Abstract
Depressive symptoms and memory impairments are associated with heightened stress hormone levels during aging. A factor that is related to memory deficits during aging is internalized negative aging stereotypes; the idea people have about the process of aging. In this study, we assessed the associations between internalized negative aging stereotypes, depressive symptoms, subjective and objective memory assessments, and cortisol concentration among older adults. Forty older adults aged between 58 and 85 years (18 females and 22 males; mean age ± SD: 71.25 ± 8.80 years) were assessed in this study. Measures of internalized negative aging stereotypes, depressive symptoms, and both subjective and objective memory performance were assessed. Salivary samples were obtained for measurement of cortisol concentration. Stepwise linear regressions were executed in our main analyses. Internalized negative aging stereotypes were associated with increased depressive symptoms and subjective memory complaints. No significant differences were observed for objective memory performance, or cortisol concentration. Internalized negative aging stereotypes are associated with increased depressive symptomatology and subjective complaints of memory; however, they do not predict increased cortisol concentration nor objective memory performance during aging. These results indicate that the mechanism underlying the association between internalized negative aging stereotypes and cognitive impairments may not be related to dysregulations of cortisol secretion among older adults.
Introduction
Aging is associated with alterations in the functioning of the hypothalamic–pituitary–adrenal (HPA)-axis production of the stress hormone cortisol (Chahal and Drake Citation2007). A considerable amount of evidence links aging with increases in mean basal cortisol levels (Touitou et al. Citation1982; Van Cauter et al. Citation1996, Citation2000; Baranowska et al. Citation2007). Under basal conditions, cortisol measurements show a 24-h circadian rhythm in which peak levels are reached in the morning, approximately 30 min after awakening (the awakening cortisol response), with gradual decrements throughout the day until trough levels are reached late at night (around midnight; Weitzman et al. Citation1971; Wust et al. Citation2000; Edwards et al. Citation2001; Fries et al. Citation2009). Evidence shows that the circadian rhythm of older adults attains an advanced phase characterized by higher concentration of cortisol in the evening which flattens the diurnal pattern (Deuschle et al. Citation1997; Raff et al. Citation1999). Notwithstanding these findings, a few studies have also reported lower cortisol concentration during aging (Drafta et al. Citation1982; Sharma et al. Citation1989), whereas others find no changes or inconsistent cortisol patterns (Sherman et al. Citation1985; Waltman et al. Citation1991; Ice et al. Citation2004).
In a longitudinal study by Lupien and colleagues (Citation1996), a sample of healthy older adults was followed annually over 7 years and cortisol concentration was measured hourly over a 24-h period. Results showed that plasma basal cortisol concentration was categorized into three subgroups: (1) high current cortisol concentration with annual increases (increasing/high group), (2) moderate current cortisol concentration with annual increases (increasing/moderate group), and (3) moderate current cortisol concentration with annual decreases (decreasing/moderate group). These findings reveal substantial inter-individual variability in HPA-axis functioning among older adults (Lupien et al. Citation1996). Despite the observed alterations in older adults' HPA-axis functioning, the underlying mechanisms associated with such changes still remain unclear. Consequently, several modulating psychosocial factors and conditions have been investigated.
Depression has been proposed as a factor that may explain changes in HPA-axis functioning. It is a highly prevalent and important mental health condition among older adults with numerous effects on physical and cognitive functioning (Blazer Citation2003). Geriatric depression is often characterized by hyperactivity of the HPA axis, resulting in increased cortisol concentration in the afternoon and evening (Holsboer Citation2001; Young Citation2004). Milder forms of depressive symptoms may also be associated with functional declines (Gallo et al. Citation1997). However, depression does not adequately explain the link between cortisol and aging because a significant proportion of nondepressed healthy older adults also show high levels of cortisol (Lupien et al. Citation1996; Raff et al. Citation1999; Fiocco et al. Citation2006).
Another factor that has been investigated is subjective memory complaints. Evidence shows that older adults who report subjective memory complaints show altered basal cortisol concentration (Wolf et al. Citation2005). Similar to previous results in older depressed subjects (Balardin et al. Citation2010), those reporting subjective memory complaints or depressive symptoms show normal elevations in cortisol concentration in the morning, yet evening levels remain high and fail to decrease normally (Fiocco et al. Citation2006). In addition, memory complaints and depression are commonly shown to predict cognitive decline over time (Lupien et al. Citation1996; Geerlings et al. Citation2000; Jonker et al. Citation2000; Jorm Citation2000; Wilson et al. Citation2003).
In accordance, cross-sectional and longitudinal studies demonstrate that older adults who have age-related elevations in cortisol levels also experience deficits in memory performance (Dori et al. Citation1994; Lupien et al. Citation1994, Citation1998; Kalmijn et al. Citation1998). Both cognitive impairments and high levels of cortisol are consistently implicated with increased subjective memory complaints and depressive symptoms (Dori et al. Citation1994; Lupien et al. Citation1994, Citation1998; Deuschle et al. Citation1998; Kalmijn et al. Citation1998; Holsboer Citation2001; Fiocco et al. Citation2006). Taken together, depression, subjective memory complaints, and objective memory performance appear intricately connected to elevated cortisol concentration in human aging. Yet, these factors do not comprehensively explain the association nor adequately address how the experience of aging itself can be stressful. Indeed, an important factor that has not yet been investigated is the role of aging perceptions.
Negative aging stereotypes represent general ideas about the process of aging, including the notion that aging equates illness, disabilities, deteriorating health, and an inability to learn new information. Aging stereotypes make the assumption that older adults are a homogeneous group while failing to take into consideration well-documented variability in age-related cognitive functioning or health status (Ory et al. Citation2003). Scientific evidence from the MacArthur Foundation studies on successful aging reveals that such beliefs may be inaccurate (Rowe and Kahn Citation1998). Regardless of existing evidence, aging stereotypes are prevalent in Western culture. Negative aging stereotypes might even demoralize our elders and inadvertently compromise their health and well-being.
Aging stereotypes play an important role in the formation of older adults' perceptions of their age group and their own aging processes (Levy et al. Citation2002a, Citation2002b). Internalizing negative aging stereotypes into self-schemas or constructs of one's self-perceptions on aging (SPA) may have a detrimental impact on cognitive and functional abilities, confidence, mood, and self-image (Palmore Citation1999), thus resulting in a “self-fulfilling prophecy” of the stereotype (Allport Citation1958; Levy et al. Citation2002a). Furthermore, SPA are likely to remain stable or even worsen over time (Levy Citation2008).
Aging stereotypes are furthermore associated with cognitive performance in older adults. When stereotype stimuli are presented (as primes below awareness), older adults exposed to negative stereotype primes performed more poorly on a memory task compared with the group exposed to positive aging stereotypes (Levy Citation1996). Similarly, worse memory performance is observed after reading negative reports on aging and memory as opposed to positive ones (Hess et al. Citation2003).
Aging stereotypes can also influence physiological functioning. Older adults who were exposed to negative aging stereotypes had higher autonomic responses (systolic and diastolic blood pressure and skin conductance) and less efficient physiological recovery compared with participants exposed to positive stereotypes (Levy et al. Citation2000). Interestingly, although the negative stereotypes group displayed greater autonomic activity, they rated the task to be as stressful as the positive stereotypes group. Taken together, these studies indicate that negative aging stereotypes are processed pre-consciously as distressing and ultimately impede physiological, cognitive, and psychosocial functioning (Levy Citation2003).
On the basis of the current literature, it is unclear to what extent cortisol concentration is related to depressive symptoms, aging perceptions, and memory. For example, although it may be that aging perceptions predict depressive symptoms and higher cortisol concentration, it is equally plausible that depressive symptoms during aging may alter experiences and increase negative perceptions. Consequently, each factor may exert bidirectional effects ().
Figure 1. Diagrams demonstrating possible directions for the relationship between cortisol, aging perceptions, memory, and depressive symptoms.
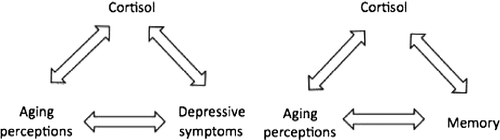
To date, no study has assessed how negative aging perceptions relate to cortisol concentration, depressive symptoms, and subjective memory complaints in a single sample of older adults. Therefore, the first goal of this study was to investigate whether associations exist between negative aging perceptions, cortisol concentration, and depressive symptoms. The second goal was to evaluate the relationship between negative aging perceptions, subjective memory complaints as well as objective measures of declarative memory performance. We hypothesized that individuals presenting internalized negative aging stereotypes would have increased salivary cortisol concentration, depressive symptoms, memory complaints, and memory impairments. Furthermore, increased age was expected to moderate internalization of negative aging stereotypes.
Materials and methods
Participants
Measures were obtained from participants of the Douglas Hospital Longitudinal Study of Normal and Pathological Aging (Lupien et al. Citation2005). A sample of 40 (18 males and 22 females) normal and healthy older adults aged between 58 and 85 years was assessed for the present analysis. The group mean age ( ± SEM) was 71.25 ( ± 1.39 years). The mean age of the males was 67.49 ( ± 1.845 years). The mean age of the females was 75.85 ( ± 1.57 years). The group mean number of years of education was 14.81 ( ± 0.56 years). The Research Ethic Board of the Douglas Hospital approved this study on an annual basis. All participants provided written informed consent before their participation in the study.
Participants were excluded from this longitudinal study if they had a history of head injury or trauma, cerebral vascular accident, alcohol abuse, or use of medications that have a potential impact on cortisol secretion or cognitive performance. Participants who reported any use of general anesthesia during the previous year were also excluded. The screening procedure additionally included a clinical neuropsychological examination. Individuals who showed any signs of dementia and scored below average (lower than 24) on the Mini Mental State Exam were not included in the study.
Salivary cortisol collection and analysis
Participants were provided with home kits to collect basal salivary cortisol samples. Participants were asked to sample their saliva on two consecutive routine days in their home environment using the Salivettes® (Starstedt, Germany; Lupien et al. Citation1998). Samples were collected at the following time points: awakening, awakening +30 min (combined as AM cortisol), 14:00 h, 16:00 h, and before bedtime (combined as PM cortisol). For each sampling time point, the average of the two sampling days was used for further analyses. To prevent contamination with food or blood, participants were asked not to smoke, eat nor brush their teeth immediately before saliva collection. Moreover, participants were given logbooks to record the exact time of sampling. Participants were then asked to store the samples in their home freezers until their next visit to the laboratory, 2–3 days after sampling. Once participants returned to the laboratory for their next session, saliva samples were stored at − 20°C until they were later analyzed using sensitive radioimmunoassay, a reliable method of analysis (Lupien et al. Citation1997, Citation1998). Samples were thawed and spun at 605 g for 20 min at 4°C. A radioimmunoassay kit DSL 2000 from the Diagnostic Systems Laboratories, Inc. (Webster, TX, USA) was used to measure cortisol concentrations. All samples were assayed in duplicates. Cortisol samples were mixed with 50 μL of 125I-tracer DSL 2020 and 50 μL of antibody DSL 2010. Then samples were vortexed and incubated for 2 h before decanting and counting in a gamma counter. Total binding and nonspecific binding typically ranged between 47% and 63% and 0.5% and 1.5%, respectively. Cross-reactivity is less than 4% with 11-deoxycortisol and less than 1% with other naturally occurring steroids. The intra- and inter-assay coefficients of variation were 4.6 and 5%, respectively. The limit of detection of the assay was 0.01 μg/dL. Participants were excluded from the analysis if they had not provided the five cortisol samples over the 2-day period for home salivary cortisol sample collection. Raw cortisol values were used for analyses.
Measures
Aging perceptions
An aging perceptions questionnaire was created for the purposes of this study. The questionnaire was developed by the Centre for Studies on Human Stress, based on the items in the Attitude Toward Own Aging subscale of the Philadelphia Geriatric Center Morale Scale (Lawton Citation1975; Liang and Bollen Citation1983).
The questionnaire is divided into two main sections, each containing four items answered on a four-point Likert scale (1 = strongly disagree to 4 = strongly agree). The first section contains statements with regard to general perceptions of aging (GPA), and the second section asks the same four questions regarding one's SPA, thus measuring internalized perceptions of aging. The questionnaire instructions are as follows: “We are presently conducting a study on the perceptions of aging and we would like to have your opinion on what you believe the process of aging entails. For each statement, you will have four answers to choose from.”
The questionnaire items for the GPA are as follows: (1) As people get older, their life becomes less enjoyable; (2) as people get older, they are less useful to society; (3) as people get older, their memory declines; and (4) as people get older, they become more dependent on others.
The questionnaire items for the SPA are as follows: (1) As you get older, do you think your life is less enjoyable? (2) As you get older, do you think you are less useful to society? (3) As you get older, do you think your memory is declining? (4) As you get older, do you think you are more dependent on others?
Subjective complaints of memory deficits
The Everyday Memory Questionnaire (EMQ; Sunderland et al. Citation1983) rates the frequency of memory problems across a number of functional domains. Thirty-five items are rated on a Likert scale ranging from (0) never to (5) several times in a day. The result is a total score, which may range from 0 to 175. Higher scores reflect greater subjective complaints of memory.
Depressive symptoms
The Geriatric Depression Scale (GDS; Yesavage et al. Citation1983) is a 30-item self-rating scale. It was specifically developed to screen older adults, and its items are sensitive to issues concerning geriatric depression. Answers to questions are in simple “Yes/No” format. Each question receives a score of 0 or 1. A cut-off score of 12 and above indicates depression.
Declarative memory
Declarative memory was assessed using a cued recall test. Previous research by our group demonstrated that this task is sensitive to increases in cortisol concentration among older adults (Lupien et al. Citation1994, Citation1997). The task involves presentation of 12 concrete word pairs based on Lyon's criteria (Citation1976). The list consists of six moderately related word pairs and six unrelated word pairs (for more details on task development and validation, see Fiocco et al. Citation2006). The words were presented on a computer screen, and participants were asked to read the words aloud. The list was presented twice in a different order. After each presentation, participants were asked to make a cued recall of (word) member of pairs. This category of nonassociative tasks (concerning the word pairs) is generally considered the primary indicator of declarative memory deficits.
Procedure
Participants went to the Douglas Hospital Research Centre for an administration of cognitive tests and questionnaires. Furthermore, participants were asked to collect saliva samples at home for the assessment of diurnal cortisol rhythms.
Statistical analyses
Descriptive statistics for the aging perceptions showed that when the four GPA items were summed, the total score ranged between 5 and 14 (mean = 9.575, SEM = 0.3376).
When scores for the four SPA items were added, the total score ranged from 4 to 16 (mean = 8.525, SEM = 0.4252).
In our regression analyses, we first assessed whether GPA and SPA increase with age using bivariate correlations between GPA and age, as well as SPA and age. Next, we executed one-way ANOVAs with our key variables to assess whether sex was a confounder. Our main regression analyses investigated whether increased negative perceptions of aging could predict increases in diurnal cortisol concentrations, geriatric depression symptoms, and memory complaints using five stepwise regressions.
Results
Preliminary analyses using Cronbach αs revealed medium internal consistency for GPA (α = 0.592) and strong internal consistency for SPA (α = 0.759).
Both GPA (r = 0.393, p = 0.012) and SPA (r = 0.494, p = 0.001) were correlated with age, showing that with increasing age, participants were more likely to believe as well as to internalize negative aging perceptions.
To rule out the possibility of sex differences, we ran one-way ANOVAs with sex as the between-subject variable and total GPA, SPA, geriatric depression, AM cortisol, PM cortisol, memory complaints, and the word pairs memory task. No significant sex effects were detected for general aging perceptions (p = 0.153), self-aging perceptions (p = 0.110), geriatric depression (p = 0.852), AM cortisol (p = 0.458), PM cortisol (p = 0.873), memory complaints (p = 0.852), and total unrelated (p = 0.056) and related (p = 0.178) word pairs on the explicit memory task.
To investigate whether increased negative perceptions of aging are associated with increases in diurnal cortisol levels, geriatric depression symptoms, and memory complaints, we computed five stepwise regressions. In this manner, predictor variables carrying negligible weight in explaining outcomes were automatically removed from the regression equations in a stepwise fashion to safeguard against multiple comparisons limited by low power. Total general perceptions and total SPA were entered as predictors for the following outcomes: (1) AM cortisol, (2) PM cortisol, (3) geriatric depressive symptoms, (4) memory complaints, and (5) memory impairments. We report the coefficient weights, r statistics, ANOVA outputs, and 95% confidence intervals (CI) for results significant at an α = 0.05 level.
For all of the following results, total general perceptions were automatically removed due to their lack of explanatory weight in the models: AM cortisol (p = 0.726), PM cortisol (p = 0.441), geriatric depressive symptoms (p = 0.468), memory complaints (p = 0.668), and memory impairments (related: p = 0.551; unrelated: p = 0.169).
Our first analysis revealed that total negative SPA did not significantly predict AM cortisol levels (p = 0.458) and did so only as a trend for PM cortisol levels (p = 0.052).
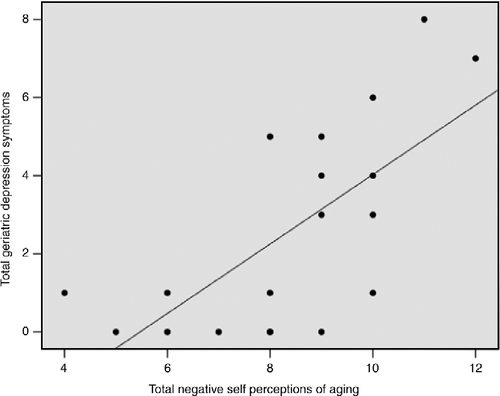
For self-reported geriatric depression, we found that increased total negative SPA (β = 0.691, t = 3.94, 95% CI: 0.413–1.37) significantly predicted increased depressive symptoms (r2 = 0.477, adjusted r2 = 0.446, F(1,17) = 15.51, p = 0.001), as shown in .
Figure 2. Regression scatter plot illustrating the significant association (p = 0.001) between total SPA measured by the aging perceptions questionnaire and total number of self-reported geriatric depression symptoms assessed by the GDS. The analysis consisted of 12 males and 10 females. No sex differences were detected.
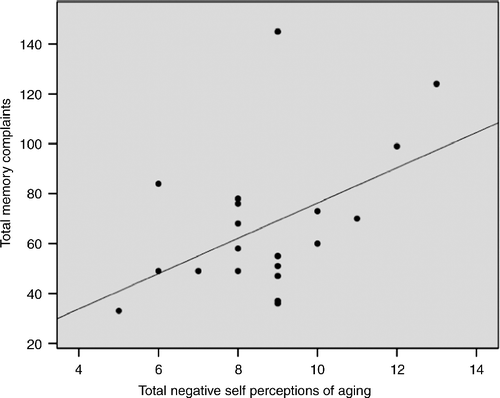
We found that increased negative total SPA (β = 0.478, t = 2.31, 95% CI: 0.629–13.51) significantly predicted increased number of self-reported memory complaints (r2 = 0.228, adjusted r2 = 0.185, F(1,19) = 5.32, p = 0.033) as shown in . For objective memory performance, negative total SPA were not associated with total related (p = 0.718) and total unrelated (p = 0.544) word pairs on the explicit memory task.
Figure 3. Regression scatter plot illustrating the significant association (p = 0.033) between total SPA measured by the aging perceptions questionnaire and total number of self-reported memory complaints assessed by the EMQ. The analysis consisted of nine males and 12 females. No sex differences were detected.
In sum, increases in total SPA, but not total general perceptions, were associated with increased geriatric depressive symptoms and memory complaints, but not to diurnal cortisol concentration nor to objective memory performance.
Discussion
The goal of this study was to assess the relationship between internalization of negative aging stereotypes and depressive symptomatology, cortisol concentration, and both objective and subjective cognitive performance in a group of healthy older adults. Our results show that older adults who internalize negative aging stereotypes present significantly higher scores on the GDS and report significantly more memory complaints. Concerning the statistical magnitude of these effects, total SPA explained approximately 25% of the variance for memory complaints, whereas it explained nearly 50% of the variance for geriatric depression. However, older adults who internalize negative aging stereotypes did not differ on measures of salivary cortisol concentration and objective memory performance.
The results of this study are in contrast with previous studies on aging showing that older adults presenting with both depressive symptomatology and subjective memory complaints demonstrate increased production of cortisol when compared with older adults with no depressive symptomatology or subjective complaints of memory deficits. Previous studies have shown that in people presenting depressive symptomatology, the diurnal cortisol cycle tends to show a flat pattern, in which cortisol concentration fails to sufficiently decrease throughout the day (Deuschle et al. Citation1997; Giordano et al. Citation2005). This study's negative results do not corroborate this literature. However, it has to be noted that although significantly associated with internalized aging stereotypes in this study, the GDS scores of participants were not in the high range. The scores of participants on the GDS scale ranged from 1 to 9, better revealing feeling of anhedonia than depression. The results that we observed on GDS scores in this study thus indicate that mild dysthymia in older adults is significantly associated with internalization of negative age stereotypes, although it may be insufficient to be significantly associated with increased production of cortisol.
It is quite unexpected to see that no significant associations were found between internalization of negative age stereotypes and objective performance on the declarative memory task. These results could be due to the time span needed for aging perceptions to negatively impact objective cognitive functioning, despite subjective complaints of memory decline. This implies that if memory performance had been measured years after the internalization of aging perceptions, we may then have detected the effects of aging perceptions on cognition in a time-lagged fashion.
Alternatively, it may be possible that the declarative memory task used in this study was not sensitive enough to capture memory deficits associated with internalization of negative age stereotypes. In this manner, internalizing negative aging perceptions might be more associated with perceptions of memory decline, even in the absence of real memory deficits. It is, therefore, important for future research studies to delineate the role personality traits (e.g. neuroticism and mastery) and other factors (e.g. quality of life, activities of daily living and social support) that may play a role in abating and maintaining negative aging perceptions.
The results of our study show that the older one gets, the more one agrees with common stereotypes related to aging. These consist of the following statements: “life becomes less enjoyable,” one becomes “less useful to society,” “memory declines,” and one becomes “more dependent on others.” That increased age in itself is a risk factor for higher rates of negative aging perceptions, and not necessarily any specific psychological or physical risk factor, is an important observation. This suggests that among aging older adults, prevalence rates of internalized negative aging perceptions can be quite high and increase over time. Our results emphasize a need for modifications of such debilitating beliefs.
In addition to the small sample size that may have increased the probability of a null finding, this study's limitations include the lack of a measure for positive aging perceptions, which would have allowed us to assess the roles of both positive and negative aging perceptions on different cognitive and health outcomes. Positive SPA have been associated with an increased life span of 7.5 years (Levy et al. Citation2002a). As well, although participants in this study were part of a longitudinal study of stress hormones and aging, we unfortunately did not assess aging stereotypes at further time points. Consequently, this did not allow us to assess change in aging stereotypes in this sample and its potential impact on depressive symptomatology, cortisol concentration, and subjective/objective memory impairments. Future longitudinal studies will more accurately explain the relationship between internalized negative age perceptions and associations with depressive symptoms and cortisol concentration in both older and young adults.
Another limitation is the reliance on self-reported provision of the time points at which cortisol samples were collected in participants' natural environments. Evidence suggests that poor compliance, deviation from sampling times, and unreliable documentation of sampling time may be common in studies on diurnal cortisol secretion (Kudielka et al. Citation2003). Despite the limitations of this study, the robust findings regarding the associations between internalized negative aging stereotypes, depressive symptoms, and memory complaints are important and require replication. Our results further support previous studies showing that aging self-perceptions are associated with psychological outcomes, but not physiological mediators (Levy et al. Citation2000; Levy Citation2003).
In conclusion, our results shed light on the functional importance of internalizing such negative stereotypes on aging. In addition to continued research, there is a need for interventions that modify these perceptions by decreasing the negative aging stereotypes and reinforcing positive aging perceptions. Such interventions can prove to be effective by targeting individuals' characteristics as well as those of their social environment (Levy and Langer Citation1994). As suggested by Ory et al. (Citation2003), there may be several methods to challenge aging stereotypes, which include public awareness campaigns, increasing awareness among health care providers, constructing opportunities for inter-generational networks, and adjusting the physical environment to better accommodate older adults. Given that negative aging stereotypes have been shown to be associated with depressive symptomatology, these types of interventions could serve to decrease the burden of depressive symptoms found in a significant proportion of older adults.
Declaration of interest: This study was funded by a grant (No. 134254) from the Canadian Institutes of Health Research to SJL. SJL holds a Senior Investigator Chair on Gender and Mental Health from the Canadian Institute of Gender and Health. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Allport GW. 1958. The nature of prejudice. Garden City, NY: Doubleday.
- Balardin JB, Vedana G, Luz C, Bromberg E. 2010. Subjective mild depressive symptoms are associated with abnormal diurnal cycle of salivary cortisol in elderly adults. J Geriatr Psychiatry Neurol. 33:143–151.
- Baranowska B, Wolinska-Witort E, Bik W, Baranowska-Bik A, Martynska L, Broczek K, Mossakowska M, Chmielowska M. 2007. Evaluation of neuroendocrine status in longevity. Neurobiol Aging. 28:774–783.
- Blazer DG. 2003. Depression in late life: Review and commentary. J Gerontol A- Biol. 58:249–265.
- Chahal HS, Drake WM. 2007. The endocrine system and ageing. J Pathol. 211:173–180.
- Deuschle M, Gotthardt U, Schweiger U, Weber B, Korner A, Schmider J, Standhardt H, Lammers CH, Heuser I. 1997. With aging in humans the activity of the hypothalamus–pituitary–adrenal system increases and its diurnal amplitude flattens. Life Sci. 61:2239–2246.
- Deuschle M, Weber B, Colla M, Depner M, Heuser I. 1998. Effects of major depression, aging and gender upon calculated diurnal free plasma cortisol concentrations: A re-evaluation study. Stress. 2:281–287.
- Dori D, Casale G, Solerte SB, Fioravanti M, Migliorati G, Cuzzoni G, Ferrari E. 1994. Chrono-neuroendocrinological aspects of physiological aging and senile dementia. Chronobiologia. 21:121–126.
- Drafta D, Schindler AE, Stroe E, Neacsu E. 1982. Age-related changes of plasma steroids in normal adult males. J Steroid Biochem. 17:683–687.
- Edwards S, Clow A, Evans P, Hucklebridge F. 2001. Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sci. 68:2093–2103.
- Fiocco AJ, Wan N, Weekes N, Pim H, Lupien SJ. 2006. Diurnal cycle of salivary cortisol in older adult men and women with subjective complaints of memory deficits and/or depressive symptoms: Relation to cognitive functioning. Stress. 9:143–152.
- Fries E, Dettenborn L, Kirschbaum C. 2009. The cortisol awakening response (CAR): Facts and future directions. Int J Psychophysiol. 72:67–73.
- Gallo JJ, Rabins PV, Lyketsos CG, Tien AY, Anthony JC. 1997. Depression without sadness: Functional outcomes of nondysphoric depression in later life. J Am Geriatr Soc. 45:570–578.
- Geerlings MI, Schoevers RA, Beekman AT, Jonker C, Deeg DJ, Schmand B, Ader HJ, Bouter LM, Van Tilburg W. 2000. Depression and risk of cognitive decline and Alzheimer's disease. Results of two prospective community-based studies in The Netherlands. Br J Psychiatry. 176:568–575.
- Giordano R, Bo M, Pellegrino M, Vezzari M, Baldi M, Picu A, Balbo M, Bonelli L, Migliaretti G, Ghigo E, Arvat E. 2005. Hypothalamus–pituitary–adrenal hyperactivity in human aging is partially refractory to stimulation by mineralocorticoid receptor blockade. J Clin Endocr Metab. 90:5656–5662.
- Hess TM, Auman C, Colcombe SJ, Rahhal TA. 2003. The impact of stereotype threat on age differences in memory performance. J Gerontol B Psychol Sci Soc Sci. 58:P3–11.
- Holsboer F. 2001. Stress, hypercortisolism and corticosteroid receptors in depression: Implications for therapy. J Affect Disord. 62:77–91.
- Ice GH, Katz-Stein A, Himes J, Kane RL. 2004. Diurnal cycles of salivary cortisol in older adults. Psychoneuroendocrino. 29:355–370.
- Jonker C, Geerlings MI, Schmand B. 2000. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry. 15:983–991.
- Jorm AF. 2000. Is depression a risk factor for dementia or cognitive decline? A review. Gerontology. 46:219–227.
- Kalmijn S, Launer LJ, Stolk RP, de Jong FH, Pols HA, Hofman A, Breteler MM, Lamberts SW. 1998. A prospective study on cortisol, dehydroepiandrosterone sulfate, and cognitive function in the elderly. J Clin Endocrinol Metab. 83:3487–3492.
- Kudielka BM, Broderick JE, Kirschbaum C. 2003. Compliance with saliva sampling protocols: Electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosom Med. 65:313–319.
- Lawton MP. 1975. Philadelphia Geriatric Center Morale Scale – revision. J Gerontol. 30:85–89.
- Levy B. 1996. Improving memory in old age through implicit self-stereotyping. J Pers Soc Psychol. 71:1092–1107.
- Levy BR. 2003. Mind matters: Cognitive and physical effects of aging self-stereotypes. J Gerontol B Psychol Sci Soc Sci. 58:P203–P211.
- Levy BR. 2008. Rigidity as a predictor of older persons' aging stereotypes and aging self-perceptions. Soc Behav Personal. 36:559–570.
- Levy B, Langer E. 1994. Aging free from negative stereotypes – successful memory in China and among the American deaf. J Pers Soc Psychol. 66:989–997.
- Levy BR, Hausdorff JM, Hencke R, Wei JY. 2000. Reducing cardiovascular stress with positive self-stereotypes of aging. J Gerontol B Psychol Sci Soc Sci. 55:205–213.
- Levy BR, Slade MD, Kasl SV. Longitudinal benefit of positive self-perceptions of aging on functional health. J Gerontol B-Psychol. 2002a; 57:P409–P417.
- Levy BR, Slade MD, Kunkel SR, Kasl SV. Longevity increased by positive self-perceptions of aging. J Pers Soc Psychol. 2002b; 83:261–270.
- Liang J, Bollen KA. 1983. The Structure of the Philadelphia Geriatric Center Morale Scale – a reinterpretation. J Gerontol. 38:181–189.
- Lupien S, Lecours AR, Lussier I, Schwartz G, Nair NP, Meaney MJ. 1994. Basal cortisol levels and cognitive deficits in human aging. J Neurosci. 14:2893–2903.
- Lupien S, Lecours AR, Schwartz G, Sharma S, Hauger RL, Meaney MJ, Nair NPV. 1996. Longitudinal study of basal cortisol levels in healthy elderly subjects: Evidence for subgroups. Neurobiol Aging. 17:95–105.
- Lupien SJ, Gaudreau S, Tchiteya BM, Maheu F, Sharma S, Nair NP, Hauger RL, McEwen BS, Meaney MJ. 1997. Stress-induced declarative memory impairment in healthy elderly subjects: Relationship to cortisol reactivity. J Clin Endocrinol Metab. 82:2070–2075.
- Lupien S, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ. 1998. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1:69–73.
- Lupien SJ, Schwartz G, Ng YK, Fiocco A, Wan N, Pruessner JC, Meaney MJ, Nair NP. 2005. The Douglas Hospital Longitudinal Study of normal and pathological aging: Summary of findings. J Psychiatry Neurosci. 30:328–334.
- Lyons J. 1976. Semantics. Cambridge: Cambridge University PressVol. 12.
- Ory M, Kinney Hoffman M, Hawkins M, Sanner B, Mockenhaupt R. 2003. Challenging aging stereotypes: Strategies for creating a more active society. Am J Prev Med. 25:164–171.
- Palmore EB. 1999. Ageism: Negative and positive. New York: Springer Publishers.
- Raff H, Raff JL, Duthie EH, Wilson CR, Sasse EA, Rudman I, Mattson D. 1999. Elevated salivary cortisol in the evening in healthy elderly men and women: Correlation with bone mineral density. J Gerontol A Biol Sci Med Sci. 54:M479–M483.
- Rowe JW, Kahn RL. 1998. Successful aging. Aging (Milano). 10:142–144.
- Sharma M, Palacios-Bois J, Schwartz G, Iskandar H, Thakur M, Quirion R, Nair NP. 1989. Circadian rhythms of melatonin and cortisol in aging. Biol Psychiatry. 25:305–319.
- Sherman B, Wysham C, Pfohl B. 1985. Age-related changes in the circadian rhythm of plasma cortisol in man. J Clin Endocrinol Metab. 61:439–443.
- Sunderland A, Harris JE, Baddeley AD. 1983. Do laboratory tests predict everyday memory – a neuropsychological study. J Verb Learn Verb Be. 22:341–357.
- Touitou Y, Sulon J, Bogdan A, Touitou C, Reinberg A, Beck H, Sodoyez JC, Demey-Ponsart E, Van Cauwenberge H. 1982. Adrenal circadian system in young and elderly human subjects: A comparative study. J Endocrinol. 93:201–210.
- Van Cauter E, Leproult R, Kupfer DJ. 1996. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocr Metab. 81:2468–2473.
- Van Cauter E, Leproult R, Plat L. 2000. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. J Am Med Assoc. 284:861–868.
- Waltman C, Blackman MR, Chrousos GP, Riemann C, Harman SM. 1991. Spontaneous and glucocorticoid-inhibited adrenocorticotropic hormone and cortisol secretion are similar in healthy young and old men. J Clin Endocr Metab. 73:495–502.
- Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. 1971. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab. 33:14–22.
- Wilson RS, Bienias JL, Mendes de Leon CF, Evans DA, Bennett DA. 2003. Negative affect and mortality in older persons. Am J Epidemiol. 158:827–835.
- Wolf OT, Dziobek I, McHugh P, Sweat V, de Leon MJ, Javier E, Convit A. 2005. Subjective memory complaints in aging are associated with elevated cortisol levels. Neurobiol Aging. 26:1357–1363.
- Wust S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. 2000. The cortisol awakening response – normal values and confounds. Noise Health. 2:79–88.
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. 1983. Development and validation of a geriatric depression screening scale – a preliminary-report. J Psychiat Res. 17:37–49.
- Young AH. 2004. Cortisol in mood disorders. Stress. 7:205–208.