?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Outer layer of cellular membrane contains ordered domains enriched in cholesterol and sphingolipids, called ‘lipid rafts’, which play various biological roles, i.e., are involved in the induction of cell death by apoptosis. Recent studies have shown that these domains may constitute binding sites for selected drugs. For example alkylphosphocholines (APCs), which are new-generation antitumor agents characterized by high selectivity and broad spectrum of activity, are known to have their molecular targets located at cellular membrane and their selective accumulation in tumor cells has been hypothesized to be linked with the alternation of biophysical properties of lipid rafts. To get a deeper insight into this issue, interactions between representative APC: erucylphosphocholine, and artificial lipid raft system, modeled as Langmuir monolayer (composed of cholesterol and sphingomyelin mixed in 1:2 proportion) were investigated. The Langmuir monolayer experiments, based on recording surface pressure-area isotherms, were complemented with Brewster angle microscopy results, which enabled direct visualization of the monolayers structure. In addition, the investigated monolayers were transferred onto solid supports and studied with AFM. The interactions between model raft system and erucylphosphocholine were analyzed qualitatively (with mean molecular area values) as well as quantitatively (with ΔGexc function). The obtained results indicate that erucylphosphocholine introduced to raft-mimicking model membrane causes fluidizing effect and weakens the interactions between cholesterol and sphingomyelin, which results in phase separation at high surface pressures. This leads to the redistribution of cholesterol molecules in model raft, which confirms the results observed in biological studies.
Introduction
Cancer – just after ischaemic heart disease – is the second most common cause of death (approx. 21% of all deaths was caused by non-communicable diseases in 2010, according to the World Health Organization [WHO] data), and despite the development of modern medicine, the number of cancer deaths increases every year. The commonly used chemotherapy is based on cytotoxic drugs that act directly on the DNA, carrying the risk of healthy tissue damage. Therefore, it is of utmost importance to develop anticancer drugs with significantly limited side-effects. Without a doubt, alkylphosphocholines (APCs) belong to a group of most promising drugs in this respect (Gajate & Mollinedo, Citation2002, Van Blitterswijk & Verheij, Citation2008).
APCs represent a class of antineoplastic synthetic phospholipid analogs (Eibl et al., Citation1992). They are derivatives of lysolecithins, occurring naturally in human organisms as intermediate product of phosphatidylcholine metabolism, which were found to cause cell lysis and affect the fluidity and permeability of biological membranes (Poole et al., Citation1970, Dick & Lawrence, Citation1992). APCs, due to structural changes as compared to natural phospholipids (i.e. lack of glycerol backbone) do not undergo metabolic lysis by enzymes but accumulate in tumor tissues (Jendrossek & Handrick, Citation2003). Selected APCs have been successfully used in anticancer therapy performed on various cell lines, i.e. against fibrosarcoma (van der Luit et al., Citation2007), leukemia (Eibl et al., Citation1992, Nieto-Miguel et al., Citation2006) and other cancerous cell lines, e.g., lung (Nieto-Miguel et al., Citation2006), breast (Eibl et al., Citation1992), colon (Hilgard et al., Citation1997), brain (Rübel et al., Citation2006) and prostate (Kondapaka et al., Citation2003). The most promising candidates of APCs are hexadecylphosphocholine (miltefosine – HePC, possessing C16 alkyl chain) and erucylphosphocholine (ErPC, with C22 alkyl chain and double cis bond between 13 and 14 carbon atoms) (Eibl & Unger, Citation1990, Eibl & Kaufmann-Kolle, Citation1995, van der Luit et al., Citation2007) ().
Although these family of drugs was synthesized in the ’80s and ’90s of the 20th century (Pachioni et al., Citation2013), their exact mode of action and mechanism of high selectivity is not fully recognized, although they are known to act on membrane level. These compounds act selectively on cancerous cells, inducing the process of programmed cell death (apoptosis), while healthy cells are saved (Gajate & Mollinedo, Citation2002). Several hypotheses have been put forward to explain this issue.
Firstly, it has been suggested that membrane composition itself is a natural barrier preventing APCs from incorporation into normal cells and facilitating penetration into cancerous cells, having more fluid membrane as compared to healthy cell lines (Inbar et al., Citation1977). The observed fluidity of malicious membrane results from changes in composition and proportion of major membrane constituents (cholesterol and phospholipids) during neoplastic transformation (Klock & Pieprzyk, Citation1979, Johnson & Robinson, Citation1979, Agatha et al., Citation2001). The reason for membrane fluidization is manifold: (i) Decreased cholesterol level and cholesterol-to-phospholipids mole ratio; (ii) increased percentage of unsaturated versus saturated fatty acids chains of membrane phospholipids; (iii) increased phosphatidylcholines and decreased phosphatidylethanolamines content; and (iv) deficiency of sphingomyelin. Although these observations have been drawn based on studies on leukemia cells, the same tendency was also noticed for other cancerous cell lines, e.g., lung cancer (Sok et al., Citation1999).
Secondly, it has been suggested that the drugs are selectively attracted to cancer cells by the binding sites located at the cellular membrane. Unfortunately, these molecular targets have not been unambiguously characterized. One hypothesis holds that gangliosides, which are overexpressed in tumor progression, are responsible to target these drugs selectively to cancer cells (Fish, Citation1996). However, recent studies indicate that also lipid rafts may be important in this aspect. Namely, in cancer cells elevated level of lipid rafts have been reported (Li et al., Citation2006) and APCs’ activity could be linked with alteration of their biophysicals properties (van der Luit et al., Citation2007). These lipid domains form specific, ordered structures that consist of laterally linked sphingolipids (mainly sphingomyelin and glycosphingolipids) containing predominantly straight hydrocarbon chain, and most free spaces between them are filled with cholesterol molecules (Simons & Ikonen, Citation1997, Simons & Toomre, Citation2000). Therefore mixtures of sphingomyelin (SM) and cholesterol (Chol) serve as the simplest model of lipid raft system of mammals (Jablin et al., Citation2010, Hąc-Wydro et al., Citation2011), although more complex ternary (POPC + Chol + SM) or quaternary (POPC + Chol + SM + GM1) mixtures of different proportions of its components are also applied (Thakur et al., Citation2011). Specifically for fungi, in model rafts, cholesterol is exchanged by ergosterol, while in plants ternary mixtures of phytosterol mixed with glucosylcerebroside and DPPC are applied (Beck et al., Citation2007). Due to strong interactions between their components, lipid rafts are very closely packed. Biophysical mechanism by which cells regulate the size of lipid rafts (from 10–200 nm [Pike, Citation2006]), durability and spatial location are not clearly understood. According to Gómez et al. (Citation2008), lipid rafts distribution and their dynamics are controlled by the spatial variation of cholesterol molecules and lipid-lipid interactions. Lipid rafts are involved in many important cellular processes, such as: Signal transduction (Simons & Toomre, Citation2000), protein sorting (Simons & Ikonen, Citation1997) membrane transport (Hanzal-Bayer & Hancock, Citation2007) and – last but not least – in activation of programmed cell death (apoptosis) by an external apoptotic pathway (Mollinedo & Gajate, Citation2006).
Studies performed both on living cells and membrane models, usually liposomes, clearly demonstrate that synthetic antitumor lipids – both alkyl-lysophospholipids, ALPs (in which ester bonds in the C1 and C2 positions of the glycerol backbone are replaced by ether linkages and the hydroxyl group in C2 position is transformed into a short-chain ether, e.g., methoxyl group, represented by edelfosine) (Gajate & Mollinedo, Citation2002) and APCs (like HePC, ErPC or perifosine), that lack the glycerol unit, act on membrane level. A common conclusion drawn based on a number of articles, involving mainly edelfosine, is that anticancer lipids:
Promote changes in fluidity of the cancerous cell membrane (Castro et al., Citation2013), which has been confirmed in studies involving Langmuir monolayers as model membrane systems (Hąc-Wydro et al., Citation2011);
Associate with lipid rafts, and in this way enter the cell. Studies performed by Ausili et al. show high level of edelfosine accumulation in these domains ([21 ± 4.3]% of the total lipid content) (Ausili et al., Citation2008). Van der Luit carried out similar experiments with perifosine, indicating similar level of drug accumulation (van der Luit et al., Citation2007). Interestingly, the pretreatment of cells with raft-disruptive agents (such as β-cyclodextrin derivative, bacterial sphingomyelin or filipin) was found to reduce significantly the drugs uptake (van der Luit et al., Citation2007); and
Affect the properties of lipid rafts when incorporated in the cell membrane. In this aspect, however, controversial results have been reported.
Firstly, it was shown that antitumor lipids induced stability of sphingomyelin-cholesterol ordered domains (Heczková & Slotte, Citation2006). Upon incorporation, the phase status of the raft is drastically altered. Namely, it is known that rafts display liquid ordered (Lo) – liquid disordered (Ld) phase coexistence (Gaus et al., Citation2005). X-ray diffraction study (Ausili et al., Citation2008) indicates the formation of two separated phases in model raft system (composed of POPC/sphingomyelin/cholesterol) upon incorporation of a representative ALP (edelfosine). In a recent study Gomide et al. (Citation2013), using vesicles composed of DOPC/sphingomyelin/cholesterol as model rafts, showed that ALPs induced a lipid and domain redistribution, followed by vesicle disruption. This, however, contradicts the work by Castro et al. (Citation2013) reporting that biophysical properties of model raft and cellular membrane are not significantly altered upon incorporation of anticancer lipids, and only a slight increase in membrane fluidity was noticed. These discrepancies may be due to different experimental materials applied, i.e., different kind of the studied antitumor lipids and their concentration as well as different composition of model rafts (DOPC or POPC mixed with sphingomyelin and cholesterol).
To verify the influence of anticancer alkylphosphocholines on lipid rafts we have chosen one of the most potent drug – erucylphosphocholine (ErPC) – and studied its behavior in the simplest raft-imitating system containing cholesterol and sphingomyelin mixed in 1:2 proportion, using the Langmuir monolayer technique, which serves as a very useful, easy to handle and controllable model of biomembranes (Maget-Dana, Citation1999).
Materials and methods
Materials
Cholesterol (> 98%) and egg sphingomyelin (> 99%) were purchased from Avanti Polar Lipids; whereas the sample of 1-erucylphosphocholine (ErPC) was a generous gift of Aeterna Zentaris GmbH (Frankfurt, Germany). The lipids were applied for investigations without any further purification. Chloroform of spectroscopic purity stabilized by ethanol and the p.a. methanol were provided by Sigma-Aldrich.
Methods
Langmuir technique
The lipids were dissolved in mixture of chloroform/methanol in a proportion in volume of 9:1 in concentration of 0.2–0.5 mg/ml. Mixed solutions containing various amount of erucylphosphocholine (mole fraction, XErPC, ranged from 0–1 with the increment of 0.2) were prepared by mixing proper volumes of respective stock solutions. Langmuir monolayers were obtained by spreading an aliquot of the above-mentioned solutions with a Hamilton microsyringe (precise to ± 2 μl) onto the surface of ultrapure water of the resistivity ≥18.2 MΩ cm produced by the Millipore system. The isotherms were recorded using 611 Langmuir-Blodgett trough (NIMA, Coventry, UK, total area = 600 cm2) placed on an anti-vibration table. In routine measurements, monolayers were compressed with the barrier speed of 20 cm2/min (equivalent to 11 Å2/molecule·minute). Surface pressure was measured with ashless chromatography paper by the Wilhelmy method, with sensitivity of ± 0.1 mN/m. Temperature of the aqueous subphase was held constant to 20 °C ± 0.1 °C by a circulating water system from Julabo. Each measurement was repeated 2–3 times to ensure high reproducibility of the obtained isotherms to ± 2 Å2.
In the first step of our investigations, a set of control experiments have been performed to figure out any potential influence of the experimental conditions on the isotherms for binary (Chol:SM = 1:2) as well as ternary mixtures containing ErPC. One has to be aware of the fact that a change of surface density of molecules dropped onto the free water surface, speed of compression (Dynarowicz-Łątka et al., Citation1999), kind of a spreading solvent (Vila Romeu et al., Citation1997), material of moving barrier (Teflon or Delrin) (Hardy et al., Citation2006) or Wilhelmy plate (filter paper, platinum or glass plate) (Brzozowska & Figaszewski, Citation2002) may alter the position and course of the pressure-area isotherms. In our experiments we observed that neither changing of the number of molecules deposited on the surface within the range of 3–6·1016 nor the variation in compression speed from 10–70 cm2/min influenced the isotherms for binary (Chol:SM = 1:2) as well as ternary mixtures containing ErPC. Moreover, coinciding isotherms were obtained upon changing the barrier material from Teflon to Delrin as well as using either of the troughs (NIMA/KSV).
Brewster angle microscopy
BAM experiments were performed with ultraBAM instrument (Accurion GmbH, Goettingen, Germany) equipped with a 50 mW laser emitting p-polarized light at a wavelength of 658 nm, a 10× magnitude objective, polarizer, analyzer and CCD camera. The spatial resolution of the BAM image was 2 μm. The BAM instrument was installed over a KSV 2000 700 cm2 double-barrier Langmuir trough (KSV, Helsinki, Finland). Surface pressure was measured upon monolayers compression and simultaneously BAM images were recorded. The spatial resolution of BAM was 2 μm. BAM images presented here show monolayers fragments of 720 × 400 μm2.
Langmuir-Blodgett (LB) transfer and imaging of transferred monolayers with Atomic Force Microscopy (AFM)
Langmuir monolayers of model lipid raft system alone and containing erucylphosphocholine (XErPC = 0.3) were transferred from the free water surface by the Langmuir-Blodgett method, using the LB trough from NIMA, described above. In order to perform the LB transfer, the mica substrate was placed in the aqueous subphase and each of the investigated monolayer was compressed to the surface pressure of 30 mN/m. After 20 min of film stabilization, the monolayer was transferred to mica using a deeper speed of 1 mm·min−1. Topographic images of the transferred films were recorded in air under ambient conditions using Atomic Force Microscopy (Agilent 5500) working in non-contact mode. The Al-coated force modulation silicon probes (Nanosensors) with spring constant about 2 N/m, resonant frequencies about 60 kHz and the tip radius equal to 7 nm were used. The set point and all gains were adjust to obtain minimal noise and high-quality images of examined surfaces. The topography images of each sample were recorded in several arbitrarily chosen locations.
The vertical structure of surfaces examined with AFM is described by the distribution of height in topographic images and characterized in terms of its mean value and spread (Bergkvist et al., Citation2001, Lee et al., Citation2002, Ouerghi et al., Citation2002). Hence, the average height and its standard deviation, given by root mean square (RMS) roughness are determined for the representative height distribution using the PicoImage software provided with the AFM equipment. In addition, the asymmetry of the AFM height distribution characterized by skewness (SK) is also specified.
Results
Cholesterol (Chol) and sphingomyelin (SM) are known to exhibit high affinity to each other (Smaby et al., Citation1994, Petelska & Figaszewski, Citation2013). In mixed monolayers strong attractive interactions between both lipids occur; the strongest are observed for Chol-SM films of 1:2 proportion (Hąc-Wydro & Dynarowicz-Łątka, Citation2008). It has been postulated that at this particular composition, highly stable ‘surface complexes’ between cholesterol and sphingomyelin are formed. As it has already been mentioned, this very composition is attributed to the simplest model of lipid raft system in mammals.
Cholesterol is known to form very condensed, solid-type monolayers (Cadena-Nava et al., Citation2006). Regarding sphingomyelin, one has to be aware of the fact that the shape of the π/A isotherm depends on the source of this lipid, i.e., whether this is a natural product (e.g., milk, brain or egg SM), or a synthetic one – saturated or not. For sphingomyelins with saturated fatty acid chains, both the lift-off area and the limiting area are very similar, however, depending on the fatty acid alkyl chain, a characteristic plateau transition from liquid-expanded to liquid-condensed state occurs at a different surface pressure as reported in References Ramstedt & Slotte (Citation2002) and Li et al. (Citation2000). In monolayer experiments egg SM is most frequently applied, and although one has to bear in mind that this is a product with fatty acid distribution, it contains mostly (∼90%) the N-palmitoyl (16:0) molecular species. The isotherms presented herein () are in a good agreement with those reported elsewhere for cholesterol (Cadena-Nava et al., Citation2006) and egg SM (Prenner et al., Citation2007, Jurak et al., Citation2014).
Figure 2. Surface pressure (π)–area (A) isotherms for model lipid rafts and their constituents: Chol and SM; inset: Molecular arrangement in lipid raft modeled with HyperChem [HyperChem 8.0: Professional version, a molecular visualization and simulation software package. Hypercube Inc., Gainesville, FL, 1995–2011].
![Figure 2. Surface pressure (π)–area (A) isotherms for model lipid rafts and their constituents: Chol and SM; inset: Molecular arrangement in lipid raft modeled with HyperChem [HyperChem 8.0: Professional version, a molecular visualization and simulation software package. Hypercube Inc., Gainesville, FL, 1995–2011].](/cms/asset/14f226dc-3839-4a80-8f2b-da2415f95e9f/imbc_a_1125537_f0002_c.jpg)
As it can be seen in , the isotherm for model lipid raft (Chol:SM = 1:2) lies in-between the isotherms for its pure components. The mixture modeling lipid raft form a surface film of relatively high stability (as evidenced by the value of the surface pressure collapse ≈54 mN/m) and high degree of ordering as proved by value of the calculated compression modulus, Cs−1 (defined as CS−1 = −A (dπ/dA)) (Davies & Rideal, Citation1963), reaching ∼350 mN/m (), resulting from favorable geometric packing of SM and Chol molecules. Critical packing parameters s defined as (Israelachvili, Citation2011) (where a is the head group, V denotes volume and lc is critical length of the hydrocarbon chain) for cholesterol (s = 1.22) and SM (s = 0.57) indicate their inverted conical shape and truncated conical shape, respectively ().
Table 1. Parameters of geometrical molecular packing; hydrophobic chain length and hydrophobic volume were calculated by formulae 
 and
and 
 .
.
This explains favorable arrangement of molecules forming lipid rafts. In order to gain insight into the effect of ErPC on model lipid raft, the investigated drug was added into a monolayer mimicking sphingomyelin-cholesterol domains in the amount of XErPC = 0.1; 0.3; 0.5; 0.7; 0.9 and 1. The resultant π-A isotherms are shown in .
Figure 3. Surface pressure (π)–area (A) isotherms for monolayer mimicking lipid raft and ErPC (a) surface pressure–area isotherms; (b) compressibility modulus vs. surface pressure plots.
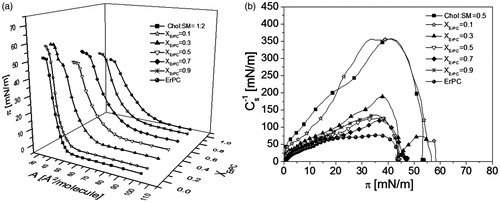
The isotherms for mixed films lie in-between those for pure ErPC and monolayer mimicking lipid raft. Upon incorporation of ErPC, the Chol-SM mixture becomes more expanded as well as more fluid, as evidenced by decreasing Cs−1 values (). Interestingly, the isotherm registered for XErPC = 0.3 is characterized by the presence of two collapses [πcoll(1) ∼43 mN/m and πcoll(2) ∼58 mN/m], which are also seen in the plots of compressibility modulus (Cs−1) vs. surface pressure (). Since Cs−1 is proportional to the first derivative of π vs. A function, these two collapses reflect in zero compressibility moduli values. In general, the presence of two independent collapses in the course of an isotherm from two film-forming molecules is an evidence of their immiscibility in a monolayer (Dynarowicz-Łątka & Kita, Citation1999). When these collapse pressures correspond exactly to the values observed in one-component monolayers, it indicates that the expulsion of a component collapsing at a lower pressure from mixed monolayer occurs at the first collapse, while the other component (collapsing at a higher pressure value) is expelled at the final collapse. For the investigated here monolayer, the value of the first collapse (∼46 mN/m) does not match exactly the value for pure ErPC (∼43 mN/m) (Wnętrzak et al., Citation2012), however, corresponds to the mixed ErPC-cholesterol system (∼ 46 mN/m) (Wnętrzak et al., Citation2013). Similarly, the second collapse is also slightly different to that for Chol:SM 1:2 model raft system (58 vs. 54 mN/m). Since our previous work (Wnętrzak et al., Citation2013) proved that ErPC strongly associates with cholesterol, it may be assumed that in ternary film of ErPC/Chol/SM, the drug does not separate from the system alone, in its free form, but bound to cholesterol molecules. This phase separation is additionally confirmed by collapse pressure values that do not change with mixed film composition () and is clearly visible with BAM as illustrated in at π ≈45 mN/m.
Figure 4. Collapse pressure values vs. film composition for monolayer mimicking lipid raft and ErPC.
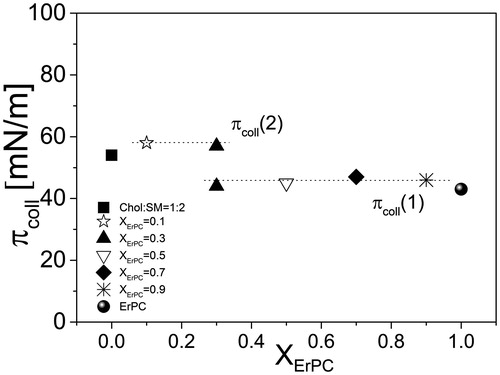
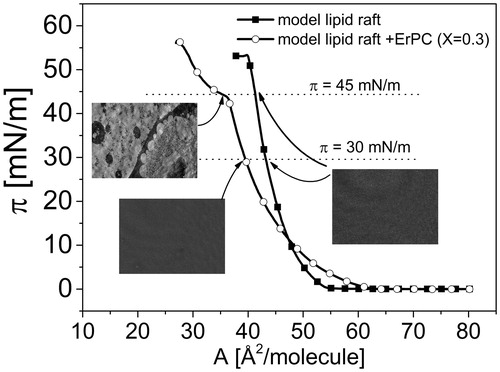
Figure 5. Surface pressure (π)–area (A) isotherms for monolayer mimicking lipid raft and lipid raft with ErPC (X = 0.3) together with BAM images at 30 mN/m and 45 mN/m.
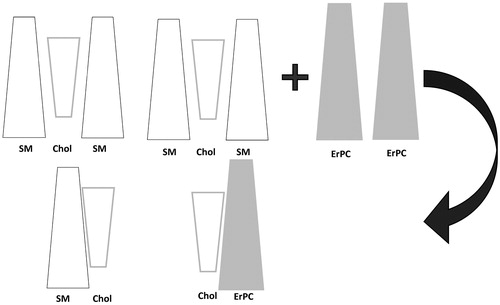
Therefore it can be concluded that in the investigated ternary system of ErPC/Chol/SM two distinct phases are being formed: one consisting of ErPC/Chol and the other one of Chol/SM system. Since the cholesterol content in the latter phase is decreased as compared to model raft monolayer, its collapses at a higher pressure as compared to model raft mixture, as it contains elevated amount of SM, which collapses at higher pressures (collapse pressure for pure SM = 69 mN/m, while for mixtures with cholesterol rich in SM oscillates around 60 mN/m). Although two collapses are seen only for mixed system of XErPC = 0.3, similar behavior can be expected for other mixed film compositions of increased ErPC content, however the second collapse is not visible as it is should occur at a very low area (due to a small proportion of SM and cholesterol), which is out of the moving barrier range.
In this way, cholesterol molecules are partitioned between SM and ErPC. One may wonder if such a distribution of Chol is possible from the thermodynamic point of view, since it is well known that raft-forming lipids are strongly bound together. Indeed, thermodynamic analysis of interactions between cholesterol and sphingomyelin revealed that the maximum excess free energy of mixing (ΔGexc) values at the pressure of 30 mN/m (which corresponds to the pressure in living cells) (Marsh, Citation1996) reach ca. −1500 J/mol (Jurak et al., Citation2014). However, as proved in our previous paper (Wnętrzak, et al., Citation2014), ErPC does not interact favorably with sphingomyelin (repulsive interactions have been observed), however, interacts strongly with cholesterol (ΔGexc ∼−2700 J/mol at 30 mN/m) (Wnętrzak et al., Citation2013). Since the interactions between ErPC and cholesterol are stronger than between cholesterol and sphingomyelin, cholesterol molecules will compete with ErPC and SM, which induces the formation of two phases: ErPC/Chol and Chol/SM. This can be schematically illustrated in .
In order to be able to relate the obtained results to the living systems, the surface pressure of 30 mN/m is of particular importance. We were interested to verify whether any differences in the structure of monolayer mimicking lipid raft occur upon incorporation of erucylphosphocholine. Unfortunately, BAM images taken at 30 mN/m do not distinguish between these two systems, and the films structure looks homogeneous in both cases (). Therefore, in the next step of our investigations, we have transferred the investigated monolayers at π = 30 mN/m, using the Langmuir-Blodgett (LB) technique. The representative images of the lipid raft system and the lipid raft system admixed with erucylphosphocholine deposited on mica substrate are presented in , respectively.
Figure 7. AFM images of LB-transferred Chol/SM (1:2) monolayer (a) and ErPC/Chol/SM film (b), together with height profiles (c and d, respectively).
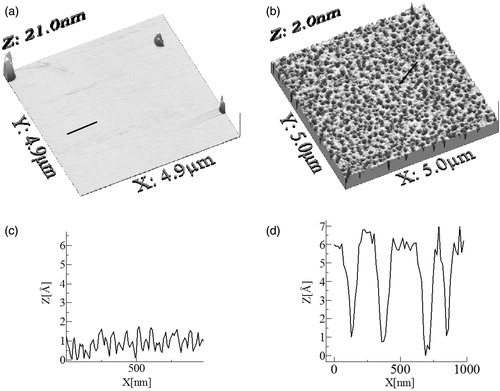
They reveal that an addition of the drug makes the lipid raft system layer more structured with hole-like pits. These features were quantified with root-mean-squared RMS roughness and skewness SK. The investigated lipid raft monolayer exhibited a smooth, uniform surface with low roughness and SK equal to 0.04 nm and 0.74, respectively. In contrast, RMS and SK values of AFM images of the lipid raft admixed with erucylphosphocholine grow to 0.17 and 13.9 nm, respectively. A similar conclusion can be drawn from the comparison of locally observed topography changes (cross sections in ).
From the AFM measurements, we conclude that model raft monolayers, formed on mica substrates, were uniform and globally homogenous. On the other hand, the quality of the model raft layer treated with erucylphosphocholine was lower, as evidenced by the presence of surface heterogeneities. The observed distinctiveness of BAM and AFM images results from the spatial resolutions of these methods. The AFM lateral resolution depends strongly on the tip radius. Relatively small tip radius of an AFM cantilever enables to image and measure roughness with nanoscale spatial resolution (Lee et al., Citation2002), while BAM spatial resolution is equal to 2 μm.
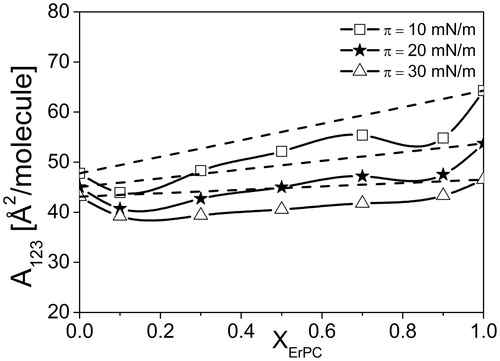
From the analysis based on pressure-area isotherms it is evident that at high pressure region, i.e., above the first collapse, the investigated ErPC/Chol/SM system phase separates. To get insight into the behavior of the investigated mixture at lower pressures, qualitative (mean molecular areas, A123) and quantitative (ΔGexc) parameters of interactions have been calculated and presented in and .
Values of A123 were obtained directly from the experimental curves π-A. The dashed lines in the graphs correspond to the ideal behavior, described by the equation:
For the studied system, the course of function A123 = f(XErPC) deviates from linearity, proving the existence of attractive interactions between the components and their mutual miscibility in the range of surface pressure ≤ 30 mN/m.
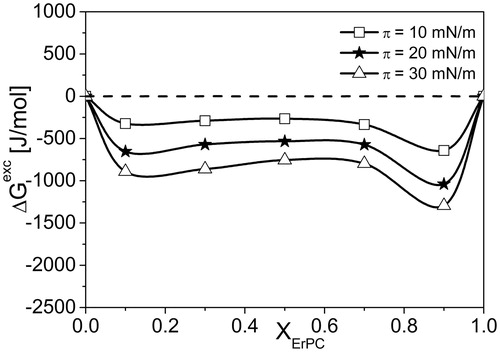
To quantify the interactions, the excess free energy of mixing values (ΔGExc) were calculated as follows:
where NA is the Avogadro number. The results are presented in .
Negative values of ΔGExc indicate that resultant interactions in ternary mixture ErPC/Chol/SM are attractive, although slightly weaker than between Chol and SM devoid of ErPC (Ramstedt & Slotte Citation2002). Although both systems are stable from thermodynamic point of view, but as it can be seen, in the presence of the drug, the stability of model raft monolayer is slightly decreased (ΔGExc ∼−1500 J/mol for model raft mixture (Jurak et al., Citation2014) vs. −1000 J/mol for model raft containing ErPC.
Discussion
Our experiments were carried out to verify the affinity of potent antitumor alkylphosphocholine, erucylphosphocholine (ErPC), to lipid domains and examine its effect on domains ordering. The obtained results proved an increase of lipid rafts fluidization caused by ErPC incorporation (observed in the Cs−1 (π) graphs), which is in agreement with biological data obtained by Ausili et al. (Citation2008) for other antitumoral-edelfosine. NMR spectroscopy studies performed for more complicated raft model: ternary POPC: Chol:SM system show that – at higher temperatures – the addition of the drug to the system disrupts the molecular order (Ausili et al., Citation2008). The observed fluidization can be explained by the geometry of molecular packing (). Our calculations () show that cholesterol molecule possesses an inverted cone shape, while SM possesses a truncated cone shape. This combination provides a favorable geometrical packing. The incorporation of ErPC molecules, possessing a truncated cone shape, reorganizes molecules in the raft, and finally leads to phase separation.
The presence of two collapses in the course of the isotherm for ternary mixture: ErPC/Chol/SM together with BAM observation indicates that at high pressures region, above the first collapse, phase separation occurs. This phase separation leads to redistribution of cholesterol molecules in the model membrane, which has been also observed in model studies involving another antitumor lipid (edelfosine) and different membrane model (vesicles) (Gomide et al., Citation2013). Results obtained in biological studies confirm that the accumulation of antitumor lipids in membrane leads to redistribution of sterols and therefore contributes to changing of the biophysical properties of lipid rafts.
At lower pressures, negative values of ΔGexc proves that ErPC/Chol/SM mixtures are thermodynamically stable, although their stability is slightly lower as compared to raft-mimicking membrane devoid of the drug. Analysis of mutual interactions in binary ErPC:Chol and ErPC:SM binary mixtures indicate that ErPC has a strong affinity to cholesterol (highly negative values of ΔGexc in the whole range of investigated mole fractions [Wnętrzak et al., Citation2013] contrary to SM [positive values of ΔGexc indicating repulsion between molecules; Marsh, Citation1996]). Van der Luit et al. (Citation2007) have shown that the incorporation of another representative of APCs – perifosine – is independent on the amount of sphingomyelin in lipid domain. Thus, it is clear that SM molecules present in lipid rafts do not affect significantly the process of penetration of APCs into the cell.
Conclusions
The obtained results, aimed at verifying the influence of a potent antitumor lipid – erucylphosphocholine – on artificial lipid raft modeled as Langmuir monolayer, confirm experiments performed on living cells, indicating membrane fluidization and destabilization followed by redistribution of cholesterol molecules. Similar effects on lipid rafts can be expected for other synthetic antitumor lipids and naturally occurring lysophosphatidylcholines. These compounds could therefore be used as potential biophysical tools for probing lipid rafts structures in biological membranes. However, natural phospholipids are not metabolically stable as they undergo enzymatic degradation and therefore are not suitable for such applications.
Acknowledgements
Aeterna Zentaris GmbH (Frankfurt, Germany) is gratefully acknowledged for providing a sample of erucylphosphocholine for investigations. The research was carried out with the equipment (Langmuir trough and BAM) purchased thanks to the financial support of the European Regional Development Fund in the framework of the Polish Innovation Economy Operational Program (contract no. POIG.02.01.00-12-023/08).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Agatha G, Häfer R, Zintil F. 2001. Fatty acid composition of lymphocyte membrane phospholipids in children with acute leukemia. Cancer Lett 173:139–144
- Ausili A, Torrecillas A, Aranda FJ, Mollinedo F, Gajate C, Corbalan-Garcia S, et al. 2008. Edelfosine is incorporated into rafts and alters their organization. J Phys Chem B 112:11643–11654
- Beck JG, Mathieu D, Loudet C, Buchoux S, Dufourc EJ. 2007. Plant sterols in “rafts”: a better way to regulate membrane thermal shocks. FASEB J 21:1714–1723
- Bergkvist M, Carlsson J, Oscarsso S. 2001. A method for studying protein orientation with atomic force microscopy using relative protein volumes. J Phys Chem B 105:2062–2069
- Brzozowska I, Figaszewski ZA. 2002. The equilibrium of phosphatidylcholine-cholesterol in monolayers at the air/water interface. Colloids Surf B 23:51–58
- Cadena-Nava RD, Martin-Mirones JM, Vázques-Martinez EA, Roca JA, Ruiz-Garcia J. 2006. Direct observations of phase changes in Langmuir films of cholesterol. Rev Mex Fis 52:32–40
- Castro BM, Fedorov A, Hornillos V, Delgado J, Acuña AU, Mollinedo F, Prieto M. 2013. Edelfosine and miltefosine effects on lipid raft properties: membrane biophysics in cell death by antitumor lipids. J Phys Chem B 117:7929–7940
- Davies JT, Rideal EK. 1963. Interfacial phenomena. New York and London: Academic Press
- Dick DL, Lawrence DS. 1992. Physicochemical behavior of cytotoxic ether lipids. Biochemistry 31:8252–8527
- Dynarowicz-Łątka P, Dhanabalan A, Oliviera ON Jr. 1999. A study on two-dimensional phase transitions in Langmuir monolayers of a carboxylic acid with a symmetrical triphenylbenzene ring system. J Phys Chem B 103:5992–6000
- Dynarowicz-Łatka P, Kita K. 1999. Molecular interaction in mixed monolayers at the air/water interface. Adv Colloid Interface Sci 79:1–17
- Eibl H, Hilgard P, Unger C. 1992. Alkylphosphocholines: new drugs in cancer therapy. Basel: Kargel
- Eibl H, Kaufmann-Kolle P. 1995. Medical application of synthetic phospholipids as liposomes and drugs. J Liposome Res 5:131–148
- Eibl H, Unger C. 1990. Hexadecylphosphocholine: a new and selective antitumor drug. Cancer Treat Rev 17:233–242
- Fish RG. 1996. Role of gangliosides in tumour progression: a molecular target for cancer therapy? Med Hypotheses 46:140–144
- Gajate C, Mollinedo F. 2002. Biological activities, mechanisms of action and biomedical prospect of the antitumor ether phospholipid ET-18-OCH3 (edelfosine), a proapoptotic agent in tumor cells. Curr Drug Metab 3:491–526
- Gaus K, Chklovskaia E, Fazekas de St. Groth B, Jessup W, Harder T. 2005. Condensation of the plasma membrane at the site of T lymphocyte activation. J Cell Biol 171:121–131
- Gomide AB, Thomé CH, dos Santos GA, Ferreira GA, Faça VM, Rego EM, et al. 2013. Disrupting membrane raft domains by alkylphospholipids. Biochim Biophys Acta – Biomembranes 1828:1384–1389
- Gómez J, Sagués F, Reigada R. 2008. Actively maintained lipid nanodomains in biomembranes. Phys Rev E Stat Nonlin Soft. Matter Phys 77:1–5
- Hanzal-Bayer MF, Hancock JF. 2007. Lipid rafts and membrane traffic. FEBS Lett 581:2098–2104
- Hardy NJ, Richardson TH, Grunfeld F. 2006. Minimising monolayer collapse on Langmuir troughs. Colloids Surf A 284–285:202–206
- Hąc-Wydro K, Dynarowicz-Łątka P, Wydro P, Bąk K. 2011. Edelfosine disturbs the sphingomyelin-cholesterol model membrane system in a cholesterol-dependent way – the Langmuir monolayer study. Coll Surf B 88:635–640
- Hąc-Wydro K, Dynarowicz-Łątka P. 2008. The impact of sterol structure on the interactions with sphingomyelin in mixed Langmuir monolayers. J Phys Chem B 112:11324–11332
- Heczková B, Slotte JP. 2006. Effect of anti-tumor ether lipids on ordered domains in model membranes, FEBS Lett 580:2471–2476
- Hilgard P, Klenner T, Stekar J, Nössner G, Kutscher B, Engel J. 1997. D-21266, a new heterocyclic alkylphospholipid with antitumour activity. Europ J Cancer 33:442–446
- Israelachvili JN. 2011. Intermolecular and surface forces, New York: Academic Press
- Jablin MS, Flasiński M, Dubey M, Ratnaweera DL, Broniatowski M, Dynarowicz-Łątka P, Majewski J. 2010. Effects of β-cyclodextrin on the structure of sphingomyelin/cholesterol model membranes. Biophys J 99:1475–1481
- Inbar M, Goldman R, Inbar L, Bursuker I, Goldman B, Akstein E, et al. 1977. Fluidity difference of membrane lipids in human normal and leukemic lymphocytes as controlled by serum components. Cancer Res 37:3037–3041
- Jendrossek V, Handrick R. 2003. Membrane targeted anticancer drugs: potent inducers of apoptosis and putative radiosensitisers. Curr Med Chem – Anti-Cancer Agents 3:343–353
- Johnson SM, Robinson R. 1979. The composition and fluidity of normal and leukemic or lymphomatous lymphocyte plasma membranes in mouse and man. Biochim Biophys Acta 558:282–295
- Jurak M, Gołąbek M, Hołysz L, Chibowski E. 2014. Properties of Langmuir and solid supported lipid films with sphingomyelin. Adv Colloid Interface Sci 222:385–397
- Klock JC, Pieprzyk JK. 1979. Cholesterol, phospholipids, and fatty acids of normal immature neutrophils: comparison with acute myeloblastic leukemia cells and normal neutrophils. J Lipid Res 20:908–911
- Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK. 2003. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther 2:1093–1103
- Lee K-B, Park S-J, Mirkin CA, Smith JC, Mrksich M. 2002. Protein nanoarrays generated by dip-pen nanolithography. Science 295:1702–1705
- Li X-M, Smaby JM, Momsen MM, Brockman HL, Brown RE. 2000. Sphingomyelin interfacial behavior: the impact of changing acyl chain composition. Biophys J 78:1921–1931
- Li Y-C, Park MJ, Ye S-K, Kim C-W, Kim Y-N. 2006. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Path 168:1107–1118
- Maget-Dana R. 1999. The monolayer technique: a potent tool for studying the interfacial properties of antimicrobial and membrane-lytic peptides and their interactions with lipid membranes. Biochim Biophys Acta 1462:109–140
- Marsh D. 1996. Lateral pressure in membranes. Biochim Biophys Acta 1286:183–223
- Mollinedo F, Gajate C. 2006. Fas/CD95 death receptor and lipid rafts: new targets for apoptosis-directed cancer therapy. Drug Resist Updat 9:51–73
- Nieto-Miguel T, Gajate C, Mollinedo F. 2006. Differential targets and subcellular localization of antitumor alkyl-lysophospholipid in leukemic versus solid tumor cells. J Biol Chem 281:14833–14840
- Ouerghi O, Touhami A, Othmane A, Ben Ouada H, Martelet C, Fretigny C, Jaffrezic-Renault N. 2002. Investigating antibody-antigen binding with atomic force microscopy. Sens Actuators B: Chem 84:167–175
- Pachioni JA, Magalhães JG, Lima EJC, de Moura Bueno L, Barbosa JF, de Sá MM, Rangel-Yagui CO. 2013. Alkylphospholipids – a promising class of chemotherapeutic agents with a broad pharmacological spectrum. J Pharm Sci 16:742–759
- Petelska AD, Figaszewski ZA. 2013. The equilibria of sphingolipid-cholesterol and sphingolipid-sphingolipid in monolayers at the air/water interface. J Membr Sci 246:13–19
- Pike LJ. 2006. Rafts defined: a report on the Keystone symposium on lipid rafts and cell function. J Lipid Res 4:1597–1598
- Poole AR, Howell JI, Lucy JA. 1970. Lysolecithin and cell fusion. Nature 227:810–814
- Prenner E, Honsek G, Hönig D, Möbius D, Lohner K. 2007. Imaging of the domain organization in sphingomyelin and phosphatidylcholine monolayers. Chem Phys Lipids 145:106–118
- Ramstedt B, Slotte JP. 2002. Membrane properties of sphingomyelins. FEBS Lett 531:33–37
- Rübel A, Handrick R, Lindner LH, Steiger M, Eibl H, Budach W, et al. 2006. The membrane targeted apoptosis modulators erucylphosphocholine and erucylphosphohomocholine increase the radiation response of human glioblastoma cell lines in vitro. Radiat Oncol 1:1–17
- Simons K, Ikonen E. 1997. Functional rafts in cell membranes. Nature 387:569–572
- Simons K, Toomre D. 2000. Lipid rafts and signal transduction. Nature 1:31–39
- Smaby JM, Brockman HL, Brown RE. 1994. Cholesterol’s interfacial interactions with sphingomyelins and phosphatidylcholines: hydrocarbon chain structure determines the magnitude of condensation. Biochemistry 33:9135–9142
- Sok M, Šentjurc M, Schara M. 1999. Membrane fluidity characteristics of human lung cancer. Cancer Lett 139:215–220
- Thakur G, Pao C, Micic M, Johnson S, Leblanc RM. 2011. Surface chemistry of lipid raft and amyloid Aβ (1–40) Langmuir monolayer. Coll Surf B 87:369–377
- Van Blitterswijk WJ, Verheij M. 2008. Anticancer alkylphospholipids: mechanisms of action, cellular sensitivity and resistance, and clinical prospects. Curr Pharm Des 14:2061–2074
- Van der Luit AH, Vink SR, Klarenbeek JB, Perrissoud D, Solary E, Verheij M, van Blitterswijk WJ. 2007. A new class of anticancer alkylphospholipids uses lipid rafts as membrane gateways to induce apoptosis in lymphoma cells. Mol Cancer Ther 6:2337–2345
- Vila Romeu N, Minones J, Iribarnegaray E, Conde O, Casas M. 1997. Influence of the solvent on the spreading of poly[(D,L-lactic acid)-co-(glycolic acid)] monolayers. Coll Polym Sci 275:580–586
- Wnętrzak A, Łatka K, Dynarowicz-Łatka P. 2013. Interactions of alkylphosphocholines with model membranes – the Langmuir monolayer study. J Membr Biol 246:453–466
- Wnętrzak A, Łątka K, Dynarowicz-Łątka P, Marzec M. 2012. Langmuir monolayer characteristics of erucylphosphocholine – a novel anti-tumor drug. Acta Phys Pol A 121:468–473
- Wnętrzak A, Łątka K, Dynarowicz-Łątka P. 2014. Interactions between antitumor alkylphosphocholines and membrane sphingolipids in Langmuir monolayers. Acta Phys Pol A 125:886–890