Abstract
We used 18F-fluorodeoxyglucose small-animal positron-emission tomography to determine whether different styles of coping with stress are associated with different patterns of neuronal activity in the hypothalamus. Adult rats were subjected to immobilization (IMO)-stress or to a non-immobilized condition for 30 min, in random order on separate days, each of which was followed by brain-scanning. Some rats in the immobilized condition were allowed to actively cope with the stress by chewing a wooden stick during IMO, while the other immobilized rats were given nothing to chew on. Voxel-based statistical analysis of the brain imaging data shows that chewing counteracted the stress-induced increased glucose uptake in the hypothalamus to the level of the non-immobilized condition. Region-of-interest analysis of the glucose uptake values further showed that chewing significantly suppressed stress-induced increased glucose uptake in the paraventricular hypothalamic nucleus and the anterior hypothalamic area but not in the lateral hypothalamus. Together with the finding that the mean plasma corticosterone concentration at the termination of the IMO was also significantly suppressed when rats had an opportunity to chew a wooden stick, our results showed that active coping by chewing inhibited the activation of the hypothalamic–pituitary–adrenal axis to reduce the endocrine stress response.
Introduction
Receiving sensory information about physical and psychological stressors, the hypothalamus plays a pivotal role in regulating autonomic and endocrine responses to stress. It is well accepted that the style of coping with a stressor affects the activity of hypothalamic neurons as well as the physiological response to the stress. Chewing is one active strategy for coping with stress in both humans and rodents, and it prevents both the pressor response and the stress-induced secretion of adrenocorticotropic hormone (ACTH; Ono et al. Citation2010). We have previously shown that chewing during exposure to a stressor significantly attenuates stress-increased neuronal activation in the paraventricular hypothalamic nucleus (PVN), using immunohistochemical markers of neuronal activation (Hori et al. Citation2004, Citation2005; Sasaguri et al. Citation2005). As different parts of the hypothalamus carry out different autonomic functions to maintain homeostasis (Armstrong Citation2004; Simerly Citation2004), a remaining unsolved question is how chewing modifies stress-induced neuronal activity in other parts of the hypothalamus in an animal under stress. Further investigation of the interactions within these hypothalamic areas and their influence on other regions of the brain will elucidate the causal relationship between chewing behavior and stress relief.
Functional brain imaging has recently been used to study brain areas involved in the stress response. Sung et al. (Citation2009) carried out experiments with the 18F-fluorodeoxyglucose micro-positron-emission tomography (FDG-PET) and reported that acute stress increased glucose uptake in the hypothalamus and other brain regions that process stress responses. A limitation of FDG-PET imaging in rodents has been that the spatial resolution is too low to provide adequate detail about brain structures. However, a recently developed small-animal PET system has improved spatial resolution to approximately 1.26 mm (Wang et al. Citation2006). This improvement makes possible the detection of changes in glucose metabolism rate within subregions of the hypothalamus.
The aim of the present study was to use statistical parametrical mapping (SPM) by FDG-PET to test the hypothesis that chewing could be an active method of coping with stress that alters stress-increased glucose uptake in the hypothalamus. We carried out voxel-based and region-of-interest (ROI)-based statistical analyses of glucose uptake values to investigate how active coping modifies neuronal responses in different subregions within the hypothalamus. In addition, we measured plasma concentrations of corticosterone to confirm that changes in glucose uptake in the particular subregions in the hypothalamus caused by chewing were paralleled by changes in the endocrine stress responses.
Materials and methods
Animals
We kept 10-week-old male Sprague-Dawley rats (Lasco, Inc., Taipei, Taiwan) in pairs per cage in a temperature- and humidity-controlled room (23 ± 2°C and 55 ± 5%) with a 12 h light/dark cycle (lights on at 07:00 h). The rats had free access to water and food except during the 12-h period before the PET scan, during which they were deprived of food to enhance FDG uptake in the brain (Fueger et al. Citation2006). This study was approved by the institutional review board and conformed to the guidelines for care and use of laboratory Animals of the National Institutes of Health.
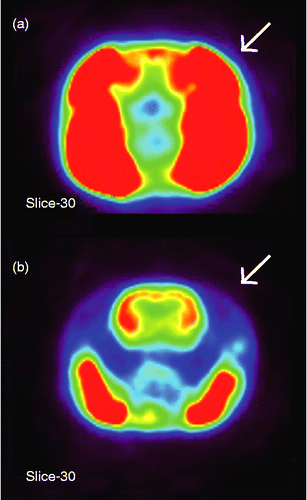
A preliminary experiment showed that chewing during immobilization (IMO) facilitates FDG uptake in the temporalis muscle, interfering with the characterization of the distribution of FDG uptake inside the brain (). Therefore, we bilaterally paralyzed the temporalis muscle of all rats by injecting botulinum toxin type A (0.5 unit dissolved in 10 μl saline for each side; BOTOX®; Allergan, Inc., Irvine, CA, USA) into the temporalis muscle to minimize this disturbance (). Each rat received a single injection of botulinum toxin 3–5 days before the initial PET scan, which allowed the toxin to completely diffuse into the muscle (Sakamoto et al. Citation2009). Even though the temporalis muscle was paralyzed in these rats, none of the rats had difficulty eating. All were able to chew a wooden stick during IMO using the masseter muscle, the major jaw-closing muscle (Thomas and Peyton Citation1983).
Figure 1. Representative transverse slices showing glucose uptake in the head of rats that chewed a wooden stick during IMO stress (a) without or (b) with the pretreatment injection of botulinum toxin type A into the temporalis muscle (indicated by arrows to the right side). Intensity of SUV is shown in a pseudocolor scaling using MIPAV software package (red-to-blue indicates high-to-low accumulation of glucose, respectively). Note the clear picture of the brain of the chewing rat after blocking of the temporalis muscle in (b).
Stress protocol and FDG-PET scan
Six rats experienced three PET scans under different experimental conditions in a randomized and counterbalanced order in a Latin square design with an inter-scan interval of at least 48 h, which represented the time necessary for the plasma stress hormone concentrations to return to the resting level (Ono et al. Citation2008). At the beginning of each scan, each rat received 2.5 mCi of FDG, a functional marker of metabolic activity, by injection into a tail vein. Immediately after the injection, each rat was assigned to one of the following three conditions for 30 min: (1) IMO (stressed; condition ST), (2) IMO with a wooden stick to chew (stressed and chewing; condition SC), or (3) no IMO (control treatment; condition CT). To produce IMO stress in conditions ST and SC, all four limbs of the rat were fixed with tape to a wooden board in a spread-eagle supine position. A wooden stick (diameter, 5 mm) was placed near the mouth of each rat in condition SC during IMO. All rats responded to the wooden stick with a rapid and repetitive sequence of jaw opening and closing movements for almost the whole period of IMO as reported previously (Ono et al. Citation2009), and consequently spent less time struggling compared with those in the ST condition. After 30 min of IMO, rats were immediately anesthetized by isoflurane inhalation (5% in 100% oxygen; IsoFlo; Abbott Laboratories, North Chicago, IL, USA) while they remained fastened to the board. The rats in the CT condition were kept undisturbed for 30 min in Plexiglas cages after FDG was administered. At the end of the 30 min, the same mixture of isoflurane and oxygen was used to anesthetize these rats. After reaching a deep state of anesthesia, the rats were placed into a PET scanner and continuously maintained with inhalation anesthesia (2% isoflurane in 100% oxygen) until the end of the scan. All experiments were carried out between 10:00 and 15:00 h.
FDG uptake in the brain was measured using an eXplore Vista Dual-Ring Small-Animal PET scanner (GE Healthcare, Waukesha, WI, USA) with average full width at half maximum resolution of 1.26 mm (Wang et al. Citation2006). The images were first anatomically standardized to achieve symmetrical midline alignment. Further improvements in the resolution and sensitivity of image processing were achieved by the 3D ordered subsets expectation maximization reconstruction algorithm. Nominal voxel size was 0.3875 × 0.3875 × 0.775 mm. The first two lengths were in transverse directions and the last was in the axial direction. Each of the 61 transverse slices in the reconstructed images contained 175 × 175 voxels. Standardized uptake values (SUVs) were calculated at each voxel in the reconstructed images as an index of FDG accumulation as follows:where FDGvoxel is the decay-corrected regional radiotracer concentration in becquerels per milliliter, FDGdose is the injected radiotracer dose in becquerels, and WT is the body weight in kilograms.
After completing all PET scans, we scanned the anatomical structure of each rat brain using a 7 T magnetic resonance (MR) imaging scanner (BioSpec 70/30 USR; Bruker, Ettlingen, Germany). We used a high-performance gradient coil mounted on the table of the scanner, whose inner diameter and maximal gradient strength were 6 cm and 1000 mTm− 1, respectively, and a quadrature coil with inner diameter of 3.5 cm for RF transmission and reception. We used a multi-slice spin echo sequence to obtain T2-weighted images covering the whole brain with the following parameters: TR/TE 2500/33 ms; slice thickness, 0.75 mm; FOV, 25 × 25 × 22.5 mm; matrix, 256 × 256 × 30; voxel dimensions, 0.098 × 0.098 × 0.75 mm.
Plasma corticosterone
We measured plasma corticosterone concentration in another group of rats to confirm the stress-relieving effect of chewing. Eight rats were treated with botulinum toxin to paralyze the temporalis muscle and were assigned to either the ST or SC condition (n = 4 each). The protocols for the administration of botulinum toxin and the stress exposure were exactly the same as those in the PET experiment. We collected blood from the tail vein of each rat at three time points, 24 h before IMO, immediately after IMO, and 1 h after IMO, using 10% volume of 0.1 M sodium citrate (Sigma-Aldrich, St. Louis, MO, USA) as an anticoagulant. Rats were anesthetized at the time of blood sampling in the same way as in the PET experiment. We centrifuged the blood, collected the plasma, and assayed the corticosterone concentration using the AssayMax Corticosterone ELISA Kit (Assaypro, St. Charles, MO, USA). The sensitivity and the intra-assay coefficient of variation of the kit were 40 pg/ml and 5.0%, respectively.
Co-registration of FDG-PET images
We used SPM software (SPM5; Friston et al. Citation1995) to co-register each individual FDG-PET image to its corresponding T2 MR image. To extract the brain regions within the FDG-PET images, we applied a mask image, which we manually generated from an individual T2 MR image using the Medical Image Processing, Analysis, and Visualization (MIPAV) software (McAuliffe et al. Citation2001), to the co-registered FDG-PET image. FDG-PET images from all rats were co-registered to the stereotaxic space (Paxinos and Watson Citation2007) with reference to their individual T2 MR image and the T2 template image of the rat brain (Schweinhardt et al. Citation2003) and re-sliced with trilinear interpolation (0.2 × 0.2 × 0.2 mm).
ROI analysis
We chose 11 subregions of the hypothalamus for ROI analysis according to the criterion that the volume of each subregion should exceed at least one original voxel size (a region consisting of more than two and four voxels in the transverse and axial directions, respectively, in the re-sliced FDG-PET image). The selected subregions were PVN, subparaventricular zone of the hypothalamus (SPa), anterior hypothalamic area (AH), hypothalamic arcuate nucleus (Arc), juxtaparaventricular part of lateral hypothalamus (JPLH), peduncular part of lateral hypothalamus (PLH), tuberal region of lateral hypothalamus (TuLH), perifornical part of lateral hypothalamus (PeFLH), ventromedial hypothalamic nucleus (VMH), dorsomedial hypothalamic nucleus (DMH), and posterior hypothalamic area (PH). All bilateral subregions except the PVN and Arc, which included voxels on the midline, were separately analyzed. We determined the coordinates of the subregions in the stereotaxic space according to the anatomical atlas of the rat brain (Paxinos and Watson Citation2007). SUVs in each subregion were summed and normalized by total sum of SUVs in the whole brain.
Statistical analysis
We used SPM to carry out voxel-based SPM of PET images based on a full-factorial general linear model with random effects analysis. Global changes in the SUV were removed using proportional normalization and grand mean scaling. We considered P values < 0.01 (uncorrected) to be statistically significant. The resulting areas of activation were characterized in terms of statistical significance between conditions and spatial extent of more than 30 contiguous voxels. T-value maps of results were superimposed on transverse views of a representative MR image to define voxels that showed significant change.
We used one-way analysis of variance (ANOVA) and post hoc Tukey's multiple comparison with Bonferroni correction to determine the group differences for plasma corticosterone concentration and the normalized SUVs. To compare the results of ROI-based and voxel-based statistical analysis, the same statistical analysis was applied to the SUVs cumulated within the ROI and to those at each voxel in the ROIs. Values are expressed as group mean ± SE of the mean. We considered P values < 0.05 to be statistically significant.
Results
Active coping reduced the corticosterone response to stress exposure
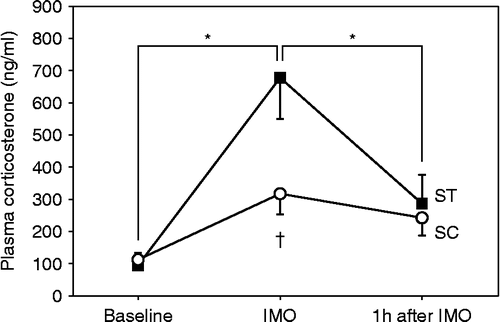
A one-way ANOVA showed a significant group difference in the time-course of plasma corticosterone concentration (F(2,24) = 3.805; P = 0.042; ). IMO stress without coping by chewing the wooden stick significantly increased the plasma corticosterone concentration to more than sevenfold than before stress exposure. However, the plasma corticosterone concentration remained at baseline concentration when rats had an opportunity to cope with stress by chewing a wooden stick. The plasma corticosterone concentration was significantly higher for the ST condition than for the SC condition immediately after IMO (P = 0.003). The plasma corticosterone concentration for both conditions returned to the baseline concentration by 1 h after termination of the IMO stress. These results indicate that chewing during IMO was effective in producing active coping to reduce stress even though the temporalis muscle was paralyzed.
Figure 2. Time-course changes of plasma corticosterone concentration in the rats that were immobilized with (SC) and without (ST) chewing (n = 4 each). Blood was collected from the tail vein three times at 24 h before IMO (Baseline), immediately after IMO for 30 min, and 1 h after the end of IMO. Asterisk indicates a statistically significant difference (P < 0.05) for values obtained at different points in time for the same condition. Dagger indicates a statistically significant difference (P < 0.05) between values obtained for two different conditions at corresponding points in time. The one-way ANOVA test and the post hoc Tukey's multiple comparison with Bonferroni correction were used to determine significant differences.
Active coping prevented stress-induced glucose uptake in the hypothalamus and increased glucose uptake in selected brain areas
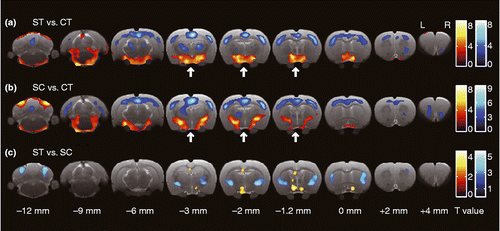
compares glucose metabolism for the three experimental conditions. IMO stress without coping activated the hypothalamus and preoptic area and deactivated the cortical areas and the thalamus (). When rats coped with stress by chewing, stress-increased glucose uptake was prevented in the hypothalamus, while the cortical area was deactivated, as was observed for rats that were not allowed to actively cope with stress (). Comparing the results of the ST condition to those of the SC condition shows that active coping increased glucose uptake in the striatum, globus pallidus, sensorimotor cortices, dorsal endopiriform nucleus, central amygdala, ventroposterior medial nuclei of the hypothalamus, posterior nuclei of the thalamus, anterior insula, and cerebellum crus 1/2 while active coping decreased glucose uptake in the anterior hypothalamus, preoptic area, and dorsal hippocampus ().
Figure 3. Transverse slices shown from caudal (left) to rostral (right), indicating brain area with significant difference for the relative value of glucose uptake for each pair of conditions. Red and blue color indicate significantly increased and decreased glucose uptake in the former condition compared with that in the latter one, respectively (P values < 0.01; uncorrected). The statistical differences were determined by SPM using a general linear-model approach with proportional normalization and grand mean scaling. Bregma was set to the point of origin in the caudal-rostral axis according to the anatomical atlas of the rat brain (Paxinos and Watson Citation2007). Arrows indicate the position of the hypothalamus. CT, control (no IMO); ST, rats under IMO without chewing; SC, rats under IMO with chewing; n = 6 each.
Active coping suppressed stress-induced glucose uptake in the paraventricular and AH nuclei but not in the lateral hypothalamic nucleus
The ROI analysis showed that the distribution of glucose uptake was different for each of the subregions of the hypothalamus and that it depended on the coping performance (). IMO stress without coping induced significant glucose uptake in all the subregions of the hypothalamus except the PH. By contrast, active coping prevented stress-induced glucose uptake in most of the subregions. The only areas in which it did not prevent significant glucose uptake were PLH and TuLH. Active coping significantly reduced glucose uptake in the PVN, in the right PeFLH, and in the SPa and AH (bilateral).
Table I. Ratio of glucose uptake in subregions of the hypothalamus to total glucose uptake in the brain (10− 2%).
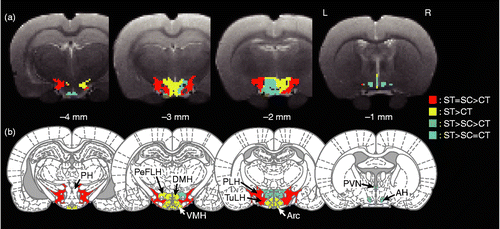
shows mapping of the relative amount of glucose uptake for the three conditions. These relationships were determined by both voxel-based and ROI-based statistical comparisons of SUVs. The results of the voxel-based statistical comparison indicated differential responses to active coping in the medial and the lateral hypothalamic structures (). The ROI-based comparison further classified the hypothalamic subregions into four different groups based on the effect of active coping on the stress-induced neuronal response (). First, in the PVN, SPa, AH, and right PeFLH, active coping with stress completely suppressed the stress-increased glucose metabolism to the control level (glucose uptake: ST>SC = CT, cyan colored region in ). Second, in the PLH and TuLH, active coping had no effect of suppressing stress-increased glucose metabolism. IMO stress significantly increased glucose uptake in these subregions regardless of the coping style (ST = SC>CT, red colored region in ). Third, in the Arc, JPLH, VMH, DMH, and left PeFLH, active coping tended to suppress stress-increased glucose metabolism but the ameliorative effect of active coping did not reach statistical significance between the coping styles (ST>CT, yellow colored region in ). Fourth, stress exposure did not alter glucose metabolism in the PH.
Figure 4. Transverse slices shown from caudal (left) to rostral (right), indicating the difference in distribution of relative glucose uptake in the hypothalamus for the three experimental conditions: (a) voxel-based and (b) ROI-based statistical comparisons. CT, control (no IMO); ST, rats under IMO without chewing; SC, rats under IMO with chewing; n = 6 each. AH, anterior hypothalamic area; Arc, arcuate hypothalamic nucleus; DMH, dorsomedial hypothalamic nucleus; PeFLH, perifornical part of lateral hypothalamus; PH, posterior hypothalamic area; PLH, posterior lateral hypothalamus; PVN, paraventricular nucleus; TuLH, tuberal region of lateral hypothalamus; VMH, ventromedial hypothalamic nucleus. Red: significant increase in ST and SC compared with CT. Yellow, significant increase in ST compared with CT; Green, significant difference between each pair of conditions, where ST is the highest and followed by SC and CT; Cyan, significant increase in ST compared with SC and CT. One-way ANOVA and post hoc Tukey's multiple comparison with Bonferroni correction were used to determine statistical differences.
Effect of paralysis of the temporalis muscle and the order of the three conditions on glucose metabolism in the brain
Although paralyzing the temporalis muscle was essential to accurately characterize the distribution of FDG uptake inside the brain, it must be acknowledged that this treatment may cause moderate chronic stress due to occlusal hypofunction (Onozuka et al. Citation2002). We, therefore, compared the distribution of glucose uptake determined in the current study with that in the preliminary study, in which another group of rats of the same strain, age, and sex were treated in exactly the same way except that we did not paralyze the temporalis muscle. Paralysis of the temporalis muscle significantly decreased glucose uptake in the CT condition in the barrel field of the somatosensory cortex, motor cortex, and motor-related subcortical areas while it increased glucose uptake in the thalamus, PVN, hippocampus, and dorsal raphe nucleus (). However, the change in glucose uptake was almost absent when compared with the ST condition.
Table II. Effect of paralysis of the temporalis muscle on the regional glucose uptake in the brain.
In order to assess the effect of prior experience of stress on brain function, we also compared the glucose uptake in the brains of rats that experienced the condition in their first scan with those in their third scan. Although many regions of the brain responded differently to the same treatment as rats that experienced different conditions, prior experience of stress did not alter the glucose metabolism in the medial hypothalamic regions in any condition ().
Table III. Effect of order of three experimental conditions on the regional glucose uptake in the brain.
Discussion
Using FDG-PET, we found that chewing during IMO stress prevented the increase in glucose uptake in the PVN and AH of the hypothalamus in the rat. The suppressed activation in the PVN, the brain region that releases corticotropin-releasing factor to induce corticotropin (ACTH) secretion from the anterior pituitary, and consequently corticosterone release from the adrenal cortex, corresponded well with the reduced plasma corticosterone concentration in the chewing rats that actively coped with stress. The present study demonstrated that FDG-PET can be used to investigate local changes in brain activity caused by different behavioral strategies in rats.
Our experiment confirms previous research showing that IMO stress increases glucose uptake in the hypothalamus and decreases it in the cortex (Sung et al. Citation2009). The new finding of the current study was that the voxel-based SPM of FDG-PET images makes it possible to detect changes in glucose metabolism within the subregions of the hypothalamus that depend on how a rat copes with stress (). Moreover, the shape of the regions distinguished by statistical significance for the three conditions in the voxel-based analysis showed a close match with the results in ROI analysis with known structures of the anatomical segmentation ().
The voxel-based SPM indicated that the most significant global change took place in the hypothalamus (, indicated by arrows). The following ROI analysis indicated that IMO stress increased glucose uptake in almost all subregions within the hypothalamus, confirming the previous studies of Briski and Gillen (Citation2001) in which they used Fos-immunoreactivity to investigate the neuronal response to IMO. The relative amount of change in glucose uptake, ranging from 3 to 20% in the ROIs showing statistically significant change between conditions, accorded well with the focal glucose consumption value upon sensory stimulation from rest in awake animals (Shulman et al. Citation1999; Shulman Citation2001). Surprisingly, active coping with stress significantly suppressed the glucose metabolism in only a few of these subregions (). These results indicate that the PVN (including the adjacent area SPa) and AH play an essential role in determining stress responses and plasma corticosterone concentration. Activation of the PVN is necessary to trigger the activation of the hypothalamic–pituitary–adrenal (HPA) axis, which is a major neuroendocrine response to acute stress. Since the PVN and AH collectively regulate the autonomic as well as he endocrine stress responses (Shintani et al. Citation1995; Jezova et al. Citation1998; Kubo et al. Citation2001; Busnardo et al. Citation2010), simultaneous suppression of these regions found in the current study further confirmed our previous finding about the suppressive effect of active coping on plasma ACTH (Ono et al. Citation2008) and pressor responses (Okada et al. Citation2007).
IMO stress similarly increased glucose uptake bilaterally in the PLH and TuLH of rats in both ST and SC conditions compared with the CT condition. The lateral hypothalamus contains peptidergic neurons expressing orexin/hypocretin, which maintain feeding and drinking behavior, the sleep–wake cycle, and the fight-or-flight response (Bonnavion and de Lecea Citation2010). Some forms of stress causing increased arousal and attention to the surrounding environment, such as contextual fear (Furlong et al. Citation2009) and IMO (Ida et al. Citation2000), have been shown to activate orexinergic neurons in the lateral hypothalamus. Since immobilized rats vigorously struggled regardless of the coping style when they were fixed to the board, the comparable increase in glucose uptake in the PLH and TuLH may indicate increased activity of orexin neurons, reflecting an IMO-induced state of high alert.
There were no obvious differences between the amount of glucose metabolism in the PH recorded for each of the three conditions in the current study. Krukoff and Khalili (Citation1997) showed that both moderate (exposure to novel environment) and severe (restraint) stress activate nitric oxide-producing neurons in the PH but the number of activated neurons was comparable regardless of the severity of stress. In the current study, we handled all the rats without anesthesia at the beginning of every measurement to inject the tracer, and even in the CT condition we left them in a novel cage until the PET scan. These moderate stimuli possibly activated the PH, resulting in similar amounts of glucose uptake in the rats of all conditions tested.
The high baseline concentration of plasma corticosterone () may reflect the moderate stress that accompanies the paralysis of the temporal muscle, as this significantly increased glucose uptake in the stress-responding regions such as the PVN and the hippocampus in the CT condition (). However, the differences according to this treatment were no longer significant when they were compared with the ST condition, indicating that the intensity of stress from the treatment was smaller than that from IMO. Together with the copious increase in the plasma corticosterone concentration, IMO is a strong enough stressor to induce further activation of the HPA axis, which is acceptable for studying the effect of active coping with stress.
It is intriguing to note that the previous immunohistochemistry study using immobilized rats showed increased c-fos gene expression in some cortical areas (Chowdhury et al. Citation2000) in which we found significant reductions in glucose uptake (,b). Immunohistochemistry using immediate early genes or gene products is a useful cellular marker of neuronal activation (Hoffman and Lyo Citation2002). Although most of the brain regions show comparable changes in metabolic glucose uptake and the expression of early genes in response to stress stimuli (Duncan et al. Citation1993), a number of previous studies have demonstrated that the neuroanatomical patterns of activation observed in these two modalities are not identical (Eells et al. Citation2000; Cochran et al. Citation2002). Considering that glucose metabolism measurement reflects net changes in neuronal activity in a particular region while expression of immediate early genes detects increased activation of specific sub-populations of cells, increases in gene expression with simultaneous reductions in glucose uptake can occur in a situation such that increased activity in the GABAergic interneurons suppresses the overall activity of the pyramidal neurons. Being a measure of cumulative glucose uptake from administering the tracer up to the time of the scan, FDG-PET provides complementary and additional information to elucidate neuronal circuits involved in neuronal and neuroendocrine functions. Moreover, SPM-based statistical analysis of FDG-PET images using a brain template makes it possible to quantitatively compare data from different study groups, which is often difficult in studies using immunohistochemistry in which the number of immunoreactive neurons varies widely depending on the experimental protocol (Dragunow and Faull Citation1989).
Another advantage of using FDG-PET is its non-invasive nature so that repetitive measurements can be made, as clearly demonstrated in the present study. This is particularly important in in vivo measurement of neuronal activity, especially for studies investigating long-term changes of neuronal responses such as those that focus on chronic stress. We utilized this advantage to determine the effect of prior experience of stress, by comparing the glucose uptake in the brains of rats that experienced the same task with or without previous experience of IMO and PET scan (). clearly indicates the effects of prior experience on glucose metabolism in various brain areas, which were counterbalanced in the analysis including all subjects regardless of the order of conditions. These results also showed that the glucose metabolism in the main stress-regulatory region of the PVN was not habituated through the three scans, confirming that each condition was perceived as a different stressor (Melia et al. Citation1994).
Active coping significantly reduced glucose uptake not only in the preoptic area and the dorsal hippocampus but also in the hypothalamus (). Since the preoptic area is a part of the basal forebrain that responds to stressful stimuli (Martinez et al. Citation1998), the suppressed response in the preoptic area reflects the ameliorative effect of active coping with stress. The hippocampus mediates the negative feedback regulation of the HPA axis via activation of glucocorticoid receptors. The reduced plasma corticosterone associated with active coping is likely to suppress neuronal activity in the hippocampus. By contrast, active coping significantly increased glucose uptake in the striatum, sensorimotor cortex, central nucleus of the amygdala (CeA), thalamus, and cerebellum crus 1/2 (). In these regions, increased activation in the striatum, somatosensory cortex, thalamus and cerebellum may affect motor control, kinesthesia, and other chewing-related neuronal activity. The CeA determines the strategy by which an animal copes with stress, and it regulates autonomic, neuroendocrine, and behavioral responses. Animals showing different coping styles have correspondingly different response patterns of neurotransmitters and humoral factors to stress stimuli in the CeA (Roozendaal et al. Citation1997; Wiersma et al. Citation1998; Morilak et al. Citation2003; Ebner et al. Citation2005). Our current result further confirms the observation of Veenema and Neumann (Citation2007) that a display of aggression, such as chewing or gnawing, is associated with high neuronal activation in the CeA. Because the CeA indirectly projects to the PVN via the anterior and lateral subdivisions of the bed nucleus of the stria terminalis (BST; Dong et al. Citation2001; Jankord and Herman Citation2008) and because lesions of these areas in the BST modify stress-induced responses of the HPA axis (Choi et al. Citation2007), chewing-induced activation of the CeA is one of the possible neuronal pathways that accounts for the suppressed activation of the PVN and reduced endocrine stress responses observed in the current study.
There remains an anatomical uncertainty that is relevant to the current study. The relatively large voxel size and the irregular shape of most hypothalamic nuclei make it possible that a voxel contains the responses of cells outside the area of interest. In addition, the ROIs are determined not by verifying the boundaries of the relevant anatomical structures in each individual brain but rather they are taken from an atlas that gives coordinates of the spatially normalized brain. This may cause additional error. Nevertheless, an important point of our study is that we were able to differentiate the hypothalamic areas into several regions, each with a different ratio of glucose uptake during stress with that during stress-coping.
In conclusion, active coping with stress by chewing prevents the stress-induced increased glucose uptake in the hypothalamus. A selective suppression of neuronal responses in the PVN and the AH area may contribute to the reduced corticosterone secretion in the rats that actively coped with stress. As chewing is a common behavior for animals as well as humans, these results suggest chewing as an easy, inexpensive, and non-pharmacological way to reduce the risk of stress-related disorders in modern society.
Acknowledgments
The authors thank Mr Chin-Chao Hsu and Mr Samuel Chen Leu for their technical help with the animal PET experiment. This work was supported by Grant-in-Aid for Young Scientists (KAKENHI 21791815) by the Ministry of Education, Culture, Sports, Science and Technology, an Open Research Center subsidy for the Research Center of Brain and Oral Science, Kanagawa Dental College of Japan, and by Neurobiology and Cognitive Science Center, National Taiwan University, and by a grant, NSC100-2311-B002-002-MY3 from National Science Council, Taiwan.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Armstrong WE. 2004. Hypothalamic supraoptic and paraventricular nuclei. In: Paxinos G. editors. The rat nervous system. San Diego, CA: Academic Press369–388.
- Bonnavion P, de Lecea L. 2010. Hypocretins in the control of sleep and wakefulness. Curr Neurol Neurosci Rep. 10:174–179.
- Briski K, Gillen E. 2001. Differential distribution of Fos expression within the male rat preoptic area and hypothalamus in response to physical vs. psychological stress. Brain Res Bull. 55:401–408.
- Busnardo C, Tavares RF, Resstel LB, Elias LL, Correa FM. 2010. Paraventricular nucleus modulates autonomic and neuroendocrine responses to acute restraint stress in rats. Auton Neurosci. 158:51–57.
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. 2007. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic–pituitary–adrenal axis activity: Implications for the integration of limbic inputs. J Neurosci. 27:2025–2034.
- Chowdhury GM, Fujioka T, Nakamura S. 2000. Induction and adaptation of Fos expression in the rat brain by two types of acute restraint stress. Brain Res Bull. 52:171–182.
- Cochran SM, McKerchar CE, Morris BJ, Pratt JA. 2002. Induction of differential patterns of local cerebral glucose metabolism and immediate-early genes by acute clozapine and haloperidol. Neuropharmacology. 43:394–407.
- Dong HW, Petrovich GD, Swanson LW. 2001. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 38:192–246.
- Dragunow M, Faull R. 1989. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 29:261–265.
- Duncan GE, Johnson KB, Breese GR. 1993. Topographic patterns of brain activity in response to swim stress: Assessment by 2-deoxyglucose uptake and expression of Fos-like immunoreactivity. J Neurosci. 13:3932–3943.
- Ebner K, Bosch OJ, Krömer SA, Singewald N, Neumann ID. 2005. Release of oxytocin in the rat central amygdala modulates stress-coping behavior and the release of excitatory amino acids. Neuropsychopharmacology. 30:223–230.
- Eells JB, Clough RW, Miller JW, Jobe PC, Browning RA. 2000. Fos expression and 2-deoxyglucose uptake following seizures in developing genetically epilepsy-prone rats. Brain Res Bull. 52:379–389.
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. 1995. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 2:189–210.
- Fueger BJ, Czernin J, Hildebrandt I, Tran C, Halpern BS, Stout D, Phelps ME, Weber WA. 2006. Impact of animal handling on the results of 18f-FDG PET studies in mice. J Nucl Med. 47:999–1006.
- Furlong TM, Vianna DM, Liu L, Carrive P. 2009. Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur J Neurosci. 30:1603–1614.
- Hoffman GE, Lyo D. 2002. Anatomical markers of activity in neuroendocrine systems: Are we all “fos-ed out”?. J Neuroendocrinol. 14:259–268.
- Hori N, Yuyama N, Tamura K. 2004. Biting suppresses stress-induced expression of corticotropin-releasing factor (CRF) in the rat hypothalamus. J Dent Res. 83:124–128.
- Hori N, Lee MC, Sasaguri K, Ishii H, Kamei M, Kimoto K, Toyoda M, Sato S. 2005. Suppression of stress-induced nNOS expression in the rat hypothalamus by biting. J Dent Res. 84:624–628.
- Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, Murakami N. 2000. Possible involvement of orexin in the stress reaction in rats. Biochem Biophys Res Commun. 270:318–323.
- Jankord R, Herman JP. 2008. Limbic regulation of hypothalamo–pituitary–adrenocortical function during acute and chronic stress. Ann N Y Acad Sci. 1148:64–73.
- Jezova D, Ochedalski T, Kiss A, Aguilera G. 1998. Brain angiotensin II modulates sympathoadrenal and hypothalamic pituitary adrenocortical activation during stress. J Neuroendocrinol. 10:67–72.
- Krukoff TL, Khalili P. 1997. Stress-induced activation of nitric oxide-producing neurons in the rat brain. J Comp Neurol. 377:509–519.
- Kubo T, Numakura H, Endo S, Hagiwara Y, Fukumori R. 2001. Angiotensin receptor blockade in the anterior hypothalamic area inhibits stress-induced pressor responses in rats. Brain Res Bull. 56:569–574.
- Martinez M, Phillips PJ, Herbert J. 1998. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur J Neurosci. 10:20–33.
- McAuliffe M, Lalonde F, McGarry D, Gandler W, Csaky K, Trus BMedical image processing, analysis and visualization in clinical research2001 Proceedings of the 14th IEEE Symposium on Computer-Based Medical Systems 381–386.
- Melia KR, Ryabinin AE, Schroeder R, Bloom FE, Wilson MC. 1994. Induction and habituation of immediate early gene expression in rat brain by acute and repeated restraint stress. J Neurosci. 14:5929–5938.
- Morilak DA, Cecchi M, Khoshbouei H. 2003. Interactions of norepinephrine and galanin in the central amygdala and lateral bed nucleus of the stria terminalis modulate the behavioral response to acute stress. Life Sci. 73:715–726.
- Okada S, Hori N, Kimoto K, Onozuka M, Sato S, Sasaguri K. 2007. Effects of biting on elevation of blood pressure and other physiological responses to stress in rats: Biting may reduce allostatic load. Brain Res. 1185:189–194.
- Ono Y, Kataoka T, Miyake S, Cheng SJ, Tachibana A, Sasaguri K, Onozuka M. 2008. Chewing ameliorates stress-induced suppression of hippocampal long-term potentiation. Neuroscience. 154:1352–1359.
- Ono Y, Kataoka T, Miyake S, Sasaguri K, Sato S, Onozuka M. 2009. Chewing rescues stress-suppressed hippocampal long-term potentiation via activation of histamine H1 receptor. Neurosci Res. 64:385–390.
- Ono Y, Yamamoto T, Kubo KY, Onozuka M. 2010. Occlusion and brain function: Mastication as a prevention of cognitive dysfunction. J Oral Rehabil. 37:624–640.
- Onozuka M, Watanabe K, Fujita M, Tonosaki K, Saito S. 2002. Evidence for involvement of glucocorticoid response in the hippocampal changes in aged molarless SAMP8 mice. Behav Brain Res. 131:125–129.
- Paxinos G, Watson C. 2007. The rat brain in stereotaxic coordinates. Amsterdam: Elsevier.
- Roozendaal B, Koolhaas JM, Bohus B. 1997. The role of the central amygdala in stress and adaption. Acta Physiol Scand Suppl. 640:51–54.
- Sakamoto T, Torii Y, Takahashi M, Ishida S, Goto Y, Nakano H, Harakawa T, Ginnaga A, Kozaki S, Kaji R. 2009. Quantitative determination of the biological activity of botulinum toxin type A by measuring the compound muscle action potential (CMAP) in rats. Toxicon. 54:857–861.
- Sasaguri K, Kikuchi M, Hori N, Yuyama N, Onozuka M, Sato S. 2005. Suppression of stress immobilization-induced phosphorylation of ERK 1/2 by biting in the rat hypothalamic paraventricular nucleus. Neurosci Lett. 383:160–164.
- Schweinhardt P, Fransson P, Olson L, Spenger C, Andersson JLR. 2003. A template for spatial normalisation of MR images of the rat brain. J Neurosci Methods. 129:105–113.
- Shintani F, Nakaki T, Kanba S, Sato K, Yagi G, Shiozawa M, Aiso S, Kato R, Asai M. 1995. Involvement of interleukin-1 in immobilization stress-induced increase in plasma adrenocorticotropic hormone and in release of hypothalamic monoamines in the rat. J Neurosci. 15:1961–1970.
- Shulman RG. 2001. Functional imaging studies: Linking mind and basic neuroscience. Am J Psychiatry.. 158:11–20.
- Shulman RG, Rothman DL, Hyder F. 1999. Stimulated changes in localized cerebral energy consumption. Proc Natl Acad Sci USA. 96:3245–3250.
- Simerly RB. 2004. Anatomical substrates of hypothalamic integration. In: Paxinos G. editors. The rat nervous system. San Diego, CA: Academic Press336–368.
- Sung KK, Jang DP, Lee S, Kim M, Lee SY, Kim YB, Park CW, Cho ZH. 2009. Neural responses in rat brain during acute immobilization stress: A [F-18]FDG micro PET imaging study. Neuroimage. 44:1074–1080.
- Thomas NR, Peyton SC. 1983. An electromyographic study of mastication in the freely-moving rat. Arch Oral Biol. 28:939–945.
- Veenema AH, Neumann ID. 2007. Neurobiological mechanisms of aggression and stress coping: A comparative study in mouse and rat selection lines. Brain Behav Evol. 70:274–285.
- Wang Y, Seidel J, Tsui BM, Vaquero JJ, Pomper MG. 2006. Performance evaluation of the GE healthcare eXplore VISTA dual-ring small-animal PET scanner. J Nucl Med. 47:1891–1900.
- Wiersma A, Konsman JP, Knollema S, Bohus B, Koolhaas JM. 1998. Differential effects of CRH infusion into the central nucleus of the amygdala in the Roman high-avoidance and low-avoidance rats. Psychoneuroendocrinology. 23:261–274.