Abstract
Experimental and clinical evidence shows that neutrophils play an important role in the mechanism of tissue injury in immune complex diseases through the generation of reactive oxygen species. In this study, we examined the influence of academic psychological stress in post-graduate students on the capacity of their blood neutrophils to release superoxide when stimulated by immune complexes bound to nonphagocytosable surfaces and investigated the modulatory effect of cortisol on this immune function. The tests were performed on the day before the final examination. The state-trait anxiety inventory questionnaire was used to examine whether this stressful event caused emotional distress. In our study, the psychological stress not only increased plasma cortisol concentration, but it also provoked a reduction in superoxide release by neutrophils. This decrease in superoxide release was accompanied by diminished mRNA expression for subunit p47phox of the phagocyte superoxide-generating nicotinamide adenine dinucleotide phosphate-oxidase. These inhibitory effects were also observed by in vitro exposure of neutrophils from control volunteers to 10− 7 M hydrocortisone, and could be prevented by the glucocorticoid receptor antagonist RU-486. These results show that in a situation of psychological stress, the increased levels of cortisol could inhibit superoxide release by neutrophils stimulated by IgG immune complexes bound to nonphagocytosable surfaces, which could attenuate the inflammatory state.
Introduction
Stress can be defined as a state of threatened homeostasis provoked by a psychological, environmental, or physiologic stimulus (Black Citation2002). Stressors can alter the immune response through the interaction between the nervous, endocrine, and immune systems. The complex stress response develops through the stimulation of the hypothalamus–pituitary–adrenal (HPA) axis and the peripheral autonomic nervous system (Glaser and Kiecolt-Glaser Citation2005). Signals in the hypothalamus induce the release of corticotropin-releasing hormone (CRH). CRH operates in the anterior pituitary by stimulating the secretion of adrenocorticotrophic hormone (ACTH). ACTH then stimulates the adrenal gland to secrete glucocorticoids, which act on target cells. The endogenous glucocorticoids, corticosterone in rodents, and cortisol in human, are among the most studied substances involved in the stress response.
In humans, there is a large body of literature that consistently shows the effects of psychological stress on immune function in general; it can be associated with changes in the leukocyte and lymphocyte subset distribution (Maes et al. Citation1999; Lucas et al. Citation2006), redistribution of T cells (Atanackovic et al. Citation2006), reduction in ex vivo lymphocyte proliferation (Knapp et al. Citation1992), and changes in the number and function of natural killer cells (Sieber et al. Citation1992). However, possible effects of psychological stress on the function of another arm of the innate immune system, namely phagocytes, were relatively less investigated.
Phagocytes produce an extraordinary variety of highly reactive oxidizing agents known as reactive oxygen species (ROS). Generation of ROS is mediated by nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase), a membrane-associated multi-component protein enzyme complex, which is inactive in unstimulated phagocytes and is only assembled upon cell stimulation (Robinson Citation2009). At least six protein components are required for NADPH oxidase function, including the membrane-bound heterodimeric flavocytochrome b558 (gp91phox and p22phox) and four cytosolic proteins (p47phox, p67phox, p40phox, and G protein Rac2). These components must assemble in the membrane for the enzyme complex to become active. Examples of ROS are the superoxide anion, hydrogen peroxide, oxidized halogens, hydroxyl radical, and singlet oxygen. Of these, the superoxide anion (O2− ) is the first to be produced by one electron reduction of molecular oxygen, and it serves as the precursor for the formation of other ROS (Babior Citation1999). These oxygen metabolites are highly reactive and act as oxidizing agents, playing an essential role in the function of phagocytes in host defense against invading microorganisms (Segal Citation2005). However, several reports show that an overproduction of ROS by phagocytes might lead to a form of “oxidative stress” and damage tissues (Kobayashi et al. Citation2003). Therefore, well-controlled production of ROS is critical for homeostasis and an imbalance in ROS production was associated with a great number of diseases such as atherosclerosis, rheumatoid arthritis, and lupus erythematosus (Halliwell and Gutteridge Citation1989).
Neutrophils play an important role in the mechanism of tissue injury in immune complex diseases through the generation of ROS and lysosomal enzyme release (Crockett-Torabi and Ward Citation1996). They accumulate at sites of antibody–antigen deposition and react with these complexes, releasing mediators of tissue damage. Because of their prominent role in contributing to the pathogenesis of immune complex diseases, there is strong interest in the study of liberation of ROS by neutrophils when they interact with immune complexes bound to tissue structures. It is known that depending on the speed of the stress response or the persistence of the stressful condition, there might be an enhancement of the vulnerability to diseases (Kloet Citation2004). The hypothesis we tested in this investigation was that academic psychological stress would have possible beneficial or harmful effects on immune complex diseases with regard to effects on ROS release by blood neutrophils. We used an in vitro model to study this mechanism.
Materials and methods
Test subjects
Subjects included in this study were post-graduate students of the Ribeirão Preto Medical School, University of São Paulo (71 females, 22 males; mean age ± SD: 27 ± 5 years; range age: 22–32 years). The number of volunteers examined varied between experiments and is presented together with each result. Volunteers were grouped into two sets: The “stress” group, post-graduate students assessed on the day before the final examination of their thesis; and the “control” group, post-graduate students that were not being submitted to the final examination. For execution of experimental assays, samples from each volunteer in the stress group were assayed together with those from a volunteer from the control group of the same sex and age (age difference ± 2years). All test subjects were in excellent health and were not on medication, with the exception of oral contraceptives. None of the female participants were pregnant or lactating. Volunteers who reported endocrine and immune disorders, or in the 2 weeks preceding the study suffered from an infection or an allergic or inflammatory reaction were excluded from the study. Test subjects were asked to refrain from drinking coffee, black tea, or alcoholic drink after 21:00 h in the night before the blood collection. All experimental procedures started at 09:00 h. ( ± 30 min). Venous blood (20–30 ml) was taken from an antecubital vein from each subject and drawn into heparin-coated vacutainers. All experiments were conducted in accordance with the ethical standards of the committee on human experimentation (Ethics Committee in Research of the Ribeirão Preto Medical School, University Hospital, University of São Paulo/Process n° 9711/2006 and CONEP—Brazilian Commission on Ethics in Research/CAAE—0218.0.004.000-06). All procedures were carried out with written informed consent of each subject.
Evaluation of psychological states
To examine whether the final thesis examination is a stressful event that causes emotional distress in post-graduate students, we used the questionnaire of the Brazilian version of Spielberger's state-trait anxiety inventory (STAI; Biaggio et al. Citation1977). The subscale STAI-S is used to measure the state of anxiety, which is a well-known emotional reaction to stress, while STAI-T is used to assess the predisposition to have anxiety. The subjects filled out these questionnaires before blood collection.
Preparation of nonphagocytosable surfaces
Nonphagocytosable surfaces were prepared according to Henson (Citation1971) with some modifications: Micropore filters (Millipore Filter Corp., Bedford, MA, USA) composed of mixed esters of cellulose, with a 13 mm diameter and a 3.0 μm pore size, were incubated with 200 μl of the solution of bovine serum albumin (BSA; 10 mg/ml in phosphate buffered saline, PBS; Sigma Chemical Co., St Louis, MO, USA) for 60 min at room temperature. All filters were washed three times by gentle swirling in 2 ml of PBS; after this, the filters were either used as such or incubated for an additional 60 min at 37°C with a dilution of the IgG anti-BSA antibody (300 μg/ml). They were again washed with PBS before use. All filters were placed in 24-well tissue culture plates and stored at 4°C for up to 7 days until use in the superoxide release assay. A polystyrene surface, the well of a tissue culture plate, was also used directly as a nonphagocytosable stimulus or pretreated for 60 min with 200 μl of the solution of BSA and washed with PBS before use.
Preparation of human neutrophils
Human neutrophils were separated from the peripheral blood of the volunteers. The isolation was performed as described by Henson (Citation1971). Briefly, the blood was centrifuged at 500g for 20 min at room temperature. The platelet-rich plasma and the “buffy coat” layer which contains mainly mononuclear cells were removed. Forty to fifty milliliters of 2.5% gelatin (Difco, Detroit, MI, USA) in PBS (pH 7.2) were added to the erythrocytes and neutrophils. The erythrocytes were allowed to sediment at 37°C for 30 min and the supernatant containing the neutrophils was taken. The cells in this supernatant were centrifuged at 400g for 10 min at room temperature. The pellet was resuspended in 0.83% ammonium chloride (pH 7.2) to lyse the remaining erythrocytes. After 5 min the cells were then cooled to 4°C and washed (400 g for 10 min) with PBS. The cells were suspended in Hanks 15 mM HEPES medium (Sigma) containing 0.1% gelatin, pH 7.2, or RPMI-1640 medium (Sigma), pH 7.2, containing sodium pyruvate (0.11 g/l; Sigma), penicillin (0.1 g/l; Sigma), streptomycin sulfate (0.1 g/l; Sigma), sodium bicarbonate (2.2 g/l; Merck, Darmstadt, Germany), and 10% heat-inactivated fetal bovine serum (Life Technologies, New York, NY, USA). About 85% of the cells were neutrophils. The percentages of neutrophils were determined by microscope identification and confirmed by flow cytometry. Viability testing with trypan blue exclusion indicated that not more than 3% nonviable cells were present in the suspensions.
Release of superoxide by neutrophils
Extracellular superoxide release by neutrophils was measured using the superoxide dismutase (SOD)-inhibitable reduction of ferricytochrome c assay, as previously described by Johnston and Lehmeyer (Citation1976). The assays were performed in 24-well tissue culture plates. Micropore filters coated with BSA or IgG–BSA immune complexes were placed in individual wells of the tissue culture plates. A mixture of 800 μM ferricytochrome c type III (Sigma) and Hanks 15 mM HEPES medium, with or without SOD (Sigma), was added to each well, and 1 × 106 neutrophils were gently added to start the reaction. The tissue culture plates were maintained at 37°C for 60 min without shaking. Then the supernatant fluid was promptly centrifuged at 730g for 10 min at 4°C, and the absorbance was measured in a spectrophotometer at a wavelength of 550 nm (Hitachi Instruments, Inc., Minato-Ku, Tokyo, Japan). In all assays, the ferricytochrome c concentration was 800 μM, and the concentration of SOD, when present, was 15 μg/ml. Wells containing neutrophils incubated with ferricytochrome c in Hanks HEPES medium plus SOD served as blanks. The results of duplicate or triplicate determinations were averaged and converted to nanomoles of ferricytochrome c reduced using ΔE550 nm = 2.1 × 104 M− 1 cm− 1 (Massey Citation1959). Superoxide release was evaluated using rates of oxidant production during stimulation (nmol/60 min per 1 × 106 cells).
Neutrophil adherence
Neutrophil adherence was tested according to a previously published method by Oez et al. (Citation1990). The assays were performed in 24-well tissue culture plates. The reaction was begun by adding neutrophils suspended (1 × 106 cell) in Hanks 15 mM HEPES medium onto the surface of a micropore filter (coated with BSA or IgG–BSA immune complexes) or directly into the well of the culture plate and incubated at 37°C without shaking. After 60 min of culture, the medium was removed to take out the nonadherent cells, and RPMI-1640 free phenol red medium (Sigma) containing sodium pyruvate (0.11 g/l), penicillin (0.1 g/l), streptomycin (0.1 g/l), sodium bicarbonate (2.2 g/l), and MTT (2 g/l) [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (Sigma) was added. The tissue culture plates were maintained for a further 180 min at 37°C in an atmosphere of 5% CO2 saturated with water vapor. After this period of incubation, the supernatant was removed; 400 μl of isopropanol/HCl 0.04 M (Merck) was promptly added, and the solution was shaken for 10 min. Finally, 200 μl of supernatant was added to each well of a 96-well plate, and the absorbance was measured in a VERSAmax microplate reader at a wavelength of 570 nm (Molecular Devices, Inc., Sunnyvale, CA, USA).
Determination of the plasma cortisol concentrations
Blood samples were centrifuged at 730g for 5 min at 4°C. The supernatants were collected and stored at − 80°C until thawed for the assay. Plasma cortisol concentrations were determined by radioimmunoassay as described by Vecsei (Citation1979). This assay has a sensitivity of 0.85 μg/dl. The intra and inter-assay coefficient variation values were 5.3 and 9.3%, respectively.
In vitro effect of glucocorticoid on the release of superoxide
Neutrophils were obtained from control volunteers as described above, and the cells were cultured in 50 ml polypropylene tubes. The cells were resuspended at 5 × 106 cell/ml in RPMI-1640 medium containing different concentrations of hydrocortisone (10− 7, 10− 6, 10− 5 M; Sigma). We used this range of concentrations in order to include the concentration obtained in plasma in the stress group (mean: 5.7 × 10− 7 M). They were incubated in culture for 120 min at 37°C in an atmosphere of 5% CO2 saturated with water vapor. After the incubation period, cells were gently resuspended in cold PBS and centrifuged at 730g for 10 min at 4°C. The cell pellet was resuspended in Hanks 15 mM HEPES medium, and the cells were used for the superoxide release assay, performed as described above. In these in vitro experiments, hydrocortisone was first dissolved in ethanol (Merck), with less than 0.09% final concentration.
In vitro effect of an antagonist of the glucocorticoid receptor on the release of superoxide
Neutrophils, obtained from control volunteers, were resuspended at 5 × 106 cell/ml in RPMI-1640 medium. The cells were pretreated with RU-486 (Mifeprestone; Sigma) at a final concentration of 7 × 10− 6 M for 30 min. After pretreatment with RU-486, the cells were treated with 10− 7 M hydrocortisone for 120 min at 37°C in an atmosphere of 5% CO2 saturated with water vapor. The cells were cultured in 50 ml polypropylene tubes. Following the incubation period, the cells were gently resuspended in cold PBS and centrifuged at 730g for 10 min at 4°C. The cell pellet was resuspended in Hanks 15 mM HEPES medium, and the cells were used for the superoxide release assay, performed as described above. RU-486 was dissolved in DMSO, and the final concentration of DMSO in the culture media was less than 0.04%.
RNA isolation
Total RNA was isolated by Trizol-LS Reagent (Invitrogen, Carisbad, CA, USA), according to the manufacturer's instructions. RNA concentration was assessed using a biophotometer (Eppendorf, Hamburg, Germany), accepting a 260/280 absorbance ratio of 2.0. Ratio of 2.0 indicates that RNA is pure for all standard molecular biology applications.
Analysis of mRNA expression by real time PCR
Total RNA, 1.2 μg of RNA from the individual samples, was reverse transcribed using the Superscript III Reverse Transcriptase kit (Invitrogen) to the first strand cDNA, and cDNA samples were amplified by real time PCR with SYBR Green PCR Master Mix (AB Applied Biosystems, Warrington, WA, UK) using the following primers: p47phox sense primer 5′-CGTACCCAGCCAGCACTATGT-3′ and p47phox anti-sense 5′-GCTGCCCGTCAAA CCACTT-3′. These primers were specific for the published p47phox sequence (NM-000265). Internal controls for equal cDNA loading in each reaction were assessed using the following primers: β-actin sense primer 5′-AGGGAAATCGTGCGTGACA-3′ and β-actin anti-sense primer 5′-GAACCGCTCATTGCCGATA-3′. Real time PCR amplification was performed using an Applied Biosystems 7500 Sequence Detection system (Applied Biosystems). Cycling conditions were 95°C for 3 min followed by 40 cycles of amplification consisting of 95°C for 12 s, 60°C for 30 s, and 72°C for 30 s. The real time PCR was performed in triplicates. Relative expression levels were calculated by the 2(-Delta Delta C(T)) method (Pfaffl Citation2001). The data were normalized to the housekeeping gene β-actin, and values were normalized to the control or vehicle-treated samples.
Statistical analysis
Each value represents the mean ± SEM (SE of the mean) of “n” independent experiments. Statistical analysis was performed using student's t-test or Mann–Whitney rank sum test (when data did not pass a normal distribution test) for comparisons between control and stress groups and one way repeated measure ANOVA for comparisons between treatments, with post hoc Bonferroni t-tests. In studies involving the variance of two factors, two-way repeated measures ANOVA with post hoc Bonferroni t tests was used. Comparisons were considered significant at the level of p < 0.05. All statistical analyses were performed using SigmaStat version 3.10 (Systat Software, Inc., Chicago, IL, USA).
Results
Evaluation of psychological states
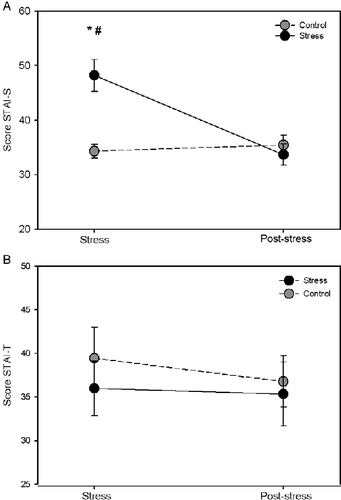
The STAI-S scores were significantly greater (t = 4.788, p < 0.001) in the stress group as measured in the stress phase when compared to the control group. However, no difference in the STAI-S scores (t = 0.613, p < 0.544) was observed among groups in the post-stress phase (45–60 days later). When we compared the STAI-S scores in the stress and post-stress phases, we observed a significant reduction in the STAI-S scores (t = 5.531, p < 0.001) for the stress group and no difference in the STAI-S scores (t = 0.422, p = 0.678) for the control group (; group effect: F(1,16) = 7.40, p = 0.015; time effect: F(1,16) = 13.052, p = 0.002; group by time interaction: F(1,16) = 17.723, p < 0.001). Therefore, we have shown that the final thesis examination is a stressful event that causes emotional distress in the post-graduate students. No difference was observed in the STAI-T scores between the stress and control groups in the stress and post-stress phase (; group effect: F(1,16) = 0.315, p = 0.583; time effect: F(1,16) = 1.691, p = 0.212; group by time interaction: F(1,16) = 0.863, p = 0.367). This result shows that all the volunteers have the same predisposition toward anxiety. The STAI-T scores were similar in the stress and post-stress phases, suggesting the reliability of the instrument (STAI) used for this evaluation.
Figure 1. STAI. (A) State-anxiety questionnaire. (B) Trait anxiety questionnaire. Data represent the mean ± SEM for nine volunteers in each group (six females and three males). *p < 0.001 compared to the control group in the stress phase (one day before the final post-graduate examination); #p < 0.001 comparison of the stress group between the stress and post-stress phase (45–60 days later). Analysis by two-way repeated measures ANOVA.
Effect of psychological stress on superoxide release by neutrophils stimulated by nonphagocytosable surfaces
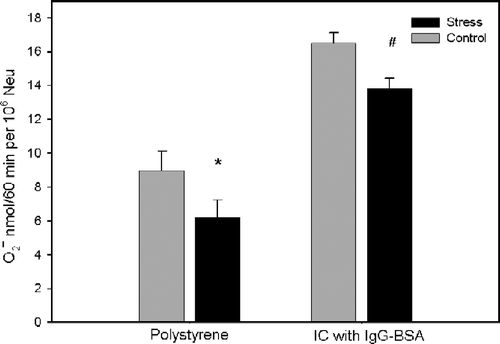
To simulate in vitro what might occur in immune complex diseases, neutrophils were incubated with a nonphagocytosable surface coated with immune complexes, micropore filters coated with BSA and opsonized with IgG anti-BSA (IgG–BSA complex). The following nonphagocytosable surfaces were used as a control for the effect of bound IgG: Micropore filters coated with BSA, a polystyrene surface and polystyrene coated with BSA. We have shown that all four kinds of surfaces promoted superoxide release by neutrophils. The superoxide release by neutrophils in contact with filter coated with immune complexes of IgG–BSA (15.22 ± 0.65 nmol/106 cells, n = 5) was significantly greater (F(3,12) = 62.134, p < 0.001) than with the polystyrene surface (6.23 ± 0.63 nmol/106 cells, n = 5), polystyrene coated with BSA (7.75 ± 0.54 nmol/106 cells, n = 5), and filter coated with BSA (6.24 ± 0.36 nmol/106 cells, n = 5). There was no difference in superoxide production by neutrophils incubated with a polystyrene surface, polystyrene coated with BSA, and filter coated with BSA. To investigate whether psychological stress alters the superoxide release in neutrophils, these cells were obtained from stress and control group volunteers and stimulated by incubation with filter coated with IgG–BSA immune complexes and with a polystyrene surface. As shown in (group effect: F(1,15) = 6.621, p = 0.021; stimulus effect: F(1,15) = 129.426, p < 0.001; group by stimulus interacts: F(1,15) = 0.000671, p = 0.980), neutrophils from the stress group showed a significant decrease in superoxide release compared to the control group when incubated with filter coated with IgG–BSA immune complexes (t = 2.164, p < 0.040) and a polystyrene surface (t = 2.192, p = 0.038).
Figure 2. Effect of psychological stress on the release of superoxide anion by human neutrophils (Neu) stimulated by nonphagocytosable surfaces. Data represent the mean+SEM for eight and nine volunteers in the control and stress groups, respectively. Polystyrene (well of tissue culture plates), IC (filter) coated with IgG–BSA immune complexes. *p < 0.05 compared to control group without stimulation and #p < 0.05 compared to control group stimulated with IC of IgG–BSA in the stress phase, as determined by two-way repeated measures ANOVA.
Effect of psychological stress on the attachment of neutrophils to nonphagocytosable surfaces
Adherence assays of neutrophils to the three types of surfaces were performed to ensure that differences in superoxide production were not dependent on differences in the number of cells involved. We found no difference (F(2,10) = 0.989, p = 0.406) in the number of neutrophils adhered to the polystyrene surface (0.810 ± 0.058 Abs 570 nm, n = 6), micropore filter coated with BSA (0.807 ± 0.052 Abs 570 nm, n = 6) or IgG–BSA immune complexes (0.875 ± 0.059 Abs 570 nm, n = 6). Thus, the filter coated with IgG–BSA immune complexes was chosen as the nonphagocytosable surface to investigate the effect of psychological stress on neutrophil adherence. No difference (t(19) = − 0.952, p = 0.353) in the cells adhered to filter coated with IgG–BSA immune complexes was observed among groups (stress: 0.790 ± 0.044 Abs 570 nm, n = 11; control: 0.848 ± 0.042 Abs 570 nm, n = 10). Therefore, the decrease in the superoxide release observed in neutrophils of the stress group is not dependent on the adherence of cells to surfaces. This result contributes to the hypothesis that stress causes changes in the intracellular signaling mechanisms regulating superoxide production.
Effect of psychological stress on mRNA expression of NADPH oxidase subunit p47phox
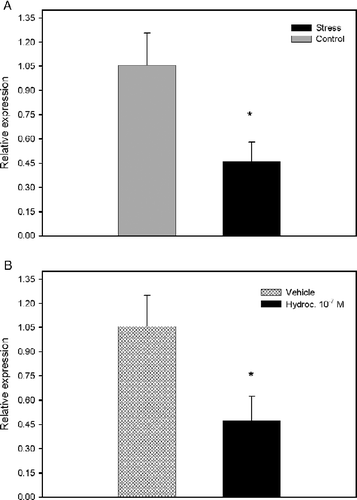
To investigate whether the decrease in release of superoxide is due to a change in the assembly of the NADPH oxidase complex, we examined the mRNA expression of p47phox subunit of the NADPH oxidase complex by real time PCR. Subunit p47phox was shown to be essential for the assembly and activity of the NADPH complex (Johnson et al. Citation1998; Sheppard et al. Citation2005). We showed that psychological stress caused a decrease in expression (t(6) = 2.543, p = 0.044) of the NADPH oxidase p47phox subunit ().
Figure 3. Analysis of expression of NADPH oxidase subunit p47phox mRNA. (A) Effect of psychological stress on expression of p47phox subunit mRNA. Data represent the mean+SEM for four volunteers in each group. *p < 0.05 as determined by Student's t-test for independent groups. (B) Effect of hydrocortisone on expression of p47phox subunit mRNA. Results are means+SEM of five independent experiments. Hydroc. 10− 7 M: Cells treated with 10− 7 M hydrocortisone. *p < 0.05 as determined by Student's t-test for independent groups.
Cortisol concentration in plasma
The plasma cortisol concentrations were significantly higher (T = 349.5, p = 0.004) in the stress group (20.59 ± 1.81 μg/dl, n = 23) when compared to the control group (13.63 ± 0.98 μg/dl, n = 21). This result suggests that this hormone could be implicated in the observed decrease in superoxide release by neutrophils from stressed post-graduate students.
In vitro effect of hydrocortisone on the release of superoxide in neutrophils stimulated by a nonphagocytosable surface
To test whether the observed decrease in superoxide release could be related to the action of glucocorticoids, we tested the effect of incubating the cells with hydrocortisone in vitro during the superoxide release assay (F(4,28) = 5.737, p = 0.002). Treatment with 10− 7 M hydrocortisone resulted in a significantly lower (t = 4.481, p < 0.001) superoxide release compared with cells treated with vehicle (). However, when neutrophils were incubated with increasing concentrations of hydrocortisone (10− 6–10− 5 M), there was no significant (vehicle vs. 10− 6 M hydrocortisone: t = 1.436, p = 0.649; vehicle vs. 10− 5 M hydrocortisone: t = 2.418, p = 0.089) alteration in superoxide release compared to cells treated with the vehicle. No significant alteration in superoxide release was observed in cultures incubated with ethanol at the final concentration of the assay (t = 1.051, p = 1.0). Additionally, the addition of hydrocortisone had no effect on neutrophil viability, as measured by the trypan blue exclusion test.
Figure 4. Superoxide release from neutrophils (Neu) stimulated by contact with immune-complex-coated filters. (A) Effect of treatment in vitro with hydrocortisone. *p < 0.001, analysis by one-way repeated measures ANOVA with multiple comparisons vs. vehicle, post-hoc Bonferroni t-test. (B) Effect of hydrocortisone on neutrophils pretreated in vitro with RU-486. *p < 0.001 and #p < 0.001, analysis by one-way repeated measures ANOVA with multiple comparisons vs. vehicle, post-hoc Bonferroni t-test. The results are mean+SEM of eight independent experiments. Hydroc. (cells treated only with hydrocortisone); RU-486 (cells treated only with RU-486); hydroc. + RU-486 (cells treated with RU486 and hydrocortisone); control (cells not treated).
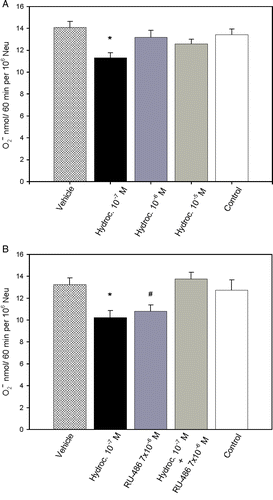
To further investigate the mechanism of the observed effect of glucocorticoid, we performed in vitro experiments pre-incubating neutrophils with RU-486 (F(4,28) = 16.199, p < 0.001). Surprisingly, neutrophils incubated only with RU-486 showed a significant (t = 4.491, p < 0.001) reduction in superoxide release. However, when neutrophils were incubated with RU-486 prior to treatment with hydrocortisone, no inhibitory effect (t = 0.965, p = 1.0) of hydrocortisone on superoxide release was found (). This result is an indication that the intracellular glucocorticoid receptor is involved in the inhibition of superoxide release caused by hydrocortisone.
In vitro effect of hydrocortisone on mRNA expression of NADPH oxidase subunit p47phox
To determine whether the effect of 10− 7 M hydrocortisone on superoxide release was correlated with changes in mRNA expression of NADPH oxidase subunits, we analyzed the mRNA expression of p47phox subunit of the NADPH oxidase complex by real time PCR. As shown in , treatment with hydrocortisone caused a decrease (t(4) = 4.254, p = 0.013) in mRNA expression of the NADPH oxidase p47phox subunit.
Discussion
Psychological stress was linked to a broad array of adverse health outcomes. To elucidate the mechanisms responsible for these effects, researchers have explored the interaction among the central nervous, endocrine, and immune systems. Psychological stress can trigger various emotional reactions, among them is anxiety. Through the use of the State questionnaire STAI, we showed that the anticipation of the final thesis examination causes anxiety in post-graduate students, generating a psychological stress. This finding is in agreement with several reports that show that academic stress is a real-life psychological stressor of moderate intensity and duration (Maes et al. Citation1999; Morita et al. Citation2005; Schoofs et al. Citation2008).
The deposition of antigen–antibody complexes in tissues was found to be associated with inflammation and tissue injury (Ranadive and Movat Citation1979), through a mechanism involving adherence of phagocytes to immune complex-coated structures and triggering the extracellular release of lysosomal enzymes (Lucisano and Mantovani Citation1984) and ROS (Kobayashi et al. Citation2003). Thus, ROS can play an important role in inflammatory injury and was implicated in several diseases (Halliwell and Gutteridge Citation1989). In our study, on the influence of psychological stress on ROS release by neutrophils, we used antigen–antibody complexes bound to nonphagocytosable surfaces as a model of what might occur in vivo.
The interaction between human neutrophils and immune complexes bound to surfaces was shown to induce the release of ROS into the extracellular environment (Henson Citation1971). We showed that contact of neutrophils with different nonphagocytosable surfaces stimulated neutrophils to release superoxide anion. There was no difference in superoxide anion release by neutrophils incubated with polystyrene, BSA coated polystyrene, or micropore filter coated with BSA. Johnston and Lehmeyer (Citation1976) showed that the superoxide production by cells incubated in polystyrene dishes was the result of stimulation by contact with the dish rather than basal activity. The most stimulatory surface for neutrophils in our experiments was filters coated with IgG–BSA immune complexes. The cells are able to interact with this surface through Fcγ membrane receptors specific for the Fc region of IgG antibodies, triggering activation of the NADPH oxidase enzymatic complex.
Disturbances in the release of ROS by phagocytes were hypothesized to be influenced by environmental factors, including psychological stress. Conflicting results were reported on the influence of stress on ROS release by phagocytes both in humans (Kihara et al. Citation1992; Kang et al. Citation1996; Kopprasch et al. Citation1996; Atanackovic et al. Citation2002; Arranz et al. Citation2009) and laboratory animals (Coe et al. Citation1988; Lyte et al. Citation1990; Kang and McCarthy Citation1994; De Castro et al. Citation2000; Oishi et al. Citation2003; Costa-Pinto and Palermo-Neto Citation2010). The differences between these studies could be explained by differences in the stress model, animal species and cell type, stimulus, types of assay used to measure ROS release (e.g. extracellular or intracellular), or other conditions employed. According to Atanackovic et al. (Citation2003), an influence of the circadian rhythm in the generation of ROS might in part explain discrepancies in the results obtained in studies on ROS release. In our study, we observed that neutrophils from post-graduate students under psychological stress showed a decrease in superoxide released when these cells interact with nonphagocytosable surfaces.
Several studies have shown that stress can modify adherence and expression of cellular adhesion molecules in immune cells (Redwine et al. Citation2003; Stanojević et al. Citation2008; Arranz et al. Citation2009). Reduced adherence of neutrophils to nonphagocytosable surfaces is unlikely to be responsible for the stress-related decrease in ROS release observed in this study. Our results showed that the adherence of neutrophils to all four types of nonphagocytosable surfaces is the same; thus, differences in the number of cells present on the filters cannot account for the observed differences in ROS release. The reduction in superoxide release observed in neutrophils from the stress group is expected to be caused by changes in the intracellular signaling mechanism.
Since the production of superoxide is a reflection of the activity of the NADPH oxidase complex, we investigated whether stress could alter the gene expression of components of this enzymatic complex. Our results showed that stress reduced the mRNA expression of the gene that encodes for the p47phox subunit. The p47phox subunit plays a key role in the translocation of other cytosolic subunits and the assembly of the NADPH oxidase complex (Johnson et al. Citation1998; Sheppard et al. Citation2005). Therefore, the observed reduction in superoxide release by neutrophils from the stress group may be a consequence of decreased transcript expression of the p47phox subunit. This is only a suggestive evidence; however, because it is difficult to estimate whether a low level of mRNA would result in a low level of the corresponding protein, the turnover rates are unknown. It is known that glucocorticoids trigger genomic events and synthesis of specific proteins that are responsible for physiological effects with a lag period ranging from 30 min to several hours or even days (Yamamoto Citation1985; Baccan et al. Citation2004; Long et al. Citation2005). Johnson et al. (Citation2006) showed that p47phox subunit exogenous gene can be expressed in human neutrophils in as short as 2 h post-transfection. Further, investigation will be needed to determine the expression of p47phox subunit protein in the cells caused by stress.
In our model, the decrease in superoxide anion release in the stress group was accompanied by an increased level of plasma cortisol, which is an indication of the stimulation of the HPA axis. This suggests a direct effect of this hormone on superoxide release by the neutrophils following activation of the HPA axis. Nevertheless, other hormones may be involved such as catecholamines. Our finding that neutrophils treated with 10− 7 M hydrocortisone can reproduce in vitro approximately the same effect as that obtained in stress conditions is an indication that this hormone might be an important factor in the inhibition of superoxide release. Higher concentrations of hydrocortisone (10− 6–10− 5 M), however, did not produce this effect. Our result is in line with the results described by Békési et al. (Citation2000) with human neutrophils treated with 10− 7 M hydrocortisone. Similar results were also described in murine macrophages treated with 10− 10–10− 4 M corticosterone (Long et al. Citation2005) and with human monocytes treated with 3 × 10− 7–10− 5 M cortisone (Szefler et al. Citation1989). Our result is at odds with earlier reports of the immunostimulatory effect of hydrocortisone on sheep neutrophils in the same concentration ranges that we used (Huber et al. Citation2006). Fleming et al. (Citation1991) did not observe any changes in ROS release by treatment of murine peritoneal inflammatory cells with 2 × 10− 6–7 × 10− 6 M corticosterone. These conflicting results indicate that the sensitivity to glucocorticoids for ROS release by phagocytes may depend on the type of cells and the stimulus used.
To further investigate the mechanism of action of glucocorticoids in the decrease in superoxide release, we incubated neutrophils with RU-486 in vitro because pharmacological and endocrinological studies have shown that RU-486 is able to antagonize the effects of glucocorticoids. We showed that RU-486 alone in culture at 7 × 10− 6 M had an agonistic effect on superoxide release by neutrophils. Schaison (Citation1989) described that RU-486 displayed agonistic effects in the absence of glucocorticoids. This result was also reported by Van Voorhis et al. (Citation1989) for human mononuclear cells treated with 10− 5 M RU-486, but for lower concentrations of RU-486 (10− 7–10− 6 M), they observed an antagonist effect. These findings indicate that RU-486 may have dose-dependent mixed agonist/antagonist effects. In our study, cultures treated only with RU-486 showed an agonist profile. However, the decrease in superoxide release that we have observed after in vitro treatment with hydrocortisone could be prevented by pretreatment of neutrophils with RU-486. This result therefore supports the participation of the intracellular glucocorticoid receptor in the cortisol-dependent modulation of superoxide release in neutrophils stimulated by contact with a nonphagocytosable surface.
There are several reports on the action of steroids in the expression of the NADPH oxidase complex (Dandona et al. Citation2001; Wagner et al. Citation2001; Sumi et al. Citation2003). Our results showed, for the first time, that 10− 7 M hydrocortisone promotes a reduction in p47phox mRNA expression, which suggests an explanation for the decrease in ROS release caused by this hormone.
In conclusion, in the present study, we demonstrated that academic psychological stress promotes an increase in plasma levels of cortisol and a reduction in superoxide release by human neutrophils stimulated by nonphagocytosable surfaces. We presented evidence that this decrease in superoxide release may be at least in part due to the action of glucocorticoids because treating neutrophils with hydrocortisone in vitro approximately reproduced this effect. In addition, reversal of the decrease in superoxide release by treatment with RU-486 indicates the participation of the intracellular glucocorticoid receptor.
Excess ROS generated by phagocytes may cause lipid peroxidation and protein oxidation. It results in altered membrane fluidity and cell membrane-related function, causing damage in the tissues in different ways (Sahin and Gümüslü Citation2007). As ROS release may be implicated in tissue damage in immune complex diseases, we may conclude that the kind of psychological stress in this study would decrease any possible harmful effect of these reactive species through activation of the HPA axis and the action of cortisol. However, appropriate production of ROS by phagocytes is also important for defense mechanisms by the host immune cells and as intracellular messengers needed for cellular functioning. Superoxide release is the main bactericidal activity specific for neutrophils in defense by the host (Segal Citation2005). Therefore, it is not surprising that patients with decreased ROS release often complain about infectious illnesses and delayed wound healing (White and Gallin Citation1986). So, the final result of stress, whether beneficial or harmful will be dependent on the physiological conditions.
In this study, we used a simplified model for a mechanism that may be implicated in immune complex diseases. More studies are needed to investigate how these findings can be extrapolated to in vivo inflammatory responses in order to understand the influence of psychological stress in these diseases.
Ethical considerations in the conduct and reporting of research: Protection of human subjects in research
All experiments were conducted in accordance with the ethical standards of the committee on human experimentation (Ethics Committee in Research of the Ribeirão Preto Medical School, University Hospital, University of São Paulo/Process n° 9711/2006 and CONEP—Brazilian Commission on Ethics in Research/CAAE—0218.0.004.000-06) and with the “Declaration of Helsinki”. All procedures were carried out with written informed consent of each subject.
Acknowledgements
We are grateful to Dr Francisco Silveira Guimarães for his helpful advice in the questionnaire STAI and statistical analysis, Dra. Margaret de Castro and José Roberto da Silva for their help in the determination of plasma cortisol concentration, José A. da Silva for technical assistance. The research reported was supported by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), and FAEPA (Fundação de Apoio ao Ensino, Pesquisa e Assistência do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Arranz L, De Vicente A, Muñoz M, De La Fuente M. 2009. Impaired immune function in a homeless population with stress-related disorders. Neuroimmunomodulation. 16:251–260.
- Atanackovic D, Brunner-Weinzierl MC, Kröger H, Serke S, Deter HC. 2002. Acute psychological stress simultaneously alters hormone levels, recruitment of lymphocyte subsets, and production of reactive oxygen species. Immunol Invest. 31:73–91.
- Atanackovic D, Schulze J, Kröger H, Brunner-Weinzierl MC, Deter HC. 2003. Acute psychological stress induces a prolonged suppression of the production of reactive oxygen species by phagocytes. J Neuroimmunol. 142:159–165.
- Atanackovic D, Schnee B, Schuch G, Faltz C, Schulze J, Weber CS, Schafhausen P, Bartels K, Bokemeyer C, Brunner-Weinzierl MC, Deter HC. 2006. Acute psychological stress alerts the adaptive immune response: Stress-induced mobilization of effector T cells. J Neuroimmunol. 176:141–152.
- Babior BM. 1999. The production and use of reactive oxidants by phagocytes. In: Gilbert DL, Colton CA. editors. Reactive oxygen species in biological systems. New York: Kluwer Academic/Plenum Publishers503–518.
- Baccan GC, Oliveira RDR, Mantovani B. 2004. Stress and immunological phagocytosis: Possible nongenomic action of corticosterone. Life Sci. 75:1357–1368.
- Békési G, Kakucs R, Várbíro S, Rácz K, Sprintz D, Fehér JE, Székács B. 2000. In vitro effects of different steroid hormones on superoxide anion production of human neutrophil granulocytes. Steroids. 65:889–894.
- Biaggio AMB, Natalício L, Spielberger CD. 1977. The development and validation of experimental portuguese form of the state-trait anxiety inventory. In: Spielberger CD. editors. Cross-cultural research on anxiety. Washington, DC: Hemisphere/Wiley29–40.
- Black PH. 2002. Stress and the inflammatory response: A review of neurogenic inflammation. Brain Behav Immun. 16:622–653.
- Coe CL, Rosenberg LT, Levine S. 1988. Prolonged effect of psychological disturbance on macrophage chemiluminescense in the squirrel monkey. Brain Behav Immun. 2:151–160.
- Costa-Pinto FA, Palermo-Neto J. 2010. Neuroimmune interactions in stress. Neuroimmunomodulation. 17:196–199.
- Crockett-Torabi E, Ward PA. 1996. The role of leukocytes in tissue injury. Eur J Anaesthesiol. 13:235–246.
- Dandona P, Aljada A, Ghanim H, Mohanty P, Hamouda W, Al-Haddad W. 2001. Acute suppressive effect of hydrocortisone on p47 subunit of nicotinamide adenine dinucleotide phosphate oxidase. Metabolism. 50:548–552.
- De Castro CMMB, Castro RM, Medeiros AF, Santos AQ, Silva WTF, Lima-Filho JL. 2000. Effect of stress on the production of O2− in alveolar macrophages. J Neuroimmunol. 108:68–72.
- Fleming SD, Edelman LS, Chapes SK. 1991. Effects of corticosterone and microgravity on inflammatory cell production of superoxide. J Leukoc Biol. 50:69–76.
- Glaser R, Kiecolt-Glaser J. 2005. Stress-induced immune dysfunction: Implications for heath. Nat Rev Immunol. 5:243–251.
- Halliwell B, Gutteridge JMC. 1989. Free radicals in biology and medicine. New York: Oxford University Press416–494.
- Henson PM. 1971. The immunologic release of constituents from neutrophil leukocytes: I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 107:1535–1546.
- Huber K, Kötz-Fahning M, Hock B. 2006. Respiratory burst as a biomarker for stress responses. Protoplasma. 229:221–224.
- Johnson JL, Park JW, Benna JE, Faust LP, Inanami O, Babior BM. 1998. Activation of p47PHOX, a cytosolic subunit of the leukocyte NADPH oxidase: Phosphorylation of ser-359 or ser-370 precedes phosphorylation at other sites and is required for activity. J Biol Chem. 273:35147–35152.
- Johnson JL, Ellis BA, Munafo DB, Brzezinska AA, Catz SD. 2006. Gene transfer and expression in human neutrophils. The phox homology domain of p47phox translocates to the plasma membrane but not to the membrane of mature phagosomes. BMC Immunol. 7:28.
- Johnston RBJr, Lehmeyer JE. 1976. Elaboration of toxic oxygen by-products by neutrophils on a model of immune complex disease. J Clin Invest. 57:836–841.
- Kang DH, McCarthy DO. 1994. The effect of psychological stress on neutrophil superoxide release. Res Nurs Health. 17:363–370.
- Kang DH, Coe CL, McCarthy DO. 1996. Academic examinations significantly impact immune responses, but not lung function, in healthy and well-managed asthmatic adolescent. Brain Behav Immun. 10:164–181.
- Kihara H, Teshima H, Sogawa H, Nakagawa T. 1992. Stress and superoxide production by human neutrophils. Ann NY Acad Sci. 650:307–310.
- Kloet ER. 2004. Hormones and the stressed brain. Ann NY Acad Sci. 1018:1–15.
- Knapp PH, Levy EM, Giorgi RG, Black PH, Fox BH, Heeren TC. 1992. Short-term immunological effects of induced emotion. Psychosom Med. 54:133–148.
- Kobayashi SD, Voyich JM, De Leo FR. 2003. Regulation of the neutrophil-mediated inflammatory response to infection. Microbes Infect. 5:1337–1344.
- Kopprasch S, Graessler J, Seibt R, Naumann HJ, Wiedemann B. 1996. Laboratory stress in normotensives, borderlie hypertensives and essential hypertensives is associated with priming of phagocytic cells. Stress Health. 12:9–16.
- Long F, Wang YX, Liu L, Zhou J, Cui RY, Jiang CL. 2005. Rapid nongenomic inhibitory effects of glucocorticoids on phagocytosis and superoxide anion production by macrophages. Steroids. 10:55–61.
- Lucas A, Holtmann G, Gerken G, Pietsch A, Braun-Lang U, Gilani K, Strassburger K, Gesing S, Janssen OE, Kavelaars A, Heijnen CJ, Schedlowski M, Elsenbruch S. 2006. Visceral pain and public speaking stress: Neuroendocrine and immune cell responses in healthy subjects. Brain Behav Immun. 20:49–56.
- Lucisano YM, Mantovani B. 1984. Lysosomal enzyme release from polymorphonuclear leukocytes induced by immune complexes of IgM and IgG. J Immunol. 132:2015–2020.
- Lyte M, Nelson SG, Thampson ML. 1990. Innate and adaptive immune responses in a social conflict paradigm. Clin Immun Immunopathol. 57:137–147.
- Maes M, Van Bockstaele DR, Van Gastel A, Song C, Schotte C, Neels H, DeMeester I, Scharpe S. 1999. The effects of psychological stress on leukocytes subset distribution in humans: Evidence of immune activation. Neuropsychobiology. 39:1–9.
- Massey V. 1959. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 34:255–256.
- Morita K, Satio T, Ohta M, Ohmori T, Kawai K, Teshima-Kondo S, Rokutan K. 2005. Expression analysis of psychological stress-associated genes in peripheral blood leukocytes. Neurosci Lett. 381:57–62.
- Oez S, Welte K, Platzer E, Kalder JR. 1990. A simple assay for quantifying the inducible adherence of neutrophils. Immunobiology. 180:308–315.
- Oishi K, Nishio N, Konishi K, Shimokawa M, Okuda T, Kuriyama T, Machida K. 2003. Differential effects of physical and psychological stressors on immune fuctions of rats. Stress. 6:33–40.
- Pfaffl MW. 2001. A new mathematical model for relative quantification in real time RT-PCR. Nucleic Acids Res. 29:2002–2007.
- Ranadive NS, Movat HZ. 1979. Tissue injury and inflammation induced by immune complexes. In: Movat HZ. editors. Inflammation, immunity and hypersensitivity. Hagerstown/Marzlland/New York/San Francisco, CA/London: Harper Row Publishers409–438.
- Redwine L, Snow S, Mills P, Irwin M. 2003. Acute psychological stress: Effects on chemotaxis and cellular adhesion molecule expression. Psychosom Med. 65:598–603.
- Robinson JM. 2009. Phagocytic leukocytes and reactive oxygen species. Histochem Cell Biol. 131:465–469.
- Sahin E, Gümüslü S. 2007. Stress-dependent induction of protein oxidation, lipid peroxidation and anti-oxidants in peripheral tissues of rats: Comparison of three stress models (immobilization, cold and immobilization-cold). Clin Exp Pharmacol Physiol. 34:425–431.
- Schaison G. 1989. Antagonist and agonist effects of the antiprogesterone RU-486. Ann Endocrinol. 50:200–207.
- Schoofs D, Hartmann R, Wolf OT. 2008. Neuroendocrine stress responses to an oral academic examination: No strong influence of sex, repeated participation and personality traits. Stress. 11:52–61.
- Segal AW. 2005. How neutrophils kill microbes. Annu Rev Immunol. 23:197–223.
- Sheppard FR, Kelher MR, Moore EE, Mclaughlin NJ, Banerjee A, Silliman CC. 2005. Structural organization of the neutrophil NADPH oxidase: Phosphorylation and translocation during priming and activation. J Leukoc Biol. 78:1025–1042.
- Sieber WJ, Rodin J, Larson L, Ortega S, Cummings N, Levy S, Hiteside T, Herberman R. 1992. Modulation of human natural killer cell activity by exposure to uncontrollable stress. Brain Behav Immun. 6:141–156.
- Stanojević S, Kustrimović N, Mitić K, Miletić T, Vujić V, Kovacević-Jovanović V, Dimitrijević M. 2008. The effects of corticosterone and beta-endorphin on adherence, phagocytosis and hydrogen peroxide production of macrophages isolated from Dark Agouti rats exposed to acute stress. Neuroimmunomodulation. 15:108–116.
- Sumi D, Hayashi T, Matsui-Hirai H, Jacobs AT, Ignarro LI, Iguchi A. 2003. 17-β-Estradiol inhibits NADPH oxidase activity through the regulation of p47phox mRNA and protein expression in THP-1 cells. Biochim Biophys Acta. 1640:113–118.
- Szefler SJ, Norton CE, Ball B, Gross JM, Aida Y, Pabst MJ. 1989. IFN-gamma and LPS overcome glucocorticoid inhibition of priming for superoxide release in human monocytes. Evidence that secretion of IL-1 and tumor necrosis factor-alpha is not essential for monocyte priming. J Immunol. 142:3985–3992.
- Van Voorhis BJ, Anderson DJ, Hill JA. 1989. The effects of RU-486 on immune function and steroid-induced immunosuppression in vitro. J Clin Endocrinol Metab. 69:1195–1199.
- Vecsei P. 1979. Glucocorticoids: Cortisol, corticosterone, and compound S. In: Jafee BM, Berhrman HR. editors. Methods of hormone radioimmnunoassay. London: Academic Press393–415.
- Wagner AH, Schroeter MR, Hecker M. 2001. 17-β-Estradiol inhibition of NADPH oxidase expression in human endothelial cells. FASEB J. 15:2121–2130.
- White CJ, Gallin JI. 1986. Phagocytes defects. Clin Immunol Immunopathol. 40:50–61.
- Yamamoto KR. 1985. Steroid receptor regulated transcription genes and gene networks. Ann Rev Genet. 19:209–252.