Abstract
Few studies have assessed the effects of stress on in vivo platelet activation. In the present study, hypobaric hypoxia induced by rapid decompression during high-altitude simulated flight in a hypobaric chamber was used to evaluate the effects of environmental stress on salivary cortisol and urinary thromboxane metabolite (TXM) excretion, a noninvasive marker of in vivo platelet function. Twenty-one male aviators (mean ± SD age = 36 ± 7 years) experiencing hypoxia by removing their oxygen mask for 4–5 min during a simulated flight to 25,000 ft (7,620 m; pO2 = 59.17 mmHg) and a matched control group of thirteen flying instructors wearing oxygen masks during the challenge, were studied. Hypobaric hypoxia induced a transient significant increase (P < 0.001) in the aviators' salivary cortisol concentration; the overall pattern of diurnal cortisol fluctuation was maintained in both groups. Urinary TXM showed a significant ∼30% reduction (P < 0.01) after the chamber session in aviators exposed to hypobaric hypoxia, but not in controls. A significant inverse correlation was found between salivary cortisol and urinary TXM in aviators (r = − 0.64, P = 0.0015). Salivary cortisol was a significant predictor (P < 0.001) for urinary TXM concentrations in aviators. In conclusion, here we observed that an acute stress-induced salivary cortisol increase was associated with reduced urinary thromboxane biosynthesis, providing the first indirect evidence for an inhibitory effect of acute stress on in vivo platelet function.
Introduction
Cortisol is known to regulate diverse body functions and is a well-known modulator of the inflammatory response and stress adaptation (Cohen et al. Citation2000; McEwen Citation2008). Release and/or new synthesis of pro-inflammatory substances from circulating platelets appear to play a crucial role at the interplay between blood coagulation and inflammation processes (Cirino et al. Citation2000; Weyrich et al. Citation2009). Partial and largely conflicting data are currently available on the relationship between glucocorticoids and in vivo platelet function, including studies using a platelet-selective regimen of aspirin, e.g. 100 mg/od (Kudielka et al. Citation2007; Schuerholz et al. Citation2007; Kristo et al. Citation2008; Pozzi et al. Citation2009; Fantidis Citation2010). Human platelets were shown to express specific and functional cytoplasmic glucocorticoid receptor (GR; Moraes et al. Citation2005). Platelet GR was shown to modulate in vitro platelet function, by lowering platelet responses to certain agonists, such as adenosine diphosphate (ADP) and thromboxane (TX) A2 receptor agonists, and to reduce platelet TXA2 generation during aggregation (Moraes et al. Citation2005). However, the relationship between stress-induced cortisol release and in vivo platelet function, which can be relevant for stress-associated cardiovascular diseases, including atherosclerosis and myocardial infarction (Matthews et al. Citation2004; Mischler et al. Citation2005; Brydon et al. Citation2006; Baranyai et al. Citation2008), remains unexplored. Indeed platelets are major players in arterial atherothrombosis (Davì and Patrono Citation2007).
11-Dehydro-TXB2 is a major urinary enzymatic metabolite reflecting TXA2 generation in the human body (Ciabattoni et al. Citation1987). TXA2 in humans is enzymatically synthesized by activated platelets from arachidonic acid (AA), through the sequential coordinated action of phospholipases, which release AA from membrane phospholipids, cyclooxygenase-1 which converts AA into prostaglandin (PG) H2, and TX synthase which converts PGH2 into TXA2. TXA2 is a short-lived pro-aggregatory and vasoconstrictive autacoid, which is rapidly and nonenzymatically hydrolyzed into the biologically inactive TXB2. This latter compound is then enzymatically converted by the liver to the end-product, 11-dehydro-TXB2, which is then excreted in urine. 11-Dehydro-TXB2 was shown to closely reflect the activation status of human platelets in vivo, as it increases in clinical conditions of high risk of cardiovascular disease, where platelets are activated by damaged blood vessels, inflammatory stimuli, and agonists such as ADP, thrombin, and collagen (Patrono et al. Citation2005). 11-Dehydro-TXB2 production is largely suppressed by low-dose aspirin, which is the recommended treatment for secondary and primary prevention of atherothrombosis (Patrono et al. Citation2005). By contrast with 11-dehydro-TXB2, urinary isoprostane 8-epi-PG-F2α (8-iso-PGF2α), reflects the nonenzymatic, oxidative metabolism of AA in vivo. It can be measured in humans and constitutes a reliable and noninvasive index of oxidative stress in the whole body (Morrow et al. Citation1990; Wang et al. Citation1995; Patrono and Fitzgerald Citation1997; Audoly et al. Citation2000).
Hypoxia was reported to variably affect platelet function, largely depending on the patho-physiological model used, e.g. prolonged exposure to high altitude or prolonged apnea (Lehmann et al. Citation2006; Minoguchi et al. Citation2007; Vij Citation2009). Rapid decompression challenge during simulated flight at high altitude in aviators represents a truly unique model of an acute environmentally stressful state (Heyes et al. Citation1982), as it mimics an emergency situation (Pinter Citation1974; Biselli et al. Citation1993; Spalding et al. Citation1998; Coste et al. Citation2005). In the present study, we considered this as a suitable model to explore the effects of an environment-induced stress on both cortisol secretion and in vivo platelet activation.
Based on previously published studies, showing in vitro modulation of human platelet function by glucocorticoids (Moraes et al. Citation2005), we tested the hypothesis that stress-induced changes in cortisol secretion would affect indices of in vivo platelet function and/or lipid peroxidation. Thus, we investigated the effect of acute hypobaric hypoxia induced by a sudden reduction of barometric pressure on salivary cortisol concentration, and on urinary 11-dehydro-TXB2 and 8-epi-PGF2α excretion in healthy military aviators exposed to an altitude-induced-hypoxia challenge in a hypobaric chamber.
Materials and methods
Study population
We studied 21 healthy male military aviators who were exposed to simulated altitude-exposure-induced hypoxia (AIH) in the hypobaric chamber (Hypobaric Chamber, AMST–Systemtechnik, Linz, Austria) at the Air Force Medical Center of the Pratica di Mare Airport (Rome, Italy) and 13 healthy male military flying instructors, wearing oxygen masks during training in the hypobaric chamber as controls (C).
Medical examination, main hematological and blood chemistry parameters, and ECG of all subjects were within the normal range and metabolic, cardiovascular, and endocrine diseases were considered as exclusion criteria. Laboratory tests were similar in the two groups; normal values of these parameters are necessary to access the hypobaric chamber session. None of the study participants had received any anti-inflammatory or immunosuppressive drug in the previous 6 months, nor vasoactive drugs which could influence cortisol secretion (e.g. anti-hypertensives, antidepressants, thyroid agents). During the 2 days before and on the day of the hypobaric chamber challenge, subjects lived in the Air Force Base under complete resting conditions, without physical activity or significant psychological external inputs. All participants woke up at 06:30 h and hypobaric chamber challenge was carried out between 10:30 h and 12:30 h, keeping to a strict daily schedule. Moreover, diet was similar and standardized for all the subjects to avoid gas-forming food and drinks. The participants were instructed how to collect saliva with a Salivette sampling device and urine samples during the study period.
The protocol of the study was approved by the Ethical Committee of the General Directorate for Italian Joint Military Health Services (Rome, July 2006). All participants were fully informed about the procedures and gave their written consent to participate in the study (carried out from March 2007 to January 2009). Subject characteristics are summarized in .
Table I. Characteristics of the study subjects.
Hypoxia in the hypobaric chamber
All the subjects (controls and AIH-exposed aviators) had at least a previous experience in the hypobaric chamber. Nevertheless, on the day of the study, subjects familiarized themselves with the hypobaric chamber and instructors informed participants about the characteristics of the simulated flight.
To assess basal morning salivary cortisol concentrations, a saliva sample was collected at 08:30 h from all study subjects: Cortisol awakening response defined as cortisol secretory activity in the first 30 min immediately post-awakening (Pruessner et al. Citation1997; Wüst et al. Citation2000; Rosmalen et al. Citation2005) did not affect the morning salivary cortisol concentration as this was measured 2 h after awakening. Moreover, saliva and urine samples were simultaneously collected at 10:30 h, just before entering the chamber. During the challenge, heart rate (HR) and percent hemoglobin oxygen saturation (obtained by pulse oxymetry, Fukuda, Mod. 5700, Japan) were recorded at the same time point as saliva sample collection.
In a typical session, the simulated flight starts ascending to 5,000 ft (1,524 m, pO2 = 132.5 mmHg) above ground level (sea level pO2 = 159.21 mmHg), when a check of vital signs (HR and percent O2 saturation) and flight instrumentation is performed (5–10 min). Then, a rapid ascent to 25,000 ft (7,620 m, pO2 = 59.17 mmHg) is simulated, reproducing a sudden loss of cabin pressurization. At this time point, AIH subjects remove the oxygen mask and begin a series of standardized written logical and mathematical tests as well as manual trials. The aim of these tests is to investigate the rapid worsening of physical and psychological performance, as percent hemoglobin saturation decreases from the normal values (98–99%) to 40–50%. When the trainees reached a saturation value of ∼50% (usually after 4–5 min of hypoxia), the test was stopped to avoid loss of consciousness, and an additional saliva sample was collected. At this point, the trainees put on the oxygen mask again and descended to ground level. An additional saliva sample was collected at 12:30 h after exiting the hypobaric chamber, and then every 120 min until 20:30 h. Owing to the limitations of feasibility associated with the environment of the hypobaric chamber, urinary samples could not be collected in the chamber and, therefore, were collected, simultaneously with salivary samples, at two time points only: Before (10:30 h) and after (12:30 h) the hypobaric chamber challenge. However, owing to the relatively fast metabolism of TXA2 into its urinary metabolites (Patrono et al. Citation1986), samples collected at 12:30 h are representative of the analytes produced during the hypobaric hypoxia challenge.
Salivary sampling procedure and cortisol measurements
Samples of saliva were collected with Salivette (Sarstedt, Italy) sampling devices, which allow a quick and hygienic saliva collection with a polyester swab, followed by recovery of saliva by centrifugation of the swab at 600g for 15 min (Gröschl et al. Citation2008).
Cortisol was assayed by duplicate measurements on 25 μl of saliva with a commercial immunoenzymatic kit (Diametra, Italy). Inter-assay coefficient of variation was < 10%, and intra-assay coefficient of variation < 7%, with a minimum detectable concentration of 0.5 ng/ml.
Urinary AA metabolites
Urinary 11-dehydro-TXB2 and 8-epi-PGF2α were measured by previously validated radio-immunoassays (Ciabattoni et al. Citation1987; Wang et al.Citation1995). Briefly, 50 ml urine samples were collected and immediately frozen at − 20°C until extraction. Ten milliliter aliquots of each urine sample were adjusted to pH 4.0 with formic acid, then applied to Sep-Pak C18 cartridges (Waters Associates) and eluted with ethyl acetate. The eluates were subjected to silicic acid column chromatography and further eluted with benzene/ethyl acetate/methanol (60:40:30, vol/vol). Immuno-reactive 11-dehydro-TXB2 was assayed by radioimmunoassay in the eluates. The urinary excretion of 11-dehydro-TXB2 was expressed as pg/mg of creatinine. Urinary creatinine was determined using commercially available kit (Biosystems, Spain; Biomedis, Italy). 8-Epi-PGF2α was measured in the same eluates by a previously described radio-immunoassay (Wang et al. Citation1995). Duplicate measurements of urinary 11-dehydro-TXB2 and 8-epi-PGF2α were made by researchers blinded to subject characteristics. Inter-assay coefficients of variation were < 5%, and intra-assays coefficient of variation < 3%, with a minimum detectable concentration of 2 pg/ml.
Statistical analyses
We estimated that at least 12 subjects (10+2, expecting a 20% drop out) would be required to detect a mean absolute difference in urinary 11-dehydro-TXB2 of 60 ± 30 pg/mg creatinine between values measured at entrance and at the exit of the hypobaric chamber, corresponding to a 20% variation in normal values of 300 ± 200 pg/mg creatinine (Santilli et al. Citation2009), with a two-tailed alpha of 0.01 and 90% power. However, we recruited 21 subjects. We also recruited as controls a group of 13 military flying instructors, wearing oxygen masks and thus not experiencing hypobaric hypoxia, during challenge in the hypobaric chamber. Sigmaplot 11 software (SxST.it, Italy) was used for statistical analysis. We applied analysis of variance (ANOVA) for repeated measures followed by HDS Tukey multiple comparison test to evaluate diurnal cortisol fluctuation and urinary TX metabolite (TXM) measured before and after the hypobaric chamber session. Pearson's correlation test was used to show correlations between variables. Multiple linear regression was used to predict independent factors. Statistical significance was set at P < 0.05 (Winer Citation1971).
Results
Characteristics of the study population
The characteristics of the two groups are reported in . There were no statistically significant differences between the two groups, including basal morning cortisol concentrations measured in saliva samples collected at 08:30 h, 2 h after waking up.
In the AIH group O2 saturation decreased from 99 ± 0.10 at baseline to 58 ± 1.72% (mean + SEM) during hypoxia (P < 0.001) and returned to 99 ± 0.32% at the end of the simulated flight. HR (mean+SEM) was 77 ± 4 bpm at baseline, 120 ± 4 bpm during hypoxia (P < 0.01 vs. baseline) and 76 ± 2 bpm at the end of the session. No differences were detected in the control group during the hypobaric chamber challenge (data not shown).
Salivary cortisol concentration
As shown in , saliva was collected every 2 h over a 12-h period (08:30–20:30 h) on the day of the hypobaric chamber session. An additional saliva sample was collected during the hypoxia experienced at 25,000 ft (see Materials and methods, for details). In samples collected during the hypobaric hypoxia at 25,000 ft (7,620 m), the salivary cortisol concentrations of the AIH group showed a statistically significant 2.5-fold increase as compared to pre-chamber values (ANOVA for repeated measures: F = 11.182; P < 0.0001; post hoc HSD Tukey multiple comparison test: P < 0.001 vs. 10:30 h) and were statistically higher than those measured in the control group (P < 0.05). However, salivary cortisol concentration in AIH subjects decreased at chamber exit (12:30 h), being not significantly different from both pre-chamber values and values of the control group at the same time point. In the control group, there was a nonsignificant trend toward an increase of salivary cortisol in the samples collected inside the chamber.
Figure 1. The effect of hypobaric chamber session on salivary cortisol concentration (mean ± SEM). C, control; AIH, altitude-induced hypoxia groups. ANOVA for repeated measures: *P < 0.05 vs. C; §P < 0.001 vs. 10:30 h; †P < 0.05 vs. respective 08:30 h values; n, number of subjects.
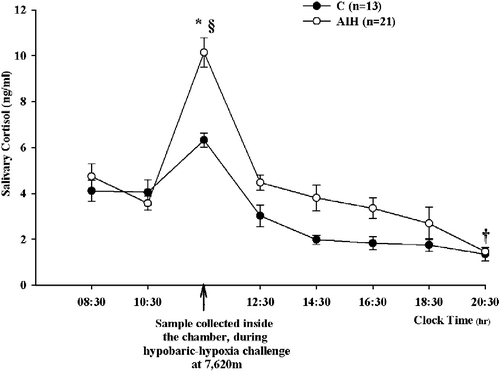
As expected (), the diurnal variation of salivary cortisol concentrations over the 12-h interval (08:00–20:00 h) was maintained in the control group (4.11 ± 0.56 at 08:30 h, vs. 1.35 ± 0.21 ng/ml at 20:30 h, ANOVA for repeated measures: F = 11.182; post hoc HSD Tukey multiple comparison test: P < 0.05). Interestingly, in spite of the hypoxia-induced stress, in the AIH group the physiological diurnal fluctuation of cortisol was also observed: 4.74 ± 0.65 at 08:30 h vs. 1.34 ± 0.27 ng/ml at 20:30 h (F = 11.182, P < 0.05); no statistically significant differences were detected between the two groups in salivary cortisol values at any time point between 12:30 and 20:30 h.
Urinary 11-dehydro-TXB2 and 8-epi-PGF2α
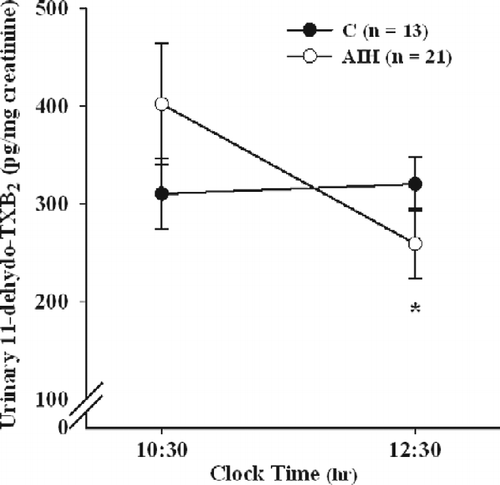
The values of urinary 11-dehydro-TXB2 concentration, an in vivo index of platelet activation, were measured after the chamber session in comparison with those before (). In the AIH group, the exposure to hypobaric hypoxia induced a statistically significant decrease in urinary 11-dehydro-TXB2 excretion rate (ANOVA for repeated measures: F = 6.084; P < 0.05; post hoc HSD Tukey multiple comparison test: P < 0.01). By contrast, no significant changes in urinary 11-dehydro-TXB2 excretion were detected in the control group between the same time points (post hoc HSD Tukey multiple comparison test: ns). Moreover, the statistical analysis did not shown any significant difference between baseline TXB2 excretion rate in the two groups before exposure to the hypobaric hypoxia challenge.
Figure 2. Mean ( ± SEM) urinary 11-dehydro-TXB2 levels in control (C) and AIH groups measured before (10:30 h) and after (12:30 h) hypobaric chamber challenge. ANOVA for repeated measures: *P < 0.01 vs. pre-chamber values; n, number of subjects.
By contrast with 11-dehydro-TXB2, the hypobaric hypoxia did not modify the urinary concentrations of 8-epi-PGF2α to any statistically significant extent in either the AIH group (465 ± 62 vs. 511 ± 60 pg/mg of creatinine, before and after the session, respectively) or in the control group (540 ± 76 vs. 550 ± 79 pg/mg creatinine, before and after the session, respectively).
Correlations between salivary cortisol, urinary TX, and isoprostane metabolites
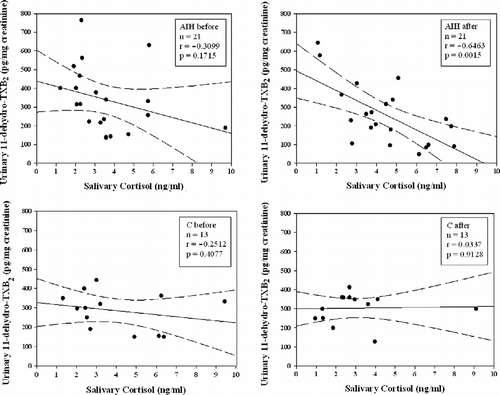
The relationship between salivary cortisol and urinary 11-dehydro-TXB2 concentrations before and after the hypobaric chamber session was tested by Pearson's correlation analysis. No significant correlation was found before challenge in the hypobaric chamber (, upper left panel). A statistically significant inverse correlation (n = 21; r = − 0.64; P = 0.0015) was found between cortisol and 11-dehydro-TXB2 concentrations in the AIH group after challenge in the hypobaric chamber (, upper right panel), indicating a relationship between stress-induced cortisol variations and TXA2 biosynthesis in vivo. No correlation was observed, before or after the challenge, in the control group (, lower left and right panels).
Figure 3. Scatterplots showing the relationship between urinary 11-dehydro-TXB2 and salivary cortisol concentrations simultaneously measured, before and after hypobaric chamber challenge, in AIH (upper graphs) and control (C; lower graphs) groups. Continuous line represents best-fit linear regression; dashed lines represent 95% confidence bands; n, number of subjects.
No statistically significant correlation was found between salivary cortisol and urinary 8-epi-PGF2α concentrations in either group, before or after the challenge.
However, a significant linear relationship between urinary 8-epi-PGF2α and 11-dehydro-TXB2 concentrations was found in the control group before (n = 13; r = 0.65; P = 0.015) and after the challenge (n = 13; r = 0.55; P = 0.048). On the contrary, no such correlation was found in the AIH group.
To determine which variable under study was able to predict urinary 11-dehydro-TXB2 excretion, a multiple linear regression analysis was carried out using urinary 11-dehydro-TXB2 concentration as the dependent variable and salivary cortisol and urinary 8-epi-PGF2α concentrations as independent variables. As reported in , in the control group, urinary 8-epi-PGF2α concentration was the sole predictor of 11-dehydro-TXB2 excretion. By contrast with the control group, in the AIH group, salivary cortisol concentration was the only predictor of urinary 11-dehydro-TXB2 excretion.
Table II. Multiple linear regression analysis for urinary 11-dehydro-TXB2 dependent variable in control group (C) and AIH.
Discussion
Previous studies from others as well as from our group showed that collection of saliva provides a noninvasive, stress-free, reliable source for monitoring changes of adrenal cortex function especially in difficult environments such as the hypobaric chamber (Hubert and De Long-Meyer Citation1989; Kirschbaum and Hellhammer Citation1989; Kirschbaum et al. Citation1995; Giubilei et al. Citation2001; Patacchioli and Martelletti Citation2006; Patacchioli et al. Citation2006). Hypoxia familiarization is an integral part of the aviator training: The experience of a rapid decompression teaches physiological self-awareness which is crucial for safety, because hypoxia has an insidious onset and dangerous consequences (Pinter Citation1974; Sive and Hatting Citation1991).
In the present study, we have shown that hypobaric hypoxia is a powerful activator of the hypothalamic–pituitary–adrenal axis, in agreement with previous observations of Coste and coworkers (Coste et al. Citation2005) who showed a large increase in cortisol after exposure to hypoxia during a simulated flight at 10,000 ft (3,048 m).
Besides the stressful, short-lasting, experience of the hypobaric hypoxia, our results showed that the overall pattern of cortisol diurnal fluctuation, characterized by higher levels in the morning and a progressive decrease thereafter, was maintained as cortisol values returned to levels comparable to the control group within 2 h after the hypobaric hypoxia experience. These data differ from a previous study (Coste et al. Citation2005) showing that an 8-h diurnal exposure to hypoxia disrupted the circadian rhythm of cortisol secretion. This discrepancy might be due to the different intensity and duration of the hypoxic experiences. Moreover, our data are in agreement with the findings of Hackney et al. (Hackney Citation2006) who observed that the hormonal effects of an acute and short-lasting stress exposure in healthy subjects are typically transient and recover within a few hours.
We report for the first time that the cortisol variation generated by the exposure to hybobaric hypoxia was associated with a significant decrease in urinary 11-dehydro-TXB2 excretion rate. These data are consistent with the in vitro studies of Moraes et al. (Moraes et al. Citation2005) reporting a decrease in TX generation and in the aggregation response to ADP and the TX receptor agonist U46619 in human platelets incubated with increasing concentrations of glucocorticoids. Our data, providing the first evidence for an inhibitory effect of acute cortisol increase on in vivo TX-dependent platelet activation, might represent an example of an adaptive adjustment in the physiology of the human stress response (McEwen Citation2005, Citation2008), with possible cardiovascular protective significance, in that platelet excitability is lower, as shown by reduced urinary TXA2 metabolite excretion.
As a biomarker of in vivo stress-induced lipid peroxidation, we measured the levels of urinary 8-epi-PGF2α that reflect primarily the nonenzymatic transformation of AA catalyzed by oxygen radicals. This parameter was not modified by the hypobaric hypoxia, indicating that the in vivo lipid peroxidation was not affected by the acute stress-induced cortisol release, nor by oxygen fluctuations. Furthermore, we observed a different relationship between the two urinary metabolites of AA in the two groups under study. Indeed there was a positive significant correlation between 8-epi-PGF2α and 11-dehydro-TXB2 in the control group while this correlation was lost in the AIH group. A positive correlation between these two metabolites was repeatedly reported in many different pathological conditions and in healthy subjects (Davì et al. Citation2001; Ciabattoni et al. Citation2007). The loss of this relationship in the AIH group further supports a selective effect of the stress-induced cortisol increase on the in vivo enzymatic pathway of AA metabolism, leaving the nonenzymatic oxidative path unaffected. Consistently, multivariate analysis indicated salivary cortisol concentration as an independent, predictor of 11-dehydro-TXB2 excretion. From a physiological point of view, this might be considered as an adaptive homeostatic response under stressful conditions driven by cortisol, possibly aimed at balancing the effects of other stress-induced hemostasis mediators (adrenergic modulators such as epinephrine, inflammatory cytokines such as CD40L, or tumor necrosis factor). Future studies might investigate the platelet pathway(s) involved in the cortisol response.
A limitation of our study is the lack of biochemical data reflecting the activation status of the adrenergic system. However, the reduction of in vivo platelet function indirectly detected in the AIH group would argue against an adrenergic increase in our experimental setting. Moreover, the adrenergic system is a known inducer of platelet activation through interaction with platelet adrenergic receptors (Spalding et al. Citation1998). Another limitation of our study is the lack of data on TXA2 generation from platelets in the peripheral blood. However, invasive procedures such as blood sampling were not feasible in our experimental setting.
The association between an acute increase of cortisol secretion and lowered in vivo TX biosynthesis, while deserving further mechanistic studies, might be a novel physiological and pharmacological path aimed at protecting the cardiovascular system under acute stress conditions.
Acknowledgements
This work was partially supported by a grant from MIUR to FRP and to BR. The Authors are grateful to professor Carlo Patrono for his critical reading of the manuscript.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Audoly LP, Rocca B, Fabre JE, Koller BH, Thomas D, Loeb AL, Coffman TM, FitzGerald GA. 2000. Cardiovascular responses to the isoprostanes iPF (2alpha)-III and iPE (2)-III are mediated via the thromboxane A(2) receptor in vivo. Circulation. 101:2833–2840.
- Baranyai R, Nonell A, Deuschle M, Lederbogen F. 2008. How depression may increase cardiac risk: Effect of hypercortisolism on platelet activation markers. Neuropsychobiology. 57:146–150.
- Biselli R, Farrace S, D'Amelio R, Fattorossi A. 1993. Influence of stress on lymphocyte subset distribution—a flow cytometric study in young student pilots. Aviat Space Environ Med. 64:116–120.
- Brydon L, Magid K, Steptoe A. 2006. Platelets, coronary heart disease, and stress. Brain Behav Immun. 20:113–119.
- Ciabattoni G, Maclouf J, Catella F, FitzGerald GA, Patrono C. 1987. Radioimmunoassay of 11-dehydrothromboxane B2 in human plasma and urine. Biochim Biophys Acta. 918:293–297.
- Ciabattoni G, Porreca E, Di Febbo C, Di Iorio A, Paganelli R, Bucciarelli T, Pescara L, Del Re L, Giusti C, Falco A, Sau A, Patrono C, Davì G. 2007. Determinants of platelet activation in Alzheimer's disease. Neurobiol Aging. 28:336–342.
- Cirino G, Napoli C, Bucci M, Cicala C. 2000. Inflammation-coagulation network: Are serine protease receptors the knot?. Trends Pharmacol Sci. 21:170–172.
- Cohen S, Hamrick N, Rodriguez MS, Feldman PJ, Rabin BS, Manuk SB. 2000. The stability of and intercorrelations among cardiovascular, immune, endocrine, and psychological reactivity. Ann Behav Med. 22:171–179.
- Coste O, Beers PV, Bogdan A, Charbuy H, Touitou Y. 2005. Hypoxic alterations of cortisol circadian rhythm in man after simulation of a long duration flight. Steroids. 70:803–810.
- Davì G, Patrono C. 2007. Mechanisms of disease: Platelet activation and atherothrombosis. N Engl J Med. 357:2482–2494.
- Davì G, Di Minno G, Coppola A, Andria G, Cerbone AM, Madonna P, Tufano A, Falco A, Marchesani P, Ciabattoni G, Patrono C. 2001. Oxidative stress and platelet activation in homozygous homocystinuria. Circulation. 104:1124–1128.
- Fantidis P. 2010. The role of the stress-related anti-inflammatory hormones ACTH and cortisol in atherosclerosis. Curr Vasc Pharmacol. 8:517–525.
- Giubilei F, Patacchioli FR, Antonini G, Sepe Monti M, Tisei P, Bastianello S, Monnazzi P, Angelucci L. 2001. Altered circadian cortisol secretion in Alzheimer's disease. J Neurosci Res. 66:262–265.
- Gröschl M, Khöler H, Topf HG, Rupprecht T, Rauh M. 2008. Evaluation of saliva collection devices for the analysis of steroids, peptides and therapeutic drugs. J Pharm Biomed Anal. 47:478–486.
- Hackney AC. 2006. Stress and the neuroendocrine system: The role of exercise as a stressor and modifier of stress. Endocrinol Metab. 1:783–792.
- Heyes MP, Farber MO, Manfredi F, Robertshaw D, Weinberger M, Fineberg N, Robertson G. 1982. Acute effects of hypoxia on renal and endocrine function in normal humans. Am J Physiol. 243:265–270.
- Hubert W, De Long-Meyer R. 1989. Emotional stress and salivary cortisol response. J Clin Chem Clin Biochem. 27:235–237.
- Kirschbaum C, Hellhammer D. 1989. Response variability of salivary cortisol under psychological conditions. J Clin Chem Clin Biochem. 27:237.
- Kirschbaum C, Pruessner JC, Stone AA, Federenko I, Gaab J, Lintz D, Schommer N, Hellhammer DH. 1995. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom Med. 57:468–474.
- Kristo C, Ueland T, Godank K, Aukrust P, Bollerslev J. 2008. Biochemical markers for cardiovascular risk following treatment in endogenous Cushing's syndrome. J Endocrinol Invest. 31:400–405.
- Kudielka BM, Fisher JE, Metzenthin P, Helfricht S, Preckel D, von Kanel R. 2007. No effect of 5-day treatment with acetylsalicylic acid (aspirin) or the beta-blocker propranolol (Inderal) on free cortisol responses to acute psychosocial stress: A randomized double-blind, placebo-controlled study. Neuropsychobiology. 56:159–166.
- Lehmann T, Mairbäurl H, Pleisch B, Maggiorini M, Bärtsch P, Reinhart WH. 2006. Platelet count and function at high altitude and in high-altitude pulmonary edema. J Appl Physiol. 100:690–694.
- Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, Markovitz JH. 2004. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 110:74–78.
- McEwen BS. 2005. Stressed or stressed out: What is the difference?. J Psychiatr Neurosci. 30:315–318.
- McEwen BS. 2008. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 583:174–185.
- Minoguchi K, Yokoe T, Tazaki T, Minoguchi H, Oda N, Tanaka A, Yamamoto M, Ohta S, O'Donnell CP, Adachi M. 2007. Silent brain infarction and platelet activation in obstructive sleep apnea. Am J Respir Crit Care Med. 175:612–617.
- Mischler K, Fischer JE, Zgraggen L, Kudielka BM, Preckel D, Von Kanel R. 2005. The effect of repeated acute mental stress on habituation and recovery responses in hemoconcentration and blood cells in healthy men. Life Sci. 77:1166–1179.
- Moraes LA, Paul-Clark MJ, Rickman A, Flower RJ, Goulding NJ, Perretti M. 2005. Ligand-specific glucocorticoid receptors activation in human platelets. Blood. 106:4167–4175.
- Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJII. 1990. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxynenase, free radical-catalyzed mechanism. Proc Natl Acad Sci USA. 87:9383–9387.
- Patacchioli FR, Martelletti P. 2006. Neuroendocrine derangement in chronic migraine: Fact or fiction?. Headache. 46:1309–1310.
- Patacchioli FR, Monnazzi P, Simeoni S, De Filippis S, Salvatori E, Coloprisco G, Martelletti P. 2006. Salivary cortisol, DHEA-S and testosterone in chronic migraine. J Headache Pain. 7:90–94.
- Patrono C, Fitzgerald GA. 1997. Isoprostanes: Potential markers of oxidant stress in atherothrombotic disease. Arterioscler Thromb Vasc Biol. 17:2309–2315.
- Patrono C, Ciabattoni G, Pugliese F, Pierucci A, Blair IA, FitzGerald GA. 1986. Estimated rate of thromboxane secretion into the circulation of normal humans. J Clin Invest. 77:590–594.
- Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C. 2005. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 353:2373–2383.
- Pinter EJ. 1974. Metabolic and endocrine changes in aerobatic flight. Aerosp Med. 45:1159–1163.
- Pozzi AO, Bernardo E, Coronado MT, Punchard MA, Gonzalez P, Fantidis P. 2009. Acute arterial thrombosis in the absence of inflammation: The stress-related anti-inflammatory hormone ACTH participates in platelet-mediated thrombosis. Atherosclerosis. 204:79–84.
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. 1997. Free cortisol levels after awakening: A reliable biological marker for the assessment of adrenocortical activity. Life Sci. 61:2539–2549.
- Rosmalen JGM, Oldehinkle AJ, Ormel J, de Winter AF, Buitelaar JK, Verhulst FC. 2005. Determinants of salivary cortisol levels in 10–12 year old children, a population-based study of individual differences. Psychoneuroendocrinology. 30:483–495.
- Santilli F, Rocca B, De Cristofaro R. 2009. Platelet cyclooxygenase inhibition by low-dose aspirin is not reflected consistently by platelet function assays: Implications for aspirin “resistance”. J Am Coll Cardiol. 53:667–677.
- Schuerholz T, Keil O, Wagner T, Klinzing S, Sumpelmann R, Oberle V, Marx G. 2007. Hydrocortisone does not affect major platelet receptors in inflammation in vitro. Steroids. 72:609–613.
- Sive WJ, Hattingh J. 1991. The measurement of psychophysiological reactions of pilots to a stressor in a flight simulator. Aviat Space Environ Med. 62:831–836.
- Spalding A, Vaitkevicius H, Dill S, MacKenzie S, Schmaier A, Lockette W. 1998. Mechanism of epinephrine-induced platelet aggregation. Hypertension. 31:603–607.
- Vij AG. 2009. Effect of prolonged stay on platelet aggregation and fibrinogen levels. Platelets. 20:421–427.
- Wang Z, Ciabattoni G, Créminon C, Lawson JA, FitzGerald GA, Patrono C, Maclouf J. 1995. Immunological characterization of urinary 8-epi-PGF2α excretion in man. J Pharmacol Exp Ther. 275:94–100.
- Weyrich AS, Schwertz H, Kraiss LW, Zimmerman GA. 2009. Protein synthesis by platelets: Historical and new perspectives. J Thromb Haemost. 7:241–246.
- Winer BJ. 1971. Statistical principles in experimental design. Tokio: McGraw-Hill.
- Wüst S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. 2000. The cortisol awakening response-normal values and confounds. Noise Health. 2:79–88.