelines for detergent selection
In order to consolidate some guidelines to select a suitable detergent for solution-state NMR structural studies of membrane proteins, we can first consider the physical properties of detergent molecules and their effects on micelle formation, protein structure and function, and quality of NMR spectra. These are summarized in . Detergent molecules that have a charged head group, smaller size of head group and shorter length of alkyl chain tend to have lower aggregation numbers and produce smaller micelles and protein-detergent micelle complexes with shorter correlation times. These harsher conditions using detergents such as SDS and its shorter chain versions often produce the best quality NMR spectra, especially for peptides and smaller membrane proteins. On the other hand, these denaturing conditions that produce micelles with a high curvature are not necessarily suitable for retaining the native structural form and activity of the protein. Detergent molecules that have a neutral head group, larger head group and longer alkyl chain tend to have higher aggregation numbers and produce larger micelles and protein-detergent micelle complexes with longer correlation times. These milder conditions using detergents such as DDM and other long chain alkyl glycosides produce micelles with lower curvature and usually retain the native structural form and activity of the protein to a better extent, but produce poorer NMR spectra. There are therefore competing tensions in selecting the most suitable detergent for structural studies of membrane proteins using solution-state NMR and other experimental techniques. The solubility and CMC values of detergent molecules, which are affected by the properties given in , also have to be considered during sample preparation. For example, the high CMC value of 20–25 mM for β-OG () means that a relatively high concentration has to be used compared with other detergents. A sample that is stable to days or weeks of NMR data acquisition time at elevated temperatures (typically ≥20 °C) is also required based on current technical capabilities.
Figure 8. Tensions in selecting a detergent for solution-state NMR structural studies with a membrane protein. This diagram illustrates how the charge on the head group, size of the head group and length of the alkyl group in detergent molecules dictate the properties of detergents and micelle complex formation that provide opposing tensions with regards to achieving good quality NMR spectra and retaining native protein structure and activity. This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
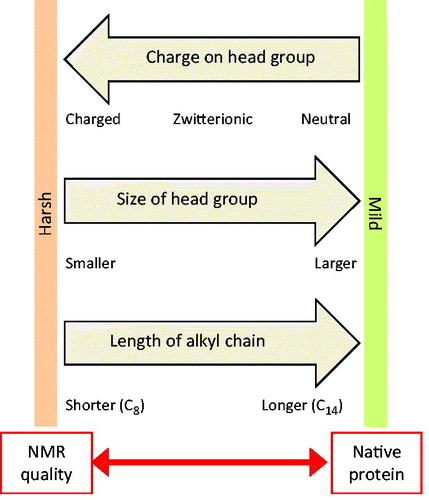
We can also look at which detergents have been most successful in producing structures of membrane proteins by solution-state NMR. As mentioned already, by far the most prolific is DPC, which is intermediate on the scale of detergent properties considered in and has solubility and CMC values that are experimentally favourable (). A detergent that has proved successful for solubilising a certain membrane protein in NMR structural studies may be assumed to be suitable for a different protein of the same type. β-barrel membrane proteins tend to accommodate a relatively wide range of detergents including those with harsher properties that favour better quality NMR spectra, for example LDAO with human VDAC-1 (). Large α-helical proteins, such as secondary transporters, are generally restricted to the mildest detergents such as DDM, hence the significant challenges in performing structural studies with these proteins using solution-state NMR. Such proteins likely require some of the native membrane lipids to be carried over into the detergent micelles for retaining their structural and functional integrity, which is facilitated by the milder detergents. Use of a deuterated detergent is generally beneficial and in some cases essential for solution-state NMR structural studies of membrane proteins, especially with larger proteins, and it is important for contrast mapping in SANS experiments. These are only guidelines and not strict rules, but they may be useful in conserving time and materials in the selection of a suitable detergent for structural studies of membrane proteins using solution-state NMR spectroscopy and other techniques. In reality, experimental investigations still have to be performed with each individual protein to identify suitable detergent or other membrane mimetic conditions. There have been some recent rigorous assessments of how different membrane mimetic environments, including detergents, affect the transmembrane domain structures of α-helical membrane proteins (Tulumello and Deber, Citation2012, Zhou and Cross, Citation2013). More work in this area will help in selecting suitable detergents and other membrane mimetics for structural studies of membrane proteins and for achieving native-like structures. With the continued development of NMR techniques for high molecular weight systems, we can envisage a migration to the more common use of milder detergents in solution-state NMR studies of membrane proteins.
Conclusions
This work has emphasized the crucial roles that detergents have in the extraction, purification and stabilization of integral membrane proteins and in experimental studies of their structure and function, especially using solution-state NMR spectroscopy. It has also highlighted the important role of deuterated detergents in these studies, even though deuterated detergents are relatively expensive and not always commercially available due to challenges associated with their chemical synthesis. Deuterated detergents provide better resolution and sensitivity in NMR spectra, access to overlapped areas of the protein spectrum and simplify the use of more advanced pulse sequences. Proton-detected NMR experiments usually require a deuterated detergent whilst heteronuclear experiments on larger proteins with 13C/15N labelling usually benefit from a deuterated detergent. Methyl-TROSY based experiments that are required for structure determination of larger membrane proteins also benefit from a deuterated detergent, which is likely to be essential for the most complex proteins. Deuterated detergents will continue to play important roles in structural and functional studies of membrane proteins along with deuterated forms of other membrane mimetics including organic solvents, lipids, nanodiscs, fluorinated surfactants and amphipols. Developments in the synthesis and production of deuterated forms of membrane mimetics is relatively unexplored and will continue to evolve and expand along with the world of membrane protein structure, mechanism, ligand interactions and dynamics.
Acknowledgements
This work was supported by the EU EDICT consortium (contract 201924).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alexandrov AI, Mileni M, Chien EY, Hanson MA, Stevens RC. 2008. Microscale fluorescent thermal stability assay for membrane proteins. Structure 16:351–359
- Almeida FC, Opella SJ. 1997. fd coat protein structure in membrane environments: structural dynamics of the loop between the hydrophobic trans-membrane helix and the amphipathic in-plane helix. J Mol Biol 270:481–495
- Andreeva BM. 2001. Separation of hydrogen isotopes in H2O-H2S system. Separat Sci Technol 36:1949–1989
- Arachea BT, Sun Z, Potente N, Malik R, Isailovic D, Viola RE. 2012. Detergent selection for enhanced extraction of membrane proteins. Protein Expr Purif 86:12–20
- Arnold T, Linke D. 2008. The use of detergents to purify membrane proteins. Curr Protoc Protein Sci Chapter 4:Unit 4.8.1–4.8.30
- Arora A. 2013. Solution NMR spectroscopy for the determination of structures of membrane proteins in a lipid environment. Methods Mol Biol 974:389–413
- Arora A, Abildgaard F, Bushweller JH, Tamm LK. 2001. Structure of outer membrane protein A transmembrane domain by NMR spectroscopy. Nat Struct Biol 8:334–338
- Arora A, Tamm LK. 2001. Biophysical approaches to membrane protein structure determination. Curr Opin Struct Biol 11:540–547
- Arseniev AS, Barsukov IL, Bystrov VF, Lomize AL, Ovchinnikov YuA. 1985. 1H-NMR study of gramicidin A transmembrane ion channel. Head-to-head right-handed, single-stranded helices. FEBS Lett 186:168–174
- Atzrodt J, Derdau V, Fey T, Zimmermann J. 2007. The renaissance of H/D exchange. Angew Chem Int Ed 46:7744–7765
- Baldwin JE, Gallagher SS, Leber PA, Raghavan AS, Shukla R. 2004. Deuterium kinetic isotope effects and mechanism of the thermal isomerization of bicyclo[4.2.0]oct-7-ene to 1,3-cyclooctadiene. J Org Chem 69:7212–7219
- Bay DC, Budiman RA, Nieh MP, Turner RJ. 2010. Multimeric forms of the small multidrug resistance protein EmrE in anionic detergent. Biochim Biophys Acta 1798:526–535
- Bayburt TH, Sligar SG. 2010. Membrane protein assembly into nanodiscs. FEBS Lett 584:1721–1727
- Bayrhuber M, Meins T, Habeck M, Becker S, Giller K, Villinger S, Vonrhein C, Griesinger C, Zweckstetter M, Zeth K. 2008. Structure of the human voltage-dependent anion channel. Proc Natl Acad Sci USA 105:15370–15375
- Berardi MJ, Shih WM, Harrison SC, Chou JJ. 2011. Mitochondrial uncoupling protein 2 structure determined by NMR molecular fragment searching. Nature 476:109–113
- Bettaney KE, Sukumar P, Hussain R, Siligardi G, Henderson PJ, Patching SG. 2013. A systematic approach to the amplified expression, functional characterization and purification of inositol transporters from Bacillus subtilis. Mol Membr Biol 30:3–14
- Bigeleisen J, Goeppert-Mayer M. 1947. Calculation of equilibrium constants for isotopic exchange reaction. J Phys Chem 15:261
- Bond PJ, Sansom MS. 2003. Membrane protein dynamics versus environment: simulations of OmpA in a micelle and in a bilayer. J Mol Biol 329:1035–1053
- Bondarenko V, Mowrey D, Tillman T, Cui T, Liu LT, Xu Y, Tang P. 2012. NMR structures of the transmembrane domains of the α4β2 nAChR. Biochim Biophys Acta 1818:1261–1268
- Breyton C, Gabel F, Lethier M, Flayhan A, Durand G, Jault JM, Juillan-Binard C, Imbert L, Moulin M, Ravaud S, Härtlein M, Ebel C. 2013. Small angle neutron scattering for the study of solubilised membrane proteins. Eur Phys J E Soft Matter 36:71
- Brown LR. 1979. Use of fully deuterated micelles for conformational studies of membrane proteins by high resolution 1H nuclear magnetic resonance. Biochim Biophys Acta 557:135–148
- Brown LR, Wüthrich K. 1981. Melittin bound to dodecylphosphocholine micelles. H-NMR assignments and global conformational features. Biochim Biophys Acta 647:95–111
- Buck-Koehntop BA, Mascioni A, Buffy JJ, Veglia G. 2005. Structure, dynamics, and membrane topology of stannin: a mediator of neuronal cell apoptosis induced by trimethyltin chloride. J Mol Biol 354:652–665
- Bystrov VF, Arseniev AS, Barsukov IL, Lomize AL. 1986. 2D NMR of single and double stranded helices of gramicidin A in micelles and solutions. Bull Magn Reson 8:84–94
- Call ME, Schnell JR, Xu C, Lutz RA, Chou JJ, Wucherpfennig KW. 2006. The structure of the zetazeta transmembrane dimer reveals features essential for its assembly with the T cell receptor. Cell 127:355–368
- Call ME, Wucherpfennig KW, Chou JJ. 2010. The structural basis for intramembrane assembly of an activating immunoreceptor complex. Nat Immunol 11:1023–1029
- Chen L, Lai C, Lai J, Tian C. 2011. Expression, purification, detergent screening and solution NMR backbone assignment of the human potassium channel accessory subunit MiRP1. Protein Expr Purif 76:205–210
- Chen W, Gamache E, Rosenman DJ, Xie J, Lopez MM, Li YM, Wang C. 2014. Familial Alzheimer’s mutations within APPTM increase Aβ42 production by enhancing accessibility of ɛ-cleavage site. Nat Commun 5:3037
- Chill JH, Louis JM, Miller C, Bax A. 2006. NMR study of the tetrameric KcsA potassium channel in detergent micelles. Protein Sci 15:684–698
- Ciaccafava A, De Poulpiquet A, Infossi P, Robert S, Gadiou R, Giudici-Orticoni MT, Lecomte S, Lojou E. 2012. A friendly detergent for H2 oxidation by Aquifex aeolicus membrane-bound hydrogenase immobilized on graphite and Self-Assembled-Monolayer-modified gold electrode. Electrochimica Acta 82:115–125
- Cierpicki T, Liang B, Tamm LK, Bushweller JH. 2006. Increasing the accuracy of solution NMR structures of membrane proteins by application of residual dipolar couplings. High-resolution structure of outer membrane protein A. J Am Chem Soc 128:6947–6951
- Clantin B, Delattre AS, Rucktooa P, Saint N, Méli AC, Locht C, et al. 2007. Structure of the membrane protein FhaC: a member of the Omp85-TpsB transporter superfamily. Science 317:957–961
- Cleland WW. 2005. The use of isotope effects to determine enzyme mechanisms. Arch Biochem Biophys 433:2–12
- Columbus L, Lipfert J, Jambunathan K, Fox DA, Sim AY, Doniach S, Lesley SA. 2009. Mixing and matching detergents for membrane protein NMR structure determination. J Am Chem Soc 131:7320–7326
- Cowan SW, Garavito RM, Jansonius JN, Jenkins JA, Karlsson R, König N, et al. 1995. The structure of OmpF porin in a tetragonal crystal form. Structure 3:1041–1050
- Damberg P, Jarvet J, Gräslund A. 2001. Micellar systems as solvents in peptide and protein structure determination. Methods Enzymol 339:271–285
- de Ghellinck A, Schaller H, Laux V, Haertlein M, Sferrazza M, Maréchal E, et al. 2014. Production and analysis of perdeuterated lipids from Pichia pastoris cells. PLoS One 9:e92999
- Demura M, Yoshida T, Hirokawa T, Kumaki Y, Aizawa T, Nitta K, et al. 2005. Interaction of dopamine and acetylcholine with an amphiphilic resorcinarene receptor in aqueous micelle system. Bioorg Med Chem Lett 15:1367–1370
- Dürr UH, Gildenberg M, Ramamoorthy A. 2012. The magic of bicelles lights up membrane protein structure. Chem Rev 112:6054–6074
- Dürr UH, Soong R, Ramamoorthy A. 2013. When detergent meets bilayer: birth and coming of age of lipid bicelles. Prog Nucl Magn Reson Spectrosc 69:1–22
- Fisher SJ, Helliwell JR. 2008. An investigation into structural changes due to deuteration. Acta Crystallogr A 64(Pt 3):359–367
- Franzin CM, Teriete P, Marassi FM. 2007. Structural similarity of a membrane protein in micelles and membranes. J Am Chem Soc 129:8078–8079
- Gabel F, Lensink MF, Clantin B, Jacob-Dubuisson F, Villeret V, Ebel C. 2014. Probing the conformation of FhaC with small-angle neutron scattering and molecular modeling. Biophys J 107:185–196
- Garavito RM, Ferguson-Miller S. 2001. Detergents as tools in membrane biochemistry. J Biol Chem 276:32403–32406
- Gayen S, Li Q, Kim YM, Kang C. 2013. Structure of the C-terminal region of the Frizzled receptor 1 in detergent micelles. Molecules 18:8579–8590
- Gong XM, Ding Y, Yu J, Yao Y, Marassi FM. 2015. Structure of the Na,K-ATPase regulatory protein FXYD2b in micelles: implications for membrane-water interfacial arginines. Biochim Biophys Acta 1848(1 Pt B):299–306
- Goñi FM, Alonso A. 2000. Spectroscopic techniques in the study of membrane solubilization, reconstitution and permeabilization by detergents. Biochim Biophys Acta 1508:51–68
- Guengerich FP. 2013. Kinetic deuterium isotope effects in cytochrome P450 oxidation reactions. J Labelled Comp Radiopharm 56:428–431
- Hagn F, Etzkorn M, Raschle T, Wagner G. 2013. Optimized phospholipid bilayer nanodiscs facilitate high-resolution structure determination of membrane proteins. J Am Chem Soc 135:1919–1925
- He X, Morris JJ, Noll BC, Brown SN, Henderson KW. 2006. Kinetics and mechanism of ketone enolization mediated by magnesium bis(hexamethyldisilazide). J Am Chem Soc 128:13599–135610
- He Y, Wang K, Yan N. 2014. The recombinant expression systems for structure determination of eukaryotic membrane proteins. Protein Cell 5:658–672
- Hiller S, Garces RG, Malia TJ, Orekhov VY, Colombini M, Wagner G. 2008. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science 321:1206–1210
- Hiller S, Wagner G. 2009. The role of solution NMR in the structure determinations of VDAC-1 and other membrane proteins. Curr Opin Struct Biol 19:396–401
- Hines HD, Thomas RK, Garrett PR, Rennie GK, Penfold J. 1997. Investigation of mixing in binary surfactant solutions by surface tension and neutron reflection: anionic/nonionic and zwitterionic/nonionic mixtures. J Phys Chem B 101:9215–9223
- Hiruma-Shimizu K, Kalverda AP, Henderson PJF, Homans SW, Patching SG. 2014. Synthesis of uniformly deuterated n-dodecyl-β-D-maltoside (d39-DDM) for solubilisation of membrane proteins in TROSY NMR experiments. J Label Compd Radiopharm 57:737–743
- Hochuli M, Szyperski T, Wüthrich K. 2000. Deuterium isotope effects on the central carbon metabolism of Escherichia coli cells grown on a D2O-containing minimal medium. J Biomol NMR 17:33–42
- Hoffman JM, Habecker CN, Pietruszkiewicz AM, Bolhofer WA, Cragoe EJ Jr, Torchiana ML, Hirschmann R. 1983. A deuterium isotope effect on the inhibition of gastric secretion by N,N-dimethyl-N′-[2-(diisopropylamino)ethyl]-N′-(4,6-dimethyl-2-pyridyl)urea. Synthesis of metabolites. J Med Chem 26:1650–1653
- Holding AFC, Ross WA. 1958. The laboratory preparation of lithium deuteride and lithium aluminium deuteride, J Appl Chem 8:321–324
- Hori Y, Demura M, Iwadate M, Ulrich AS, Niidome T, Aoyagi H, Asakura T. 2001. Interaction of mastoparan with membranes studied by 1H-NMR spectroscopy in detergent micelles and by solid-state 2H-NMR and 15N-NMR spectroscopy in oriented lipid bilayers. Eur J Biochem 268:302–309
- Horst R, Horwich AL, Wüthrich K. 2011. Translational diffusion of macromolecular assemblies measured using transverse-relaxation-optimized pulsed field gradient NMR. J Am Chem Soc 133:16354–16357
- Horst R, Stanczak P, Serrano P, Wüthrich K. 2012. Translational diffusion measurements by microcoil NMR in aqueous solutions of the Fos-10 detergent-solubilized membrane protein OmpX. J Phys Chem B 116:6775–6780
- Howell SC, Mesleh MF, Opella SJ. 2005. NMR structure determination of a membrane protein with two transmembrane helices in micelles: MerF of the bacterial mercury detoxification system. Biochemistry 44:5196–5206
- Hsiao CY, Ottaway CA, Wetlaufer DB. 1974. Preparation of fully deuterated fatty acids by simple method. Lipids 9:913–915
- Huang C, Mohanty S, Banerjee M. 2010. A novel method of production and biophysical characterization of the catalytic domain of yeast oligosaccharyl transferase. Biochemistry 49:1115–1126
- Hwang PM, Choy WY, Lo EI, Chen L, Forman-Kay JD, Raetz CR, et al. 2002. Solution structure and dynamics of the outer membrane enzyme PagP by NMR. Proc Natl Acad Sci USA 99:13560–13565
- Imai S, Osawa M, Takeuchi K, Shimada I. 2010. Structural basis underlying the dual gate properties of KcsA. Proc Natl Acad Sci USA 107:6216–6221
- Inagaki S, Ghirlando R, Grisshammer R. 2013. Biophysical characterization of membrane proteins in nanodiscs. Methods 59:287–300
- Isaacs NS. Physical organic chemistry. New York: Longman; 1987
- Jaremko L, Jaremko M, Giller K, Becker S, Zweckstetter M. 2014. Structure of the mitochondrial translocator protein in complex with a diagnostic ligand. Science 343:1363–1366
- Kalverda AP, Gowdy J, Thompson GS, Homans SW, Henderson PJ, Patching SG. 2014. TROSY NMR with a 52 kDa sugar transport protein and the binding of a small-molecule inhibitor. Mol Membr Biol 31:131–140
- Kang HJ, Lee C, Drew D. 2013. Breaking the barriers in membrane protein crystallography. Int J Biochem Cell Biol 45:636–644
- Katz JJ. 1965. Chemical and biological studies with deuterium. Pennsylvania: Pennsylvania State University, University Park. 1–100
- Keana JF, Roman RB. 1978. Improved synthesis of n-octyl-beta-D-glucoside: a nonionic detergent of considerable potential in membrane biochemistry. Membr Biochem 1:323–327
- Kelly SM, Price NC. 2000. The use of circular dichroism in the investigation of protein structure and function. Curr Protein Pept Sci 1:349–384
- Kim HJ, Howell SC, Van Horn WD, Jeon YH, Sanders CR. 2009. Recent advances in the application of solution NMR spectroscopy to multi-span integral membrane proteins. Prog Nucl Magn Reson Spectrosc 55:335–360
- Klammt C, Schwarz D, Fendler K, Haase W, Dötsch V, Bernhard F. 2005. Evaluation of detergents for the soluble expression of alpha-helical and beta-barrel-type integral membrane proteins by a preparative scale individual cell-free expression system. FEBS J 272:6024–6038
- Klammt C, Maslennikov I, Bayrhuber M, Eichmann C, Vajpai N, Chiu EJ, et al. 2012. Facile backbone structure determination of human membrane proteins by NMR spectroscopy. Nat Methods 9:834–839
- Koch HJ, Stuart RS. 1978. The synthesis of per-C-deuterated D-glucose. Carbohyd Res 64:127–134
- Korchuganov DS, Gagnidze IE, Tkach EN, Schulga AA, Kirpichnikov MP, Arseniev AS. 2004. Determination of protein rotational correlation time from NMR relaxation data at various solvent viscosities. J Biomol NMR 30:431–442
- Krueger-Koplin RD, Sorgen PL, Krueger-Koplin ST, Rivera-Torres IO, Cahill SM, Hicks DB, et al. 2004. An evaluation of detergents for NMR structural studies of membrane proteins. J Biomol NMR 28:43–57
- Krumbiegel P. 2011. Large deuterium isotope effects and their use: a historical review. Isotopes Environ Health Stud 47:1–17
- Lee D, Hilty C, Wider G, Wüthrich K. 2006. Effective rotational correlation times of proteins from NMR relaxation interference. J Magn Reson 178:72–76
- Leitch LC, Morse AT. 1952. Synthesis of organic deuterium compounds III. 1,2-dibromoethane-d and its derivatives. Can J Chem 30:924–932
- le Maire M, Champeil P, Moller JV. 2000. Interaction of membrane proteins and lipids with solubilizing detergents. Biochim Biophys Acta 1508:86–111
- Liang B, Tamm LK. 2007. Structure of outer membrane protein G by solution NMR spectroscopy. Proc Natl Acad Sci USA 104:16140–16145
- Lin SH, Guidotti G. 2009. Purification of membrane proteins. Methods Enzymol 463:619–629
- Linke D 2009. Detergents: an overview. Methods Enzymol 463:603–617
- Liu L, Westler WM, den Boon JA, Wang X, Diaz A, Steinberg HA, Ahlquist P. 2009. An amphipathic alpha-helix controls multiple roles of brome mosaic virus protein 1a in RNA replication complex assembly and function. PLoS Pathog 5:e1000351
- Magolda RL, Johnson PR. 1985. A new efficient and versatile synthesis of alkyl phosphorylcholines. Tet Lett 26:1167–1170
- Manley PW, Blasco F, Mestan J, Aichholz R. 2013. The kinetic deuterium isotope effect as applied to metabolic deactivation of imatinib to the des-methyl metabolite, CGP74588. Bioorg Med Chem 21:3231–3239
- Maslennikov I, Kefala G, Johnson C, Riek R, Choe S, Kwiatkowski W. 2007. NMR spectroscopic and analytical ultracentrifuge analysis of membrane protein detergent complexes. BMC Struct Biol 7:74
- Maslennikov I, Choe S. 2013. Advances in NMR structures of integral membrane proteins. Curr Opin Struct Biol 23:555–562
- Matsuo K, Gekko K. 2013. Circular-dichroism and synchrotron-radiation circular-dichroism spectroscopy as tools to monitor protein structure in a lipid environment. Methods Mol Biol 974:151–176
- Mészáros R, Varga I, Gilányi T. 2005. Effect of polymer molecular weight on the polymer/surfactant interaction. J Phys Chem B 109:13538–13544
- Meyer A, Dierks K, Hussein R, Brillet K, Brognaro H, Betzel C. 2015. Systematic analysis of protein-detergent complexes applying dynamic light scattering to optimize solutions for crystallization trials. Acta Crystallogr F Struct Biol Commun 71(Pt 1):75–81
- Miles AJ, Wallace BA. 2006. Synchrotron radiation circular dichroism spectroscopy of proteins and applications in structural and functional genomics. Chem Soc Rev 35:39–51
- Miskolzie M, Lucyk S, Kotovych G. 2003. NMR conformational studies of micelle-bound orexin-B: a neuropeptide involved in the sleep/awake cycle and feeding regulation. J Biomol Struct Dyn 21:341–351
- Mitsuda Y, Kono J. 2012. Anionic surfactant compositions with good fluidity and storage stability. Japan patent 2012092163
- Mittermaier A, Kay LE. 2002. Effect of deuteration on some structural parameters of methyl groups in proteins as evaluated by residual dipolar couplings. J Biomol NMR 23:35–45
- Miyamoto K, Togiya K. 2011. Solution structure of LC4 transmembrane segment of CCR5. PLoS One 6:e20452
- Montserret R, Saint N, Vanbelle C, Salvay AG, Simorre JP, Ebel C, Sapay N, et al. 2010. NMR structure and ion channel activity of the p7 protein from hepatitis C virus. J Biol Chem 285:31446–31461
- Moreland JL, Gramada A, Buzko OV, Zhang Q, Bourne PE. 2005. The Molecular Biology Toolkit (MBT): a modular platform for developing molecular visualization applications. BMC Bioinformatics 6:21
- Murad S, Chen RH, Kishimoto Y. 1977. alpha-Hydroxylation of fatty acids in brain. Substrate specificity and deuterium isotope effect. J Biol Chem 252:5206–5210
- Nadeau VG, Rath A, Deber CM. 2012. Sequence hydropathy dominates membrane protein response to detergent solubilization. Biochemistry 51:6228–6237
- Nadezhdin KD, Bocharova OV, Bocharov EV, Arseniev AS. 2011. Structural and dynamic study of the transmembrane domain of the amyloid precursor protein. Acta Naturae 3:69–76
- Nadezhdin KD, Bocharova OV, Bocharov EV, Arseniev AS. 2012. Dimeric structure of transmembrane domain of amyloid precursor protein in micellar environment. FEBS Lett 586:1687–1692
- Neale C, Ghanei H, Holyoake J, Bishop RE, Privé GG, Pomès R. 2013. Detergent-mediated protein aggregation. Chem Phys Lipids 169:72–84
- Newstead S, Ferrandon S, Iwata S. 2008. Rationalizing alpha-helical membrane protein crystallization. Protein Sci 17:466–472
- Nietlispach D, Gautier A. 2011. Solution NMR studies of polytopic α-helical membrane proteins. Curr Opin Struct Biol 21:497–508
- Oliver RC, Lipfert J, Fox DA, Lo RH, Doniach S, Columbus L. 2013. Dependence of micelle size and shape on detergent alkyl chain length and head group. PLoS One 8:e62488
- O’Malley MA, Helgeson ME, Wagner NJ, Robinson AS. 2011. Toward rational design of protein detergent complexes: determinants of mixed micelles that are critical for the in vitro stabilization of a G-protein coupled receptor. Biophys J 101:1938–1948
- O’Neil JD, Sykes BD. 1989. Side-chain dynamics of a detergent-solubilized membrane protein: measurement of tryptophan and glutamine hydrogen-exchange rates in M13 coat protein by 1H NMR spectroscopy. Biochemistry 28:6736–6345
- Orädd G, Lindblom G, Arvidson G, Gunnarsson K. 1995. Phase equilibria and molecular packing in the N,N-dimethyldodecylamine oxide/gramicidin D/water system studied by 2H nuclear magnetic resonance spectroscopy. Biophys J 68:547–557
- OuYang B, Xie S, Berardi MJ, Zhao X, Dev J, Yu W, et al. 2013. Unusual architecture of the p7 channel from hepatitis C virus. Nature 498:521–525
- Oxenoid K, Chou JJ. 2005. The structure of phospholamban pentamer reveals a channel-like architecture in membranes. Proc Natl Acad Sci USA 102:10870–10875
- Page RC, Moore JD, Nguyen HB, Sharma M, Chase R, Gao FP, et al. 2006. Comprehensive evaluation of solution nuclear magnetic resonance spectroscopy sample preparation for helical integral membrane proteins. J Struct Funct Genomics 7:51–64
- Pagliano C, Barera S, Chimirri F, Saracco G, Barber J. 2012. Comparison of the α and β isomeric forms of the detergent n-dodecyl-D-maltoside for solubilizing photosynthetic complexes from pea thylakoid membranes. Biochim Biophys Acta 1817:1506–1515
- Parker JL, Newstead S. 2012. Current trends in α-helical membrane protein crystallization: an update. Protein Sci 21:1358–1365
- Pascal RA, Baum MW, Wagner CK, Rodgers LR, Huang DS. 1986. Measurement of deuterium kinetic isotope effects in organic and biochemical reactions by natural abundance deuterium NMR spectroscopy. J Am Chem Soc 108:6477–6482
- Patching SG. 2011. NMR structures of polytopic integral membrane proteins. Mol Membr Biol 28:370–397
- Patching SG, Edara S, Ma P, Nakayama J, Hussain R, Siligardi G, Phillips-Jones MK. 2012. Interactions of the intact FsrC membrane histidine kinase with its pheromone ligand GBAP revealed through synchrotron radiation circular dichroism. Biochim Biophys Acta 1818:1595–1602
- Patzelt H, Simon B, terLaak A, Kessler B, Kühne R, Schmieder P, Oesterhelt D, Oschkinat H. 2002. The structures of the active center in dark-adapted bacteriorhodopsin by solution-state NMR spectroscopy. Proc Natl Acad Sci USA 99:9765–9770
- Pautsch A, Schulz GE. 1998. Structure of the outer membrane protein A transmembrane domain. Nat Struct Biol 5:1013–1017
- Pautsch A, Schulz GE. 2000. High-resolution structure of the OmpA membrane domain. J Mol Biol 298:273–282
- Pebay-Peyroula E, Garavito RM, Rosenbusch JP, Zulauf M, Timmins PA. 1995. Detergent structure in tetragonal crystals of OmpF porin. Structure 3:1051–1059
- Pedras MS, Minic Z, Sarma-Mamillapalle VK. 2011. Brassinin oxidase mediated transformation of the phytoalexin brassinin: structure of the elusive co-product, deuterium isotope effect and stereoselectivity. Bioorg Med Chem 19:1390–1399
- Persky A, Kuppermann A. 1974. An apparatus for the production of high isotopic purity deuterium, J Phys E: Scientific Instruments 7:889–890
- Popot JL. 2010. Amphipols, nanodiscs, and fluorinated surfactants: three nonconventional approaches to studying membrane proteins in aqueous solutions. Annu Rev Biochem 79:737–775
- Postis VL, Deacon SE, Roach PC, Wright GS, Xia X, Ingram JC, et al. 2008. A high-throughput assay of membrane protein stability. Mol Membr Biol 25:617–624
- Privé GG. 2007. Detergents for the stabilization and crystallization of membrane proteins. Methods 41:388–397
- Raman P, Cherezov V, Caffrey M. 2006. The Membrane Protein Data Bank. Cell Mol Life Sci 63:36–51
- Reardon PN, Sage H, Dennison SM, Martin JW, Donald BR, Alam SM, et al. 2014. Structure of an HIV-1-neutralizing antibody target, the lipid-bound gp41 envelope membrane proximal region trimer. Proc Natl Acad Sci USA 111:1391–1396
- Reckel S, Hiller. 2013. Perspectives of solution NMR spectroscopy for structural and functional studies of integral membrane proteins. Molec Phys 111:843–849
- Renaud, RN, Leitch LC. 1968. Reinvestigation of the Polonovski reaction. Synthesis of deuterated dimethylamine and formaldehyde. Can J Chem 46:385–390
- Roche J, Louis JM, Grishaev A, Ying J, Bax A. 2014. Dissociation of the trimeric gp41 ectodomain at the lipid-water interface suggests an active role in HIV-1 Env-mediated membrane fusion. Proc Natl Acad Sci USA 111:3425–3430
- Roosild TP, Greenwald J, Vega M, Castronovo S, Riek R, Choe S. 2005. NMR structure of Mistic, a membrane-integrating protein for membrane protein expression. Science 307:1317–1321
- Rouse SL, Marcoux J, Robinson CV, Sansom MS. 2013. Dodecyl maltoside protects membrane proteins in vacuo. Biophys J 105:648–656
- Rosevear P, VanAken T, Baxter J, Ferguson-Miller S. 1980. Alkyl glycoside detergents: a simpler synthesis and their effects on kinetic and physical properties of cytochrome c oxidase. Biochemistry 19:4108–4115
- Saunders WH Jr, Edison DH. 1960. Mechanisms of elimination reactions. IV. Deuterium isotope effects in E2 reactions of some 2-phenylethyl derivatives. J Am Chem Soc 82:138–142
- Schubert M, Kolbe M, Kessler B, Oesterhelt D, Schmieder P. 2002. Heteronuclear multidimensional NMR spectroscopy of solubilized membrane proteins: resonance assignment of native bacteriorhodopsin. Chembiochem 3:1019–1023
- Seddon AM, Curnow P, Booth PJ. 2004. Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta 1666:105–117
- Shao L, Hewitt MC. 2010. The kinetic isotope effect in the search for deuterated drugs. Drug News Perspect 23:398–404
- Sharma R, Strelevitz TJ, Gao H, Clark AJ, Schildknegt K, Obach RS, Ripp SL, Spracklin DK, Tremaine LM, Vaz AD. 2012. Deuterium isotope effects on drug pharmacokinetics. I. System-dependent effects of specific deuteration with aldehyde oxidase cleared drugs. Drug Metab Dispos 40:625–634
- Shenkarev ZO, Paramonov AS, Lyukmanova EN, Shingarova LN, Yakimov SA, Dubinnyi MA, et al. 2010. NMR structural and dynamical investigation of the isolated voltage-sensing domain of the potassium channel KvAP: implications for voltage gating. J Am Chem Soc 132:5630–5637
- Siligardi G, Hussain R, Patching SG, Phillips-Jones MK. 2014. Ligand- and drug-binding studies of membrane proteins revealed through circular dichroism spectroscopy. Biochim Biophys Acta 1838(1 Pt A):34–42
- Simmons EM, Hartwig JF. 2012. On the interpretation of deuterium kinetic isotope effects in C-H bond functionalizations by transition-metal complexes. Angew Chem Int Ed 51:3066–3072
- Slotboom DJ, Duurkens RH, Olieman K, Erkens GB. 2008. Static light scattering to characterize membrane proteins in detergent solution. Methods 46:73–82
- Sonoda Y, Newstead S, Hu NJ, Alguel Y, Nji E, Beis K, et al. 2011. Benchmarking membrane protein detergent stability for improving throughput of high-resolution X-ray structures. Structure 19:17–25
- Stanczak P, Horst R, Serrano P, Wüthrich K. 2009. NMR characterization of membrane protein-detergent micelle solutions by use of microcoil equipment. J Am Chem Soc 131:18450–18456
- Stanczak P, Zhang Q, Horst R, Serrano P, Wüthrich K. 2012. Micro-coil NMR to monitor optimization of the reconstitution conditions for the integral membrane protein OmpW in detergent micelles. J Biomol NMR 54:129–133
- Takei K, Tsuto K, Miyamoto S, Wakatsuki J. 1985. Anionic surfactants: lauric products. J Am Oil Chem Soc 62:341–347
- Takeuchi K, Takahashi H, Kawano S, Shimada I. 2007. Identification and characterization of the slowly exchanging pH-dependent conformational rearrangement in KcsA. J Biol Chem 282:15179–15186
- Tang P, Simplaceanu V, Xu Y. 1999. Structural consequences of anesthetic and nonimmobilizer interaction with gramicidin A channels. Biophys J 76:2346–2350
- Tate CG. 2010. Practical considerations of membrane protein instability during purification and crystallisation. Methods Mol Biol 601:187–203
- Teriete P, Franzin CM, Choi J, Marassi FM. 2007. Structure of the Na,K-ATPase regulatory protein FXYD1 in micelles. Biochemistry 46:6774–6783
- Thomas AF. 1971. Deuterium labeling in organic chemistry. New York: Appleton-Century
- Timmins GS. 2014. Deuterated drugs: where are we now? Expert Opin Ther Pat 24:1067–1075
- Török Z, Szalontai B, Joó F, Wistrom CA, Vigh L. 1993. Homogeneous catalytic deuteration of fatty acyl chains as a tool to detect lipid phase transitions in specific membrane domains: a Fourier Transform Infrared spectroscopic study. Biochem Biophys Res Commun 192:518–524
- Tulumello DV, Deber CM. 2012. Efficiency of detergents at maintaining membrane protein structures in their biologically relevant forms. Biochim Biophys Acta 1818:1351–1358
- Ujwal R, Cascio D, Colletier JP, Faham S, Zhang J, Toro L, et al. 2008. The crystal structure of mouse VDAC1 at 2.3 Å resolution reveals mechanistic insights into metabolite gating. Proc Natl Acad Sci USA 105:17742–17747
- Urey HC, Brickwedde FG, Murphy GM. 1932. A hydrogen isotope of mass 2 and its concentration. Phys Rev 39:164
- Vacklin HP, Tiberg F, Thomas RK. 2005a. Formation of supported phospholipid bilayers via co-adsorption with beta-D-dodecyl maltoside. Biochim Biophys Acta – Biomembranes 1668:17–24
- Vacklin HP, Tiberg F, Fragneto G, Thomas RK. 2005b. Composition of supported model membranes determined by neutron reflection. Langmuir 21:2827–2837
- van Heyningen WE, Rittenberg D, Schoenheimer R. 1938. The preparation of fatty acids containing deuterium. J Biol Chem 125:495–500
- Van Horn WD, Kim HJ, Ellis CD, Hadziselimovic A, Sulistijo ES, Karra MD, et al. 2009. Solution nuclear magnetic resonance structure of membrane-integral diacylglycerol kinase. Science 324:1726–1729
- Vergis JM, Purdy MD, Wiener MC. 2010. A high-throughput differential filtration assay to screen and select detergents for membrane proteins. Anal Biochem 407:1–11
- Vostrikov VV, Mote KR, Verardi R, Veglia G. 2013. Structural dynamics and topology of phosphorylated phospholamban homopentamer reveal its role in the regulation of calcium transport. Structure 21:2119–2130
- Wade D. 1999. Deuterium isotope effects on noncovalent interactions between molecules. Chem Biol Interact 117:191–217
- Walther R, Fahlbusch K, Sievert R, Gottschalk G. 1981. Formation of trideuteromethane from deuterated trimethylamine or methylamine by Methanosarcina barkeri. J Bacteriol 148:371–373
- Ward A, Sanderson NM, O’Reilly J, Rutherford NG, Poolman B, Henderson PJF. 2000. The amplified expression, identification, purification, assay and properties of hexahistidine-tagged bacterial membrane transport proteins. In: Baldwin SA, editor. Membrane transport – a practical approach. Oxford: Blackwell. pp. 141–166
- Warschawski DE, Arnold AA, Beaugrand M, Gravel A, Chartrand É, Marcotte I. 2011. Choosing membrane mimetics for NMR structural studies of transmembrane proteins. Biochim Biophys Acta 1808:1957–1974
- Washburn EW, Urey HC. 1932. Concentration of the H isotope of hydrogen by the fractional electrolysis of water. Proc Natl Acad Sci USA 18:496–498
- Westheimer FH 1961. The magnitude of the primary kinetic isotope effect for compounds of hydrogen and deuterium. Chem Rev 61:265–273
- White RD, Gandolfi AJ, Bowden GT, Sipes IG. 1983. Deuterium isotope effect on the metabolism and toxicity of 1,2-dibromoethane. Toxicol Appl Pharmacol 69:170–178
- Wiberg KB. 1955. The deuterium isotope effect. Chem Rev 55:713–743
- Yamamoto G. 2000. Preparation of sulfuric acid esters for anionic surfactants. Japan patent 2000247947
- Yang CS, Ishizaki H. 1992. Deuterium isotope effect on the metabolism of N-nitrosodimethylamine and related compounds by cytochrome P4502E1. Xenobiotica 22:1165–1173
- Yao S, Weber DK, Separovic F, Keizer DW. 2014. Measuring translational diffusion coefficients of peptides and proteins by PFG-NMR using band-selective RF pulses. Eur Biophys J 2014;43:331–339
- Yeh AP, McMillan A, Stowell MH. 2006. Rapid and simple protein-stability screens: application to membrane proteins. Acta Crystallogr D Biol Crystallogr 62(Pt 4):451–457
- Yoshimura Y, Shimizu H, Hiroshi Hinoub H, Nishimura S-I. 2005. A novel glycosylation concept; microwave-assisted acetal-exchange type glycosylations from methyl glycosides as donors. Tet Lett 46:4701–4705
- Yu PH, Durden DA, Davis BA, Boulton AA. 1987. Deuterium isotope effect in gamma-aminobutyric acid transamination: determination of rate-limiting step. J Neurochem 48:440–446
- Yu L, Sun C, Song D, Shen J, Xu N, Gunasekera A, et al. 2005. Nuclear magnetic resonance structural studies of a potassium channel-charybdotoxin complex. Biochemistry 44:15834–15841
- Zhang Q, Horst R, Geralt M, Ma X, Hong WX, Finn MG, et al. 2008. Microscale NMR screening of new detergents for membrane protein structural biology. J Am Chem Soc 130:7357–7363
- Zhou FX, Cocco MJ, Russ WP, Brunger AT, Engelman DM. 2000. Interhelical hydrogen bonding drives strong interactions in membrane proteins. Nat Struct Biol 7:154–160
- Zhou Y, Cierpicki T, Jimenez RH, Lukasik SM, Ellena JF, Cafiso DS, et al. 2008. NMR solution structure of the integral membrane enzyme DsbB: functional insights into DsbB-catalyzed disulfide bond formation. Mol Cell 31:896–908
- Zhou HX, Cross TA. 2013. Influences of membrane mimetic environments on membrane protein structures. Annu Rev Biophys 42:361–392
- Zoonens M, Popot JL. 2014. Amphipols for each season. J Membr Biol 247:759–796
