Abstract
The kynurenine (KYN) pathway, which is initiated by indoleamine 2,3-dioxygenase, is the main tryptophan (TRP) metabolic pathway. It shares TRP with the serotonin (5-HT) pathway. We investigated the influence of inescapable-predator (rat) stress on behavior and brain TRP metabolism in mice. Male ICR mice (4W) were exposed to 20-min inescapable-predator stress. Behavior on an elevated plus-maze, and TRP, KYN, and 5-HT levels in the prefrontal cortex, hippocampus, amygdala, and dorsal raphe nuclei were measured 1 and 4 weeks after stress exposure. Predator stress increased the number of open-arm entries (NOA) 4 weeks after stress exposure without altering the number of closed-arm entries (NCA). Thus, the open/closed-arm entry ratio (NOA/NCA) increased after stress exposure. Predator stress increased KYN levels in the prefrontal cortex (until 4 weeks after stress exposure) and dorsal raphe nuclei (for 1 week after stress exposure), decreased 5-HT levels in all brain regions (until 4 weeks after stress exposure). Thus, predator stress increased the KYN/5-HT ratio in all regions, in particular in the prefrontal cortex and hippocampus until 4 weeks after stress exposure. Predator stress shifted the balance between the KYN and 5-HT pathways to the KYN pathway, and induced behavioral disinhibition.
| Abbreviations | ||
| ECD | = | electrochemical detection |
| EPM | = | elevated plus-maze |
| FD | = | fluorimetric detection |
| HPLC | = | high-performance liquid chromatography |
| 5-HIAA | = | 5-hydroxyindoleacetic acid |
| 5-HT | = | serotonin |
| IDO | = | indoleamine 2,3-dioxygenase |
| ISO | = | isoproterenol |
| KYN | = | kynurenine |
| LPS | = | lipopolysaccharide |
| NCA | = | number of closed-arm entries |
| NOA | = | number of open-arm entries |
| 3-NTYR | = | 3-nitro-l-tyrosine |
| PND | = | postnatal days |
| PTEs | = | potentially traumatizing stressful experiences |
| Sert | = | serotonin transporter |
| TPH | = | tryptophan hydroxylase |
| TRP | = | tryptophan |
| UV | = | ultraviolet |
Introduction
Exposure to potentially traumatizing stressful experiences (PTEs) in early life can easily cause long-term effects on the still-developing neurobiological system.
In animal studies, early-life PTEs have been shown to cause prolonged neurobiological and behavioral changes (Liu et al. Citation1997, Citation2000a,Citationb; Francis et al. Citation1999; Caldji et al. Citation2000a,Citationb; Weaver et al. Citation2004; Imanaka et al. Citation2006). In juvenile animals, PTEs also have been demonstrated to induce long-term neurobiological and behavioral changes (Heidbreder et al. Citation2000; Maslova et al. Citation2002a,Citationb; Avital and Richter-Levin Citation2005; Avital et al. Citation2006). Such changes tend to increase the vulnerability of animals to stressors encountered during adulthood (Cohen et al. Citation2007).
In humans, early-life adversity such as child abuse and neglect is known to increase the risk of major psychiatric disorders such as depression, anxiety disorder (Young et al. Citation1997; Heim and Nemeroff Citation2001), and post-traumatic stress disorder (PTSD; Widom Citation1999; Shea et al. Citation2005). Evidence from clinical studies suggests that exposure to early-life PTEs is associated with neurobiological changes in children and adults, which may underlie the increased risk of psychopathology (Heim and Nemeroff Citation2001).
Psychological stress as well as immunological challenges is known to change brain tryptophan (TRP) metabolism. There are two metabolic pathways in TRP metabolism. One is the kynurenine (KYN) pathway, which is initiated by indoleamine 2,3-dioxygenase (IDO) (Hirata et al. Citation1974), a key TRP metabolic pathway (Stone and Darlington Citation2002). This pathway shares TRP with the serotonin (5-HT) pathway, the other TRP metabolic pathway, which is initiated by TRP 5-monooxygenase [Ichiyama et al. Citation1970; tryptophan hydroxylase (TPH)]. Activation of the KYN pathway by proinflammatory cytokines induces depressive symptoms (Bonaccorso et al. Citation2002; Capuron et al. Citation2002, Citation2003; Wichers et al. Citation2005). Such activation of the KYN pathway (Konsman et al. Citation2002; Widner et al. Citation2002; Wichers and Maes Citation2004) elicit shifts in the balance between the KYN and 5-HT pathways of TRP metabolism toward the KYN pathway, and these shifts are closely related to the etiology of depression. Indeed, psychological stress (Miura et al. Citation2008a) as well as systemic administration of lipopolysaccharide (LPS; Kita et al. Citation2002; Miura et al. Citation2009, Citation2010; O'Connor et al. Citation2009) have been shown to increase brain KYN levels and shift the balance between KYN and 5-HT pathways to the KYN pathway. Thus, the effects of psychological stress and immunological challenges share in common that they change brain TRP metabolism. However, the influence of PTEs in early life (i.e. in juveniles) on brain TRP metabolism remains unknown.
The aim of the present study was to investigate the long-term effects of acute, inescapable predator stress as PTEs on behavior and brain TRP metabolism in juvenile mice. As a behavioral measure, the elevated plus-maze (EPM) was used, because this test is suitable for the detection of anxiety-like behaviors elicited by stress in a mouse model. For this series, four brain regions were selected, the first three of which possess 5-HT nerve terminals: the prefrontal cortex, which is involved in behavioral motivation; the amygdala, which is involved in emotion; the hippocampus, which regulates the hypothalamo–pituitary–adrenal (HPA) axis, and the hyperactivity of this axis is closely related to the etiology and pathophysiology of depression; and the dorsal raphe nuclei, because these structures contain the cell bodies of 5-HT neurons and are the center of brain 5-HT synthesis.
Materials and methods
Animals
A total of 100 male specific pathogen-free (SPF) ICR mice for the experimental/control animals and 20 male Wistar rats for the predators were used in the present experiments. At 21 postnatal days (PND), the mice were transported from the breeding company to our experimental animal center. After a habituation period of 1 week, the mice were used for experiments. The mice were housed in groups of 4–5 per cage. The animals were divided into two groups as follows: a stress group exposed to predator stress session and a control group lacking the predator stress session. Recent studies have shown that 25% of Sprague-Dawley, 50% of Lewis, and 10% of Fisher F344 rats became maladaptive, based on the results of two behavioral tests (EPM and acoustic startle response tests) conducted 7 days after predator exposure, and these maladaptive effects remained for 30 days (Cohen et al. Citation2004, Citation2006). Therefore, we expected that some ICR mice would also exhibit maladaptive responses on the EPM test after being exposed to predator stress (i.e. as previously demonstrated in rats). We based our selection of the number of mice used for each group on the previously mentioned rat experiments, as follows: about 30% (approximately expected by the mean of 10 and 50%) of the total number of animals exposed to predator stress corresponded to the number of control group. Furthermore, the mice were divided into two groups as follows: the 1W group received 1 week of follow-up and the 4W group received 4 weeks of follow-up. Finally, by combining the above conditions, the mice were divided into four groups: 1W controls (n = 12); 1W stress (n = 38); 4W controls (n = 12); and 4W stress (n = 38). At 7 weeks of age, rats were transported from the breeding company to our experimental animal center, and were reared in a dedicated room. After a 1-week habitation period, the rats were used for the experiments. The rats were housed in groups of 2–3 per cage.
Cages for the mice had the following dimensions: 17 × 29 × 13 cm. Cages for the rats measured 26 × 42 × 20 cm. Cage exchange was performed 2 times per week. Food and water were provided ad libitum. The animals were kept on a 12-h light/dark cycle (lights on at 07.00 h, off at 19.00 h), and room temperature was maintained at 21–23°C. All efforts were made to minimize both the number of animals used and the degree of their suffering. All of the experiments were conducted in accordance with the European Communities Council Directive of November 24, 1986 (86/609/EEC). The study was approved by the ethical committee of Nagoya University Graduate School of Medicine.
Inescapable-predator stress
At 4 weeks of age, mice in the stress group were exposed to a predator stress session. Mice were held in a 50-ml transparent plastic centrifugal tube with holes drilled in the cap and at the bottom for respiration, and the tube was fixed by adhesive tape in the center of the floor of a transparent plastic box (28 × 35 × 30 cm). A rat was placed into the box as soon as possible after introduction of the mouse, and the rat was allowed to freely explore the box for 20 min. Thus, the mice were held in a manner in which they could not escape from a predator rat for 20 min. Although the mice were able to see and smell the predator, they were not directly attacked. After the stress session, mice were returned to their home cages. After every session, the floor of the box and the tube were wiped clean with ethanol and a wet cloth to minimize odor. For each session, one rat was selected as a predator from a group of 20 that had been reared together. The selected rat was never allocated to more than one session per day. Each rat rested at least 1 week until used for the next stress session. Thus, the rats used for the stress sessions ranged from 8 to 12 weeks of age. The inescapable-predator stress session was performed between 13.00 and 18.00 h.
Elevated plus-maze
The EPM (50 × 50 × 8 cm black wood, plus-sign-shaped apparatus, wherein the floor of the arms was covered with a gray resin sheet, and the apparatus was raised above the ground by 0.4 m). This maze was used for the analysis of animal behavior. The EPM session was performed 1 week (1W group) or 4 weeks (4W group) after the mouse had been exposed to an inescapable-predator stress session. Mice were placed in the center of the EPM, and could face either an open or a closed arm by turning. Mice were allowed to freely explore the maze for 5 min. Behaviors were recorded for 5 min by a digital video camera (ivis DC300, Canon, Tokyo, Japan) placed approximately 1 m above the center of the floor of the EPM. The session was carried out between 22.00 and 24.00 h in a dark room with red dim light (7 W) placed approximately 1 m above the central floor of the EPM. After every session, the EPM was wiped clean with ethanol and a wet cloth to minimize the odor. The following parameters were analyzed: number of open-arm entries (NOA), number of closed-arm entries (NCA), and open/closed-arm entry ratio (NOA/NCA). Entry into one of the two arms of the maze was defined as the animal placing four paws into the arm.
Sample preparation
Four brain regions (prefrontal cortex, hippocampus, amygdala, and dorsal raphe nuclei) were dissected from the whole brain. The mice were sacrificed by decapitation on the day following the EPM session; mice were decapitated under brief anesthesia. The precise method for brain dissection has been described elsewhere (Miura et al. Citation2009).
The above-mentioned brain regions were removed as quickly as possible on glass plates over ice. The samples were weighed and treated with 1000 μl of an ice-cold 0.2 M trichloroacetic acid solution containing 0.2 mM sodium pyrosulfite, 0.01% EDTA-2Na; 0.5 μM isoproterenol (ISO) and 3-nitro-l-tyrosine (3-NTYR) as an internal standard per 100 mg of wet tissue. The solution was sonicated and then centrifuged at 10,000g for 20 min at 4°C. The supernatant was filtered through a Millipore HV filter (pore size, 0.45 μm) and then subjected to both high-performance liquid chromatography (HPLC) with electrochemical detection (ECD) of 5-HT, and HPLC with fluorimetric detection (FD) of TRP, and with ultraviolet (UV) detection of KYN.
The standard solution was prepared using the above-mentioned ice-cold 0.2 M trichloroacetic acid solution containing 0.5 μM internal standards (ISO, 3-NTYR). The concentration was adjusted to 0.5 μM for 5-HT and KYN, and 10 μM for TRP.
HPLC determination of brain levels of 5-HT
The levels of 5-HT in the brain extracts were measured by HPLC with ECD. The system employed for HPLC-ECD consisted of a CMA/200 autosampler (CMA/Microdialysis AB, Stockholm, Sweden), a micro LC pump (BAS, West Lafayette, IN, USA), an LC-4C ECD (BAS), a Bio-Phase ODS-4 51-6034 column (4.0 × 110 mm; BAS), a CR-6A recorder (Shimadzu, Kyoto, Japan), an LC-26A vacuum degasser (BAS), and a CTO-10A column heater set at 35°C (Shimadzu). The mobile-phase solution consisted of 0.1 M tartaric acid–0.1 M sodium acetate buffer, pH 3.2, containing 0.5 mM EDTA-2Na, 555 μM sodium 1-octane sulfonate, and 5% acetonitrile. The flow rate was 700 μl/min. The concentration of each compound was calculated by comparison with both the internal (ISO) and external standards. The sensitivity of 5-HT measurements was 150 fmol.
HPLC determination of brain levels of TRP and KYN
We measured the levels of TRP and KYN according to the methods of Widner et al. (Citation1997) and those improved by Laich et al. (Citation2002). The HPLC pump was an LC-10AD (Shimadzu). For separation, reversed-phase column LiChroCART 55-4 cartridges filled with Purospher STAR Rp-18e (55 mm length, 3 μm grain size) together with a reverse-phase LiChroCART 4-4 precolumn filled with Purospher STAR RP-18e (5 μm grain size; Merck, Rahway, NJ, USA) were used. TRP was detected by RF-535 FD (Shimadzu) at an excitation wavelength of 285 nm and an emission wavelength of 365 nm. Both KYN and 3-NTYR were detected using a SPD-10A UV-detector (Shimadzu) at a wavelength of 360 nm. Both detectors were connected in a series to allow for simultaneous measurement. The mobile-phase solution consisted of a 15 mM l-acetic acid-sodium acetate buffer, pH 4.0, containing 2.7% acetonitrile. The flow rate was 900 μl/min at room temperature. The sensitivity of the TRP and KYN measurements were 50 and 100 fmol, respectively.
Statistical analyses
In order to examine differences in NOA, NCA, and in the ratio of NOA/NCA for the EPM behavioral test, two-way MANOVA (Wilks's lambda) for the stress and time course (duration of follow-up after stress exposure) was conducted on the dependent measures, followed by the Tukey–Kramer test. To examine differences in the levels of TRP, 5-HT, and KYN, and in the ratios of KYN/5-HT, two-way MANOVA (Wilks's lambda) for the stress and time course was conducted on the dependent measures in each brain region, followed by the Tukey–Kramer test. Furthermore, to examine differences elicited by stress at each time point (1 and 4 weeks after the stress session), an unpaired two-tailed t-test of control and stress groups was conducted. Data are presented as the means ± SEM. All P values less than 0.05 were accepted as significant.
Results
Inescapable predator stress was found to influence behavior and brain TRP metabolism in experimental mice.
Behavioral changes in the EPM
Although we expected that it would be easy to select the maladaptive animals from all mice exposed to predator stress based on behavior exhibited during the EPM, we were unable to detect any apparent maladaptive behavior (data not shown), as was observed in previous studies of rats (e.g. avoidance of entry into the open arm of the maze after a 5-min EPM session) (Cohen et al. Citation2004, Citation2006). Thus, we used all animals in the stress group in the subsequent trial.
The results of the two-way MANOVA of stress and the time course were as follows: stress (F(3, 94) = 2.706, P = 0.0497) significantly altered the dependent measures, and time course (F(3, 94) = 9.991, P < 0.0001) also significantly altered the dependent measures. The interaction between stress and time course (F(3, 94) = 0.092, P = 0.9642) was not significant. The post-hoc test revealed that stress significantly increased the NOA, whereas the NCA was not altered by stress (). Thus, stress significantly increased the ratio of NOA/NCA (). The post-hoc test also revealed that time course significantly decreased the NOA () and the ratio of NOA/NCA ().
Figure 1. Changes in the behavior of mice in the EPM (NOA and NCA), elicited by inescapable-predator stress and time course (duration of follow-up after stress exposure). Each bar indicates a group defined according to stress exposure and time course. White bar, non-stress; black bar, stress. Values are shown as means ± SEM. The results of the Tukey–Kramer test for predator stress and time course are shown. **, P < 0.01 for the effects of stress. ##, P < 0.01 for the effects of time course. Unpaired two-tailed t-test between non-stress and stress groups at each time point, 1 and 4 weeks after the stress session. +, P < 0.05. NCA, number of closed-arm entries; NOA, number of open-arm entries.
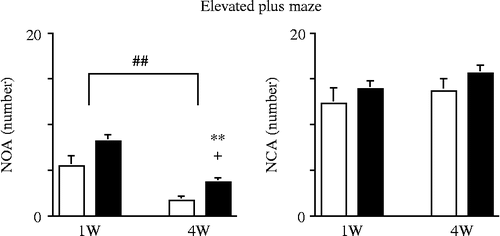
Table I. Changes in the behavior of mice in the EPM, based on NOA/NCA, elicited by inescapable-predator stress and the differences between acute (1 week) and chronic (4 weeks) effects.
Furthermore, 4 weeks after the stress session, the NOA significantly increased, although there had been no significant increase 1 week after the stress session ().
Thus, the inescapable-predator stress elicited behavioral changes, and increased the NOA without altering the NCA, at 4 weeks after the stress session.
Brain TRP metabolism
Prefrontal cortex
The results of the two-way MANOVA of stress and the time course were as follows: stress (F(4, 93) = 19.371, P < 0.0001) significantly altered the dependent measures, whereas time course (F(4, 93) = 1.253, P = 0.2940) did not significantly alter the dependent measures. The interaction between stress and time course (F(4, 93) = 1.731, P = 0.1497) was not significant. The post-hoc test revealed that stress significantly increased KYN levels, and significantly decreased 5-HT levels, whereas it did not significantly alter TRP levels (). Thus, stress significantly increased the KYN/5-HT ratio ().
Figure 2. Changes in TRP, KYN, and 5-HT levels elicited by inescapable-predator stress and time course (duration of follow-up after stress exposure). Each bar indicates a group defined by stress and time course. White bar, non-stress; black bar, stress. Values are shown as means ± SEM. The results of the Tukey–Kramer test for predator stress and time course are shown. **, P < 0.01 for the effects of stress. #, P < 0.05; ##, P < 0.01 for the effects of time course. Unpaired two-tailed t-test between non-stress and stress groups at each time point, 1 and 4 weeks after stress session. +, P < 0.05; ++, P < 0.01. (A) prefrontal cortex, (B) hippocampus, (C) amygdala, (D) dorsal raphe nuclei. 5-HT, serotonin; KYN, kynurenine; TRP, tryptophan.
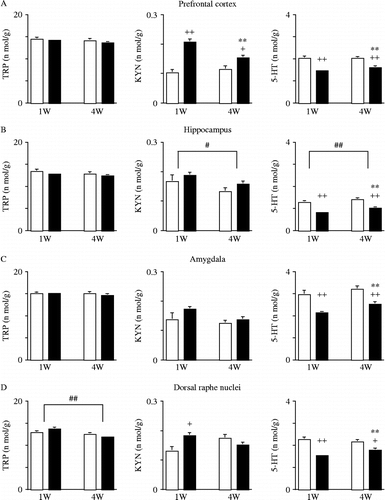
Table II. Changes in the KYN/5-HT ratio elicited by inescapable-predator stress and the differences between acute (1 week) and chronic (4 weeks) effects.
Furthermore, at 4 weeks, stress was still significantly associated with increased KYN and decreased 5-HT levels, as had been observed 1 week after the stress session (). Thus, the KYN/5-HT ratio remained increased at 4 weeks, as had been the case 1 week after the stress session ().
Hippocampus
The results of the two-way MANOVA of stress and the time course were as follows: stress (F(4, 93) = 8.989, P < 0.0001) significantly altered the dependent measures, and the time course (F(4, 93) = 2.583, P = 0.0421) also significantly altered the dependent measures. The interaction between stress and time course (F(4, 93) = 0.144, P = 0.9654) was not significant. The post-hoc test revealed that stress significantly decreased 5-HT levels, whereas it did not significantly alter either TRP or KYN levels (). Thus, stress significantly increased the KYN/5-HT ratio (). The post-hoc test also revealed decreased KYN levels, and increased 5-HT levels, with time whereas it did not alter TRP levels (). Thus, the KYN/5-HT ratio significantly decreased over time ().
Furthermore, at 4 weeks post-stress session, stress remained a factor that significantly decreased 5-HT levels, as had also been seen 1 week after the stress session (). Thus, the KYN/5-HT ratio was still increased at 4 weeks as well as 1 week after the stress session ().
Amygdala
The results of the two-way MANOVA of stress and the time course were as follows: stress (F(4, 93) = 7.717, P < 0.0001) significantly altered the dependent measures, whereas the time course (F(4, 93) = 1.724, P = 0.1514) did not significantly alter the dependent measures. The interaction between stress and time course (F(4, 93) = 0.505, P = 0.7322) was not significant. The post-hoc test revealed that stress significantly decreased 5-HT levels, whereas it significantly altered neither TRP nor KYN levels (). Thus, stress significantly increased the KYN/5-HT ratio ().
Furthermore, stress significantly decreased 5-HT levels at 4 weeks as well as 1 week post-stress session (). Although the KYN/5-HT ratio was increased 1 week after the stress session, no such difference was observed 4 weeks after the stress session ().
Dorsal raphe nuclei
The results of the two-way MANOVA of stress and the time course were as follows: stress (F(4, 93) = 7.607, P < 0.0001) significantly altered the dependent measures, and the time course (F(4, 93) = 2.474, P = 0.0497) also significantly altered the dependent measures. The interaction between stress and time course (F(4, 93) = 2.987, P = 0.0228) was significant. The post-hoc test revealed that stress significantly decreased 5-HT levels, whereas it did not significantly alter either the TRP or KYN levels (). Thus, stress significantly increased the KYN/5-HT ratio (). The post-hoc test also revealed that the time course significantly decreased TRP levels, whereas it altered neither the KYN nor the 5-HT levels (). Thus, the time course did not alter the KYN/5-HT ratio ().
Furthermore, stress was found to significantly decrease 5-HT levels at 4 weeks as well as 1 week after the stress session, whereas the significant increase in KYN levels observed 1 week after the stress session was no longer observed 4weeks after the session (). Although the KYN/5-HT ratio was increased 1 week after the stress session, no such difference was observed 4 weeks after the stress session ().
Summary
The inescapable-predator stress trial demonstrated prolonged changes in behavior and brain TRP metabolism in mice. In the EPM, the NOA had increased, but the NCA did not, at 4 weeks after the stress session. The inescapable-predator stress shifted the balance of TRP metabolism between the KYN and 5-HT pathways to the KYN pathway in the prefrontal cortex and hippocampus for 4 weeks after the stress session, whereas the shifts in the balance to the KYN pathway that had appeared 1 week after the stress session disappeared from the amygdala and dorsal raphe nuclei by 4 weeks after the stress session.
Discussion
PTEs in early life are known to induce long-term effects on behavior and neurobiological changes in animals (Liu et al. Citation1997, Citation2000a,Citationb; Francis et al. Citation1999; Caldji et al. Citation2000a,Citationb; Weaver et al. Citation2004; Imanaka et al. Citation2006). Furthermore, PTEs also induce long-term effects in juvenile animals (Heidbreder et al. Citation2000; Maslova et al. Citation2002a,Citationb; Avital and Richter-Levin Citation2005; Avital et al. Citation2006). Shifts in the balance between the KYN and 5-HT pathways of brain TRP metabolism to the KYN pathway, when elicited by psychological stress (Miura et al. Citation2008a) or immunological challenge (Miura et al. Citation2009, Citation2010), may be associated with the etiology and pathophysiology of psychiatric disorders such as depression (Miura et al. Citation2008b). Thus, we investigated the behavioral (measured by EPM) and neurobiological (measured by brain TRP, KYN, and 5-HT levels) changes elicited by inescapable-predator stress in juvenile (4-week-old) mice.
Changes in behavioral measures observed in the EPM
Unexpectedly, inescapable-predator stress significantly increased the NOA as well as the NOA/NCA. The NOA and entry ratio have been regarded as markers of anxiety-like behavior. Most studies conducted in this field have reported that exposure to predator stress decreases the NOA and NOA/NCA; the authors of these studies have suggested that such changes are indicative of elevated anxiety (Blanchard et al. Citation2003; Korte and De Boer Citation2003; Adamec et al. Citation2004a, Citation2006a,Citationb; Armario et al. Citation2008). The present EPM results contradict those of previous studies regarding the type of changes elicited by predator stress.
Increases in the NOA and NOA/NCA in the EPM have been regarded as markers of reduced anxiety, and increased disinhibition, hyperactivity, and impulsivity (Olausson et al. Citation1999; Laviola et al. Citation2003; Sobrian et al. Citation2003; Meyer et al. Citation2008; Howells et al. Citation2009). Because the NCA in EPM studies is generally regarded as a measure of locomotor activity, the unaltered NCA observed in our study suggested that exposure to stress did not directly influence the locomotion of the mice. Thus, it is likely that our stress protocol reduced inhibition against entering the open arm, without affecting locomotor activity. Although further studies incorporating other measures (e.g. startle response, ethological risk assessment and a detailed temporal analysis of the EPM outcome) will be needed to interpret the meaning of these behavioral changes, we are suggesting that behavioral disinhibition and/or an increase in impulsivity might have taken place in the present study.
Other stressors experienced during adolescence also showed long-term behavioral effects. Social stress in adolescence (daily 1 h isolation and change of cage partner, PND30-45) tended to increase anxiety-like behavior of rats, when tested as adults several weeks after the stress session (McCormick et al. Citation2008). Predator odor exposure in periadolescence (PND40-48) changed behavior. Rats were more fearful in a novel open field and displayed increased activity in response to a predator odor stress in adulthood (Wright et al. Citation2008). Social defeated rats during mid-adolescence (PND35-39) exhibited increased locomotion in both EPM and open field tests, suggesting heightened novelty-induced behavior and deficits in anxiety responses. In particular, previously defeated adult male rats spent more time in the open arms of the EPM, suggesting increased risk-taking behavior (Watt et al. Citation2009). Thus, stress exposure in adolescence may have induced different behavioral changes by the selected stress protocol. However, one study at least showed that stress in adolescence increased the time spent in open arms of EPM, suggesting increased risk-taking behavior and deficit in anxiety.
The stress protocol of this study may be partly responsible for the differences observed between our results and those of previous studies of predator stress. First, the complexity of the present stress protocol included both restraint and predator stress. Although the mice were not directly injured by the predator (rat), they were able to both see and smell the predator while being unable to escape. In other words, the mice were inescapably exposed to the terror elicited by the presence of the predator without undergoing the immunological changes elicited by direct bodily injury. Second, the intensity and duration of the stress protocol differed in the previous and present studies. In most of the previous studies, the animals exposed to predator stress were allowed to move freely; the present protocol, which incorporated the aspect of inescapable fear, may have exposed the animals to greater stress than that experienced in trials in which the animals could freely move about. In the present study, the duration of the stress was 20 min, because we used to apply 20 min of novelty-stress session in our previous studies (Miura et al. Citation2002a,Citationb, Citation2005, Citation2008a). The duration of predator stress applied in other previous studies was shorter, i.e. from 5 to 10 min (Adamec et al. Citation2004a,Citationb,Citationc, Citation2006a,Citationb). Thus, our stress protocol may have been more intense and of longer duration than that used in previous studies.
Differences in species and strains of animals may also influence study results. In the present study, we selected ICR mice, because we have been used the strain in a series of our recent studies about alteration of TRP metabolism elicited by psychological stress and/or immunological challenges (Miura et al. Citation2008a, Citation2009, Citation2010). Even within rats, the prevalence of maladaptive behavior elicited by predator stress has been reported to range from 10 to 50% (Cohen et al. Citation2004, Citation2006); the prevalence rate in mice is equally difficult to predict. Among mouse strains, ICR mice may be unique in terms of their behavioral responses. In a recent study, the prevalence of maladaptive behaviors in mice ranged from 5.5% (DBA/2J) to 41.2% (C57BL/6J) at 7 days after exposure to 10 min of predator-scent stress (Cohen et al. Citation2008); however, the mice used in that study were adults, and not juveniles, as was the case here. Since, in previous studies, no ICR mice showed maladaptive behaviors, ICR mice may be generally less anxiogenic than other strains of mice in response to exposure to predators.
Changes in brain TRP metabolism
Exposure to inescapable-predator stress induced prolonged changes in brain TRP metabolism in juvenile (4-week-old) mice. Stress increased KYN levels, reduced 5-HT levels, and increased the KYN/5-HT ratio. Thus, stress exposure shifted the balance between the KYN and 5-HT pathways toward the KYN pathway. The shift to the KYN pathway in the prefrontal cortex and hippocampus was prolonged, i.e. lasted until 4 weeks after exposure to the stress, whereas in the amygdala and the dorsal raphe nuclei, the shift that had appeared at 1 week after the exposure to stress had already disappeared 4 weeks after the stress session.
Both psychological stress and immunological challenges are thought to activate the inflammatory response system (IRS), induce proinflammatory cytokines, and induce and activate peripheral and brain IDO (Miura et al. Citation2008b). The activated IDO metabolizes TRP into KYN, which may shift the balance between the KYN and 5-HT pathways toward the KYN pathway. Indeed, acute psychological stressors such as novelty stress and acute immunological challenges (e.g. systemic LPS injection) have been shown to shift the balance between the KYN and 5-HT pathways toward the KYN pathway in the mouse brain (Miura et al. Citation2008a, Citation2009). In accordance with our studies, acute psychological stress actually increased IDO1 mRNA expression in brain, lung, spleen, and Peyer's patches of mice, and resulted in a transient depletion of TRP and 5-HT from the plasma, while KYN levels increased (Kiank et al. Citation2010). Furthermore, unpredictable chronic mild stress increased TRP catabolism along the KYN pathway in the periphery. 5-HT and KYN were found to be strongly negatively correlated in all brain structures except the hippocampus of mice (Laugeray et al. Citation2010). As exposure to inescapable-predator stress also shifted the balance to the KYN pathway in the present study, the present results provide additional evidence in support of these previous studies.
In recent years, the developmental role of 5-HT has become apparent. During the postnatal period, transient alterations in 5-HT homeostasis cause permanent changes to adult behavior and modify the fine wiring of brain connection (Gaspar et al. Citation2003). In addition, to 5-HT neurons, a number of neurons transiently express 5-HT transporter (Sert), from embryonic day (ED) 15 to PND 10, with matching expression of Vmat2, the transporter that packages 5-HT into synaptic vesicles, but not synthesize 5-HT. So, the presence of functional Sert and Vmat2 proteins allows them to capture 5-HT that is released or leaks out of neighboring 5-HT-producing axons from the raphe nuclei (Gaspar et al. Citation2003). The control of 5-HT levels is important for the construction of the focused axonal arbors of the sensory neurons (Gaspar et al. Citation2003). During the first postnatal week, the deletion of monoamine oxidase A (MAOA), the most active subtype in 5-HT metabolism, results in the lack of barrels in the somatosensory cortex. During first 3 weeks of postnatal life, 5-HT1A receptor activation stimulates dendritic differentiation in the hippocampus. Furthermore, 5-HT1B receptors are transiently expressed in all the sensory thalamic relay nuclei during early postnatal development. Excessive stimulation of the 5-HT1B receptors disturbs barrel formation in the somatosensory cortex. Thus, juvenile (4-week-old) might still be the period that the control of 5-HT level is important for fine wiring of brain connection.
Previous studies of the effects of predator stress on the brain's 5-HT system have been reported (Rueter and Jacobs Citation1996; Linthorst et al. Citation2000; Belzung et al. Citation2001; Hayley et al. Citation2001; Adamec et al. Citation2004b,Citationc, Citation2008; Beekman et al. Citation2005). Predator stress was found to increase levels of 5-hydroxyindoleacetic acid (5-HIAA), a metabolite of 5-HT, in the prefrontal cortex and hippocampus of mice (Hayley et al. Citation2001), whereas it increased the 5-HIAA/5-HT ratio in the hippocampus without altering 5-HT and 5-HIAA levels (Belzung et al. Citation2001). In studies using in vivo microdialysis, predator stress was shown to increase 5-HT levels without altering 5-HIAA levels in the prefrontal cortex, hippocampus, amygdala, and striatum of rats (Rueter and Jacobs Citation1996) and it increased 5-HT and 5-HIAA levels in the prefrontal cortex, hippocampus, and lateral septum of mice (Linthorst et al. Citation2000; Beekman et al. Citation2005). These previously reported results suggest that predator stress activates the brain 5-HT system, i.e. increases 5-HT release and turnover in nerve terminals. Thus, in the present study, the observed decreases in 5-HT levels in the three terminal regions (prefrontal cortex, hippocampus, and amygdala) that receive neuronal projections carrying 5-HT, as well as the observed decrease in 5-HT levels in the central region that synthesizes 5-HT (dorsal raphe nuclei), suggest that the inescapable-predator stress protocol enhanced turnover in excess of the biosynthesis of 5-HT. Although the direct effects of predator stress on the biosynthesis of 5-HT remains unknown, acute as well as chronic stress are both known to induce brain TPH activity (Boadle-Biber et al. Citation1989; Evans et al. Citation2009), mRNA, and protein (Chamas et al. Citation2004). Thus, the effects of inescapable-predator stress on 5-HT release from nerve terminals and 5-HT turnover may have been greater than could be compensated for by 5-HT biosynthesis in juvenile mice 1 and 4 weeks after exposure to a stress session.
As an example of changes in the 5-HT system elicited by PTEs, those of post-weaning social isolation are known. In most laboratories, post-weaning social isolation is performed by hosing rat or mouse pups in individual cages from the first day of weaning from the dam (between PND21 and 28) for a period of 4–8 weeks (Veenema Citation2009). Thus, the experimental period in post-weaning social isolation covers that of our study (stress exposure at 4-week old, follow up 4 weeks). Post-weaning social isolation decreased extracellular 5-HT responsiveness in the hippocampus, decreased basal 5-HT turnover and increased extracellular 5-HT responsiveness in the nucleus accumbens, whereas the stress protocol increased 5-HT1A autoreceptor function in the dorsal raphe nuclei, suggesting decreased 5-HT postsynaptic (re)activity (Veenema Citation2009). In the prefrontal cortex, effects of post-weaning social isolation on 5-HT system were inconsistent (Veenema Citation2009). Although the differences in stress protocol such as chronic or acute between post-weaning social isolation and inescapable-predator stress may have influenced the differences in the neuronal activities of 5-HT terminal regions, the precise underlying mechanism remains unknown. However, the inhibitory effect of post-weaning social isolation as PTEs on dorsal raphe nuclei may be comparable to that of our inescapable predator stress.
In addition, the inescapable-predator stress induced prolonged changes in brain TRP metabolism. In the prefrontal cortex and hippocampus, the shift in the balance between the KYN and 5-HT pathways toward the KYN pathway continued for 4 weeks, although the shift seen in the amygdala and dorsal raphe nuclei 1 week after stress exposure disappeared by 4 weeks after stress exposure. The precise mechanism of the longer-lasting effects remains to be clarified. However, the intensity and duration of the stress protocol of the present study, as well as the developmental stage of these juvenile mice, may have influenced our results. As mentioned above, the complexity (both predator and restraint stress) and the relatively long duration (20 min) of the present stress protocol may have exposed the animals to a higher level of stress than did previous, simpler, predator-stress sessions with duration of 5–10 min. The mice in the present study were 4 weeks old and therefore still juveniles (post-weaning, pre-puberty), in which the neurobiological system has not yet fully developed. Thus, the animals in this study were likely to be particularly susceptible to PTEs. The present, relatively intense stress protocol may have elicited long-lasting changes in brain TRP metabolism in these mice.
It is possible that prolonged changes in brain TRP metabolism may be associated with persistent behavioral changes. Long-lasting shifts in the balance between the KYN and 5-HT systems to the KYN system, elicited by PTEs in juvenile mice, may have been related to increases in both the NOA and NOA/NCA in the present study.
Study limitations
The first limitation of the present study was the complexity of the stress session protocol. Our inescapable-predator stress session included both restraint and predator stress as potential influences. Here, we did not merely consider the effects of predator stress on behavior and brain TRP metabolism; we moreover intended to investigate the effects of acute life-threatening stress in the absence of injury by a predator. Thus, rather than using a simple stress session, we chose to expose the animals to acute, life-threatening stress without inducing any immunological changes that would be elicited by bodily injury. The second limitation was the design of control group. A better control would have been held in the 50-ml tube for 20 min without predator exposure, which would allow the effects of restraint versus predator exposure to be differentiated. The last limitation of our study was the imbalance in the number of animals allocated to the stress and control groups. We had expected that our inescapable-stress context would induce behavioral changes in the EPM in approximately 25% of maladaptive-responding animals, as reported in a previous study (Cohen et al. Citation2004) of rats. However, no apparent maladaptive behavior of ICR mice was distinguishable from that of other mice in the EPM. Thus, we were not able to reduce the number of mice in the stress group.
Conclusion
Acute, inescapable-predator stress induced long-lasting changes in the behavior in the EPM and brain TRP metabolism of juvenile (4-week-old) mice. Unexpectedly, the NOA in the EPM increased without altering the NCA after the stress session, and this increase was evident 4 weeks after stress exposure. The stress session increased levels of KYN, whereas it decreased 5-HT levels, and thus the balance between the KYN and 5-HT pathways in the brain shifted toward the KYN pathway. These neurochemical changes continued until at least 4 weeks after stress exposure. The complexity, which included both simultaneous restraint and predator stressors and longer duration of the present stress protocol may have reproduced a more inescapable, life-threatening situation than would have been obtained with a simpler predator-stress protocol. Thus, the increased intensity of the stress may have induced behavioral disinhibition rather than anxiety-like behaviors in these juvenile mice at a stress-sensitive age. These results suggest that shifting the balance of TRP metabolism to the KYN pathway was related to behavioral disinhibition elicited by acute, inescapable-predator stress.
Acknowledgements
We would like to thank Mr Ogiso, a technician in the Division of Experimental Animals at Nagoya University, who kindly advised us regarding the protocol for the animal experiments.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adamec R, Walling S, Burton P. Long-lasting, selective, anxiogenic effects of feline predator stress in mice. Physiol Behav. 2004a; 83:401–410.
- Adamec R, Bartoszyk GD, Burton P. Effects of systemic injections of vilazodone, a selective serotonin reuptake inhibitor and serotonin 1A receptor agonist, on anxiety induced by predator stress in rats. Eur J Pharmacol. 2004b; 504:65–77.
- Adamec R, Creamer K, Bartoszyk GD, Burton P. Prophylactic and therapeutic effects of acute systemic injections of EMD 281014, a selective serotonin 2A receptor antagonist on anxiety induced by predator stress in rats. Eur J Pharmacol. 2004c; 504:79–96.
- Adamec RE, Blundell J, Burton P. Relationship of the predatory attack experience to neural plasticity, pCREB expression and neuroendocrine response. Neurosci Biobehav Rev. 2006a; 30:356–375.
- Adamec R, Head D, Blundell J, Burton P, Berton O. Lasting anxiogenic effects of feline predator stress in mice: Sex differences in vulnerability to stress and predicting severity of anxiogenic response from the stress experience. Physiol Behav. 2006b; 88:12–29.
- Adamec R, Holmes A, Blundell J. 2008. Vulnerability to lasting anxiogenic effects of brief exposure to predator stimuli: Sex, serotonin and other factors-relevance to PTSD. Neurosci Biobehav Rev. 32:1287–1292.
- Armario A, Escorihuela RM, Nadal R. 2008. Long-term neuroendocrine and behavioural effects of a single exposure to stress in adult animals. Neurosci Biobehav Rev. 32:1121–1135.
- Avital A, Richter-Levin G. 2005. Exposure to juvenile stress exacerbates the behavioural consequences of exposure to stress in the adult rat. Int J Neuropsychopharmacol. 8:163–173.
- Avital A, Ram E, Maayan R, Weizman A, Richter-Levin G. 2006. Effects of early-life stress on behavior and neurosteroid levels in the rat hypothalamus and entorhinal cortex. Brain Res Bull. 68:419–424.
- Beekman M, Flachskamm C, Linthorst AC. 2005. Effects of exposure to a predator on behaviour and serotonergic neurotransmission in different brain regions of C57bl/6N mice. Eur J Neurosci. 21:2825–2836.
- Belzung C, El Hage W, Moindrot N, Griebel G. 2001. Behavioral and neurochemical changes following predatory stress in mice. Neuropharmacology. 41:400–408.
- Blanchard DC, Griebel G, Blanchard RJ. 2003. Conditioning and residual emotionality effects of predator stimuli: Some reflections on stress and emotion. Prog Neuropsychopharmacol Biol Psychiatry. 27:1177–1185.
- Boadle-Biber MC, Corley KC, Graves L, Phan TH, Rosecrans J. 1989. Increase in the activity of tryptophan hydroxylase from cortex and midbrain of male Fischer 344 rats in response to acute or repeated sound stress. Brain Res. 482:306–316.
- Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Verkerk R, Meltzer H, Maes M. 2002. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol. 22:86–90.
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry. 2000a; 48:1164–1174.
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000b; 22:219–229.
- Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. 2002. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 7:468–473.
- Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller AH. 2003. Interferon-alpha-induced changes in tryptophan metabolism. Relationship to depression and paroxetine treatment. Biol Psychiatry. 54:906–914.
- Chamas FM, Underwood MD, Arango V, Serova L, Kassir SA, Mann JJ, Sabban EL. 2004. Immobilization stress elevates tryptophan hydroxylase mRNA and protein in the rat raphe nuclei. Biol Psychiatry. 55:278–283.
- Cohen H, Zohar J, Matar MA, Zeev K, Loewenthal U, Richter-Levin G. 2004. Setting apart the affected: The use of behavioral criteria in animal models of post traumatic stress disorder. Neuropsychopharmacology. 29:1962–1970.
- Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U, Kozlovsky N, Kaplan Z. 2006. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol Psychiatry. 59:1208–1218.
- Cohen H, Kaplan Z, Matar MA, Loewenthal U, Zohar J, Richter-Levin G. 2007. Long-lasting behavioral effects of juvenile trauma in an animal model of PTSD associated with a failure of the autonomic nervous system to recover. Eur Neuropsychopharmacol. 17:464–477.
- Cohen H, Geva AB, Matar MA, Zohar J, Kaplan Z. 2008. Post-traumatic stress behavioural responses in inbred mouse strains: Can genetic predisposition explain phenotypic vulnerability?. Int J Neuropsychopharmacol. 11:331–349.
- Evans AK, Heerkens JL, Lowry CA. 2009. Acoustic stimulation in vivo and corticotropin-releasing factor in vitro increase tryptophan hydroxylase activity in the rat caudal dorsal raphe nucleus. Neurosci Lett. 455:36–41.
- Francis DD, Caldji C, Champagne F, Plotsky PM, Meaney MJ. 1999. The role of corticotropin-releasing factor–norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biol Psychiatry. 46:1153–1166.
- Gaspar P, Cases O, Maroteaux L. 2003. The developmental role of serotonin: News from mouse molecular genetics. Nat Rev Neurosci. 4:1002–1012.
- Hayley S, Borowski T, Merali Z, Anisman H. 2001. Central monoamine activity in genetically distinct strains of mice following a psychogenic stressor: Effects of predator exposure. Brain Res. 892:293–300.
- Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, Feldon J, Moran MC, Nelson P. 2000. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 100:749–768.
- Heim C, Nemeroff CB. 2001. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biol Psychiatry. 49:1023–1039.
- Hirata F, Hayaishi O, Tokuyama T, Senoh S. 1974. In vitro and in vivo formation of two new metabolites of melatonin. J Biol Chem. 249:1311–1313.
- Howells FM, Bindewald L, Russell VA. 2009. Cross-fostering does not alter the neurochemistry or behavior of spontaneously hypertensive rats. Behav Brain Funct. 5:24.
- Ichiyama A, Nakamura S, Nishizuka Y, Hayaishi O. 1970. Enzymic studies on the biosynthesis of serotonin in mammalian brain. J Biol Chem. 245:1699–1709.
- Imanaka A, Morinobu S, Toki S, Yamawaki S. 2006. Importance of early environment in the development of post-traumatic stress disorder-like behaviors. Behav Brain Res. 173:129–137.
- Kiank C, Zeden J-P, Drude S, Domanska G, Fusch G, Otten W, Schuett C. 2010. Psychological stress-induced, IDO1-dependent tryptophan catabolism: Implications on immunosuppression in mice and humans. PLos ONE. 5:e11825.
- Kita T, Morrison PF, Heyes MP, Markey SP. 2002. Effects of systemic and central nervous system localized inflammation on the contributions of metabolic precursors to the L-kynurenine and quinolinic acid pools in brain. J Neurochem. 82:258–268.
- Konsman JP, Parnet P, Dantzer R. 2002. Cytokine-induced sickness behaviour: Mechanisms and implications. Trends Neurosci. 25:154–159.
- Korte SM, De Boer SF. 2003. A robust animal model of state anxiety: Fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol. 463:163–175.
- Laich A, Neurauter G, Widner B, Fuchs D. 2002. More rapid method for simultaneous measurement of tryptophan and kynurenine by HPLC. Clin Chem. 48:579–581.
- Laugeray A, Launay J-M, Callebert J, Surget A, Belzung C, Barone PR. 2010. Peripheral and cerebral metabolic abnormalities of the tryptophan-kynurenine pathway in a murine model of major depression. Behav Brain Res. 210:84–91.
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. 2003. Risk-taking behavior in adolescent mice: Psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 27:19–31.
- Linthorst AC, Flachskamm C, Barden N, Holsboer F, Reul JM. 2000. Glucocorticoid receptor impairment alters CNS responses to a psychological stressor: An in vivo microdialysis study in transgenic mice. Eur J Neurosci. 12:283–291.
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. 1997. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic–pituitary–adrenal responses to stress. Science. 277:1659–1662.
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000a; 3:799–806.
- Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ. Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. J Neuroendocrinol. 2000b; 12:5–12.
- Maslova LN, Bulygina VV, Markel AL. Chronic stress during prepubertal development: Immediate and long-lasting effects on arterial blood pressure and anxiety-related behavior. Psychoneuroendocrinology. 2002a; 27:549–561.
- Maslova LN, Bulygina VV, Popova NK. Immediate and long-lasting effects of chronic stress in the prepubertal age on the startle reflex. Physiol Behav. 2002b; 75:217–225.
- McCormick CM, Smith C, Mathews IZ. 2008. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 187:228–238.
- Meyer JS, Piper BJ, Vancollie VE. 2008. Development and characterization of a novel animal model of intermittent MDMA (“Ecstasy”) exposure during adolescence. Ann NY Acad Sci. 1139:151–163.
- Miura H, Qiao H, Ohta T. Influence of aging and social isolation on changes in brain monoamine turnover and biosynthesis of rats elicited by novelty stress. Synapse. 2002a; 46:116–124.
- Miura H, Qiao H, Ohta T. Attenuating effects of the isolated rearing condition on increased brain serotonin and dopamine turnover elicited by novelty stress. Brain Res. 2002b; 926:10–17.
- Miura H, Qiao H, Kitagami T, Ohta T, Ozaki N. 2005. Effects of fluvoxamine on levels of dopamine, serotonin, and their metabolites in the hippocampus elicited by isolation housing and novelty stress in adult rats. Int J Neurosci. 115:367–378.
- Miura H, Ozaki N, Shirokawa T, Isobe K. Changes in brain tryptophan metabolism elicited by ageing, social environment, and psychological stress in mice. Stress. 2008a; 11:160–169.
- Miura H, Ozaki N, Sawada M, Isobe K, Ohta T, Nagatsu T. A link between stress and depression: Shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress. 2008b; 11:198–209.
- Miura H, Shirokawa T, Isobe K, Ozaki N. 2009. Shifting the balance of brain tryptophan metabolism elicited by isolation housing and systemic administration of lipopolysaccharide in mice. Stress. 12:206–214.
- Miura H, Shirokawa T, Ozaki N, Isobe K. 2010. Shifts in the balance of brain tryptophan metabolism due to age and systemic administration of lipopolysaccharide. Health. 2:225–233.
- O'Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. 2009. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 14:511–522.
- Olausson P, Engel JA, Soderpalm B. 1999. Behavioral sensitization to nicotine is associated with behavioral disinhibition; counteraction by citalopram. Psychopharmacology (Berl). 142:111–119.
- Rueter LE, Jacobs BL. 1996. A microdialysis examination of serotonin release in the rat forebrain induced by behavioral/environmental manipulations. Brain Res. 739:57–69.
- Shea A, Walsh C, Macmillan H, Steiner M. 2005. Child maltreatment and HPA axis dysregulation: Relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinology. 30:162–178.
- Sobrian SK, Marr L, Ressman K. 2003. Prenatal cocaine and/or nicotine exposure produces depression and anxiety in aging rats. Prog Neuropsychopharmacol Biol Psychiatry. 27:501–518.
- Stone TW, Darlington LG. 2002. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 1:609–620.
- Veenema AH. 2009. Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: What can we learn from animal models?. Front Neuroendocrinol. 30:497–518.
- Watt MJ, Burke AR, Renner KJ, Forster GL. 2009. Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behav Neurosci. 123:564–576.
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. 2004. Epigenetic programming by maternal behavior. Nat Neurosci. 7:847–854.
- Wichers MC, Maes M. 2004. The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-alpha-induced depression. J Psychiatry Neurosci. 29:11–17.
- Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S, Maes M. 2005. IDO and interferon-alpha-induced depressive symptoms: A shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry. 10:538–544.
- Widner B, Werner ER, Schennach H, Wachter H, Fuchs D. 1997. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem. 43:2424–2426.
- Widner B, Laich A, Sperner-Unterweger B, Ledochowski M, Fuchs D. 2002. Neopterin production, tryptophan degradation, and mental depression–what is the link?. Brain Behav Immun. 16:590–595.
- Widom CS. 1999. Posttraumatic stress disorder in abused and neglected children grown up. Am J Psychiatry. 156:1223–1229.
- Wright LD, Hebert KE, Perrot-Sinal TS. 2008. Periadolescent stress exposure exert long-term effects on adult stress responding and expression of prefrontal dopamine receptors in male and female rats. Psychoneuroendocrinology. 33:130–142.
- Young EA, Abelson JL, Curtis GC, Nesse RM. 1997. Childhood adversity and vulnerability to mood and anxiety disorders. Depress Anxiety. 5:66–72.