Abstract
Although acute stress accelerates colonic transit, the effect of chronic stress on colonic transit remains unclear. In this study, rats received repeated restraint stress (chronic homotypic stress) or various types of stress (chronic heterotypic stress) for 5 and 7 days, respectively. Vehicle saline, oxytocin (OXT), OXT receptor antagonist or corticotropin-releasing factor (CRF) receptor antagonists were administered by intracerebroventricular (ICV) injection prior to restraint stress for 90 min. Immediately after the stress exposure, the entire colon was removed and the geometric center (GC) of Na51CrO4 (a nonabsorbable radioactive marker; 0.5 μCi) distribution was calculated to measure the transit. Gene expression of OXT and CRF in the paraventricular nucleus (PVN) was evaluated by in situ hybridization. Accelerated colonic transit with the acute stressor was no longer observed following chronic homotypic stress. This restored colonic transit was reversed by ICV injection of an OXT antagonist. In contrast, chronic heterotypic stress significantly accelerated colonic transit, which was attenuated by ICV injection of OXT and by a CRF receptor 1 antagonist. OXT mRNA expression in the PVN was significantly increased following chronic homotypic stress, but not chronic heterotypic stress. However, CRF mRNA expression in the PVN was significantly increased following acute and chronic heterotypic stress, but not chronic homotypic stress. These results indicate that central OXT and CRF play a pivotal role in mediating the colonic dysmotility following chronic stress in rats.
| Abbreviations | ||
| CRF | = | corticotropin-releasing factor |
| HPA | = | Hypothalamus–pituitary–adrenal axis |
| IBS | = | Irritable bowel syndrome |
| PVN | = | Paraventricular nucleus |
| SON | = | Supraoptic nucleus |
Introduction
Functional gastrointestinal (GI) disorders including functional dyspepsia (FD) and irritable bowel syndrome (IBS) are common in the general population (Talley Citation1994). GI disorders are multifactorial, since the various pathophysiological mechanisms can be combined in each patient. Motor dysfunction of the GI tract and visceral hypersensitivity are especially considered as important factors. Stress is widely believed to play a major role in developing functional GI disorders (Thompson et al. Citation1999; Drossman et al. Citation2002).
Acute stress increases corticotropin-releasing factor (CRF) release by the paraventricular nucleus (PVN) neurons, which activates the hypothalamus–pituitary–adrenal (HPA) axis; this is followed by an increased CRF gene expression in the PVN (Gomez et al. Citation2004). Activation of the HPA axis results in the secretion of glucocorticoid (corticosterone in the rat) from the adrenal cortex to organize protective responses to stress (Gomez et al. Citation2004).
The motor responses to acute stress differ between the stomach and the colon. Acute restraint stress delays gastric emptying (Tache et al. Citation1987; Nakade et al. Citation2005), while it accelerates colonic transit in rodents (Monnikes et al. Citation1992; Nakade et al. Citation2007a). We have previously shown that delayed gastric emptying induced by acute restraint stress is mediated via central CRF type 2 receptors (CRFR2) and sympathetic pathways (Nakade et al. Citation2005). In contrast, accelerated colonic transit induced by acute restraint stress is mediated via central CRF type 1 receptors (CRFR1) and parasympathetic pathways in rats (Nakade et al. Citation2007a). Although the motor responses to acute restraint stress differ between the upper and lower GI tract, it should be noted that both responses are mediated via the same neuropeptide in the brain, which is CRF. However, it remains unclear whether central CRF plays a major role in regulating the HPA axis and colonic dysmotility following chronic stress.
Oxytocin (OXT) is synthesized in the neurosecretory cells that are located in the PVN and supraoptic nucleus (SON) of the hypothalamus. OXT attenuates stress-induced activity of the HPA axis and thus modulates the regulation of the stress response in rodents (Windle et al. Citation1997; Neumann Citation2002). Peripheral and central administration of OXT shows an anxiolytic pattern in both males (Ring et al. Citation2006; Waldherr and Neumann Citation2007) and females (McCarthy et al. Citation1996; Bale et al. Citation2001). In response to various stressors, there is a dose-dependent effect of OXT in attenuating the activation of the neural circuitry in the brain that mediates stress-induced HPA activation (Windle et al. Citation2004). OXT administration also attenuates the increase in CRF mRNA expression in the PVN in response to acute restraint stress in rats (Windle et al. Citation2004; Zheng et al. Citation2010). This suggests that its inhibitory effect on CRF expression in the brain mediates both the anxiolytic and stress-attenuating effects of OXT.
In contrast to acute stress, repeated experiences with the same stressor may produce habituation or diminution of behavioral responses. Although there is abundant evidence for a major role of central OXT as an anti-stressor, it still remains unknown whether central OXT is involved in mediating the adaptation mechanism following chronic homotypic stress. We have previously shown that delayed gastric emptying observed with acute restraint stress was completely reversed following 5 consecutive days of restraint stress (chronic homotypic stress) in rats (Zheng et al. Citation2009; Zheng et al. Citation2010) and mice (Babygirija et al. Citation2010b).
OXT mRNA expression is upregulated, while CRF mRNA expression is downregulated in the PVN following chronic homotypic stress in rats (Zheng et al. Citation2010) and mice (Babygirija et al. Citation2010b). Furthermore, we have demonstrated that chronic homotypic stress failed to restore delayed gastric emptying in OXT knockout mice (Babygirija et al. Citation2010a). These findings suggest that central OXT is involved in mediating restored gastric motility following chronic homotypic stress. We also showed that accelerated colonic transit and augmented colonic motility observed with acute restraint stress were completely recovered to the normal levels following chronic homotypic stress in rats (Masere et al. Citation2009). A recent study demonstrated that stimulation of colonic motility induced by acute water avoidance stress is attenuated by central, but not peripheral, administration of OXT in rats (Matsunaga et al. Citation2009).
In contrast to chronic homotypic stress, delayed gastric emptying was still observed when rats were exposed to different types of stressors for 7 days (chronic heterotypic stress; Zheng et al. Citation2010), suggesting maladaptation to chronic heterotypic stress. A recent study also shows that chronic heterotypic stress for 9 days enhances the contractile activity of the colonic smooth muscle in vitro in rats (Choudhury et al. Citation2009). However, it remains unknown whether chronic heterotypic stress alters colonic motility in conscious animals in vivo. We hypothesized that (1) restoration of normal colonic transit during chronic homotypic stress exposure results from increased OXT activity in the brain, and increased OXT gene expression in the hypothalamic PVN and (2) the absence of habituation of colonic transit to chronic heterotypic stress reflects enhanced central CRF action via CRFR1, and insufficient opposing central OXT action.
This study was designed to study the role of central OXT and CRF in mediating colonic transit following different types of stressors (acute stress, chronic homotypic stress, and chronic heterotypic stress). As the PVN plays a key role in stress responses of the GI tract, we studied the changes in CRF mRNA and OXT mRNA expression at the PVN following acute and chronic stress by in situ hybridization. This study suggests that the central OXT may play an important role in regulating the adaptation mechanism to restore colonic transit following chronic stress in rats.
Materials and methods
Animals
Male Sprague Dawley rats (250–300 g) were obtained from Charles River Laboratories (Raleigh, NC, USA). They were kept in group cages under conditions of controlled temperature (22–24°C), humidity, and light (12:12 h light/dark cycle and the light cycle starts at 07:00 h) with free access to laboratory chow and water. The rats were singly housed prior to the start of the experiment. All experiments were started at 09:00 h. Protocols describing the use of rats were approved by the Institutional Animal Care and Use Committee of Zablocki VA Medical Center at Milwaukee and carried out in accordance with the National Institute of Health ‘Guide for the Care and Use of Laboratory Animals.’ All efforts were made to minimize animal suffering and to reduce the number of animals used in experiments.
Chronic homotypic and heterotypic stress loading
For chronic homotypic stress, the rats received the same restraint stress loading for 5 consecutive days. For chronic heterotypic stress, the rats received different types of stressors for 7 consecutive days, as previously reported (Zheng et al. Citation2010). The stress paradigms used were water avoidance stress, forced swimming stress, cold restraint stress, and restraint stress. Rats were exposed to two different stressors each day for 7 days. The specific conditions for each type of stress were as follows:
Water avoidance stress
Rats were placed on a platform (3 × 6 cm) in the middle of a plastic container (50 × 30 × 20 cm) filled with room temperature (RT) water to 1 cm below the height of the platform for 90 min. Control rats were placed on the same platform in a waterless container for 90 min.
Forced swimming stress
Rats were placed individually in a plastic tank (52 × 37 × 20 cm) filled with RT water to a depth of 15 cm for 20 min. The depth of the water forced the rat to swim or float without the hind limbs touching the bottom of the tank. The rats were dried and warmed at the end of the forced swimming stress. Control rats were placed individually in a waterless tank for 20 min.
Cold restraint stress
Rats were kept in restraining cylinders (20 cm in length and 7.5 cm in diameter; with ventilation holes) at 4°C for 45 min. Control rats were kept at RT for 45 min.
Restraint stress
Rats were placed in a prone position on a wooden plate with their trunks wrapped in a confining harness for 90 min, as previously reported (Zheng et al. Citation2009). The rat was able to move its limbs and head but not its trunk.
The rats did not show any diarrhea during the 5–7 consecutive days of chronic homotypic or heterotypic stress loading. There was no significant body weight change in the rats exposed to the variable stress paradigm for 7 consecutive days (data not shown).
Colonic transit study
Rats were anesthetized with pentobarbital sodium (45 mg/kg, IP). An in-dwelling silastic cannula was inserted into the cecum and positioned to enter the proximal colon. The proximal portion of the tube was brought through the left abdominal wall and tunneled beneath the skin to the posterior neck and fixed to the skin. For post-operative pain relief, the rats were administered buprenorphine (0.05 mg/kg) subcutaneously every 12 h for 2 days. One week after the surgery, a nonabsorbable radioactive marker (0.5 μCi; Na51CrO4 in 0.2 ml saline) was administered into the proximal colon, and rats were restrained for 90 min, as previously described (Nakade et al. Citation2007a).
After stress loading was completed, the rats were euthanized with pentobarbital sodium (200 mg, IP). The entire colon was removed and divided into 10 equal segments. Each segment was placed into a vial, and the radioactivity was counted with a gamma counter for 1 min. The geometric center (GC) of the distribution of 51Cr within the colon is the center of gravity for the distribution of radiochromium. GC was calculated as previously reported (Nakade et al. Citation2007a). Location of the GC indicates transit rate in the colon.
Two-thirds of IBS patients are female, and gender differences are suggested to be important in mediating stress-induced GI dysmotility in humans (Chang and Heitkemper Citation2002). In a preliminary study, we compared the effects of acute stress and chronic homotypic stress on colonic transit between male and female rats. Acute stress accelerated colonic transit, which was completely restored to normal following chronic homotypic stress to the same extent in male and female rats (). As no sex differences were observed in colonic transit, we used male rats in this study.
Table I. Gender difference in colonic transit (GC) responses to acute restraint stress and chronic homotypic stress in rats.
Fecal pellet output has been widely used for assessing colonic motor function because of its simplicity in rodents. Counting fecal pellet output is based on the concept that the fecal contents in the distal colon in each rodent are the same before stress loading. However, the actual fecal contents vary between the individuals. If the initial fecal content is small, restraint stress may fail to show any significant increase in fecal pellet output. Moreover, it is frequently observed that handling itself without stress loading produces fecal pellet output. The motor activity of the distal colon is mainly regulated by the pelvic nerves, while the proximal and mid-colon are mainly regulated by vagal nerves in rats (Tong et al. Citation2010). The number of fecal pellets expelled is considered to mainly reflect distal colonic motor function (Million et al. Citation2000), while colonic transit measurement is considered to reflect the motor function of the entire colon. As vagal and pelvic nerves are both involved in regulating colonic motor function in response to stress (Nakade et al. Citation2007a), we consider that it is preferable to measure colonic transit to study the stimulatory effects of stress and central CRF on colonic motor function in rodents. Moreover, we have previously reported that the acceleration of colonic transit does not always correlate with increased output in number of fecal pellets in response to restraint stress and intracerebroventricular (ICV) injection of CRF in conscious rats (Nakade et al. Citation2007b) and mice (Babygirija et al. Citation2011).
ICV administration of OXT, OXT receptor antagonist, and CRF receptor antagonists
Following overnight fasting, rats were anesthetized with isoflurane (2%) and placed in a stereotaxic apparatus. A 24-gauge plastic sterile cannula was implanted into the left cerebral lateral ventricle (1.5 mm caudal; 2 mm lateral with respect to bregma; 6 mm ventral from the skull surface), as previously reported (Ishiguchi et al. Citation2001). The cannula was fixed with bone cement (Kyowa, Tokyo, Japan) and acrylic resin (Shofu, San Marcos, CA, USA). The rats were allowed to recover for 1 week. The rats were habituated to handling before ICV injections.
To investigate whether central OXT is involved in mediating colonic transit, OXT (0.5 μg in 5 μl saline) was injected (ICV) 30 min prior to stress loading by use of a microsyringe attached to polyethylene tubing. It has been shown that ICV injection of OXT (0.3 μg) increases cardiovascular reactivity in response to some types of stressor in rats (Petersson and Uvnas-Moberg Citation2007). Others showed that ICV injection of OXT (500 pmol; 0.5 μg) inhibits accelerated colonic motility induced by water avoidance stress in rats (Matsunaga et al. Citation2009). We also showed that ICV injection of OXT (0.5 μg) completely reversed inhibitory effects of acute restraint stress on gastric emptying in rats (Zheng et al. Citation2010). OXT dissolved in isotonic saline was injected over a period of 10 s, as described previously (Windle et al. Citation1997; Windle et al. Citation2004).
To investigate whether central OXT is involved in restoring normal colonic transit following chronic homotypic stress, an OXT receptor antagonist, [, Try(Me)2, Orn8]-OXT (100 ng/5 μl), was injected (ICV) 30 min prior to stress loading. Saline (5 μl, ICV)-injected rats served as controls. [
, Tyr(Me)2, Orn8]-OXT (1–100 ng) has been shown to inhibit the action of OXT in sexual physiological stimulation in rats (Argiolas et al. Citation1987; Melis et al. Citation1999). We previously showed that [
, Try(Me)2, Orn8]-OXT (100 ng) restored delayed gastric emptying following chronic repeated stress in mice (Babygirija et al. Citation2010b).
To investigate which CRF receptors are involved in mediating colonic dysmotility following chronic heterotypic stress, a CRFR1 antagonist (NBI-27914; 100 μg/5 μl) or a CRFR2 antagonist (astressin2-B; 10 μg/5 μl) was injected (ICV) 30 min prior to restraint stress loading on day 7. Icv-injection of NBI-27914 (50–100 μg/rat) has been shown to abolish the colonic response to CRF (ICV; Martinez and Tache Citation2001). We previously showed that intracisternal injection of astressin2-B (10 μg/rat) prevented delayed gastric emptying induced by acute restraint stress (Nakade et al. Citation2005).
At the end of the experiment, rats were euthanized with sodium pentobarbital (200 mg/kg, IP). The implantation site of the ICV cannula was confirmed by the presence of Evans blue (5%, 1 μl) after injection via the catheter, as previously reported (Ishiguchi et al. Citation2001).
Plasma corticosterone assay
We have previously shown that plasma corticosterone concentrations were no longer significantly increased following chronic homotypic stress, indicating the attenuation of HPA axis activity (Zheng et al. Citation2009). Here, we studied the plasma corticosterone levels following chronic heterotypic stress. After the restraint stress exposure on the 7th day, the rats were euthanized with pentobarbital (200 mg/kg, ip). Immediately the rats became unconscious, blood samples were drawn via cardiac puncture. Blood was collected in tubes containing ethylenediaminetetraacetic acid (EDTA) and aprotinin (500 kIU/ml), as previously reported (Zheng et al. Citation2009). After centrifugation, plasma was aliquoted and was stored at − 20°C. Plasma concentration of corticosterone was measured using a corticosterone radioimmunoassay kit (ICN Biomedicals, Inc., Costa Mesa, CA, USA). Intra- and inter-assay coefficient variances were < 10%, as previously reported (Zheng et al. Citation2009). The sensitivity of the corticosterone assay was 7.7 ng/ml.
In situ hybridization of OXT and CRF mRNAs in the PVN
Other groups of rats were deeply anesthetized with sodium pentobarbital (200 mg/kg, IP) and perfused transcardially with 0.1 M phosphate-buffered saline (PBS; pH 7.4) at 4°C for 2 min, followed by a fixative containing 4% paraformaldehyde (PFA; Sigma-Aldrich, St Louis, MO, USA) in 0.1 M phosphate buffer (PB) at 4°C for 15 min. The brains were removed and placed into a solution of 4% PFA in 0.1 PB (pH 7.4) for 24 h at 4°C. The brains were then immersed for more than 24 h in PBS containing 30% sucrose at 4°C, and then rapidly frozen on dry ice and kept at − 80°C until sectioning. Frozen serial sections (40 μm thick) were treated with Protease K (1 mg/ml) at 37°C for 15 min, and then incubated with 0.25% acetic anhydride in 0.1 M triethanolamine for 10 min. After prehybridization with hybridization buffer at 60°C, the sections were hybridized with a digoxigenin (DIG)-labeled sense or antisense OXT or CRF cRNA probe at 60°C for 18 h, as previously described (Fujiwara et al. Citation2003). Sense and antisense rat transcripts were generated from the vector pGEM-3Z (Promega, Madison, WI, USA) for OXT and CRF. The probes were transcribed using an Sp6/T7 transcription kit according to the instructions of the manufacturer (Roche Diagnostics, Indianapolis, IN, USA).
The sections were then treated with 2 × standard saline citrate (SSC) in 50% formamide at 60°C for 15 min. Following this, the sections were treated with RNase A (20 mg/ml) at 37°C for 30 min. Then, they were treated sequentially with 2 × SSC, 0.5 × SSC, and DIG-1 buffer (100 mM Tris–HCl, pH 7.5; 150 mM NaCl) twice for 15 min each. After being treated with 1.5% blocking reagent (Roche Diagnostics) for 60 min, the sections were incubated with an alkaline phosphatase conjugated anti-DIG antibody (1:1000, Roche Diagnostics) at 37°C for overnight. The sections were then washed with DIG-1 buffer, and treated with DIG-3 buffer (100 mM Tris–HCl, pH 9.5; 100 mM NaCl; 50 mM MgCl2). The sections were then treated with a chromagen solution (Nitro blue tetrazolium (NBT) 5-Bromo-4-chloro-3-indolyl phosphate (BCIP), intraperitoneal (IP) 20 μl in DIG-3 buffer 980 μl). After the detection of a visible signal, the reaction was stopped by the addition of a reaction stop solution (10 mM Tris–HCl, pH 7.6; 1 mM EDTA, pH 8.0). After processing, the sections were mounted and examined by light microscopy. As a control for non-specific labeling, a sense RNA probe was used for the same sections, but no specific signal was detected. The parvocellular and magnocellular subdivisions in the PVN were distinguished as previously reported (Sawchenko and Swanson Citation1982; Zheng et al. Citation2010).
Chemicals
[,Try(Me)2,Orn8]-OXT was purchased from Bachem (Torrance, CA, USA). CRFR1 antagonist (NBI-27914) and CRFR2 antagonist (astressin2-B) were purchased from Sigma-Aldrich and were kept in powder form. Peptides were dissolved in 0.9% saline immediately before use.
Statistical analysis
The mean densities (MDs) of OXT and CRF mRNA-containing cells were calculated bilaterally in each subdivision of the PVN on the sections between − 0.8 and − 2.1 mm from the bregma using NIH Image software (Version 1.63), as previously reported (Kiyokawa et al. Citation2004). The MD is the sum of the gray values of all the pixels in the section divided by the number of pixels.
Student's t-test was used for determination of statistical significance between unpaired variables. A multiple group comparison was performed by one-way ANOVA followed by Tukey–Kramer. All results are expressed as means ± standard error of the mean (SEM). A P-value < 0.05 was considered statistically significant.
Results
Effect of OXT and an OXT antagonist on colonic transit in control rats
Saline, OXT, and an OXT antagonist were administered via the ICV cannula 30 min prior to the intra-colonic administration of 51Cr in non-stressed control rats. Colonic transit, measured as GC, was not altered by OXT or an OXT antagonist, compared to that in saline-injected rats ().
Figure 1. Effect of ICV injection of saline, OXT, and an OXT antagonist on colonic transit. (a) In non-stressed control rats neither OXT nor an OXT antagonist altered colonic transit. GC of the distribution of 51Cr within the colon indicates colonic transit. (b)Effect of ICV injection of an OXT antagonist on colonic transit in response to chronic homotypic stress (90 min stress for 5 consecutive days). Chronic homotypic stress did not accelerate colonic transit, compared with control (a), but ICV injection of OXT antagonist (100 ng) significantly accelerated colonic transit, compared with that in saline-injected rats following chronic homotypic stress. (c) Effect of ICV injection of OXT on colonic transit in response to chronic heterotypic stress (different types of stressors for 7 consecutive days. In contrast to chronic homotypic stress, colonic transit was accelerated following chronic heterotypic stress. ICV injection of OXT (0.5 μg) significantly attenuated accelerated colonic transit, compared with that of saline (ICV)-injected rats following chronic heterotypic stress. Values are mean ± SEM; n = 6–7 rats per group. *P < 0.05 by Student's t-test.
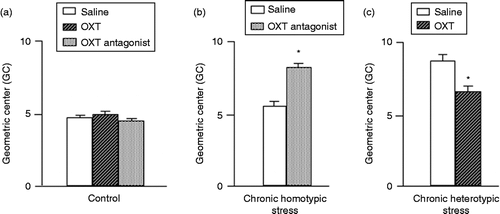
Effect of an OXT antagonist on colonic transit following chronic homotypic stress
After 5 consecutive days of chronic homotypic stress, colonic transit was no longer accelerated, compared with controls. An OXT antagonist was administered by ICV injection 30 min before the restraint stress loading. During 30 min observation, the OXT antagonist did not evoke any significant changes in general behavior patterns, including wakefulness and activity following chronic homotypic stress.
ICV injection of OXT antagonist (100 ng) significantly accelerated colonic transit (GC; n = 6, P < 0.05 by Student's t-test), compared with that of saline-injected rats following chronic homotypic stress (GC; n = 7; ).
Effect of OXT on colonic transit following chronic heterotypic stress
In contrast to chronic homotypic stress, colonic transit was significantly accelerated following chronic heterotypic stress. OXT was administered (ICV) 30 min before the last restraint stress loading. General behavior patterns were not affected by OXT administration following chronic heterotypic stress.
ICV injection of OXT (0.5 μg) significantly attenuated the accelerated colonic transit (GC; n = 7), compared with that of the saline (ICV)-injected rats following chronic heterotypic stress (GC; n = 7, P < 0.05 by Student's t-test; ).
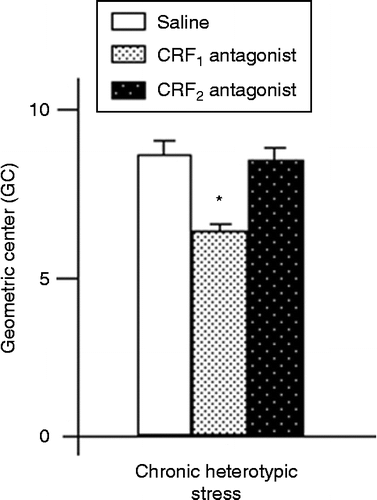
Effect of CRF receptor antagonists on accelerated colonic transit following chronic heterotypic stress
ICV injection of NBI-27914 (GC; n = 7), but not astressin2-B (GC; n = 7), reversed the accelerated colonic transit following chronic heterotypic stress (F(2,18) = 7.5, P < 0.05 by one-way ANOVA followed by Tukey–Kramer test; ).
Figure 2. Effect of ICV injection of CRFR1 and CRFR2 antagonists on colonic transit in response to chronic heterotypic stress. A CRFR1 antagonist, but not a CRFR2 antagonist, reversed accelerated colonic transit following chronic heterotypic stress. Values are mean ± SEM; n = 7 rats per group. *P < 0.05 by one-way ANOVA followed by Tukey–Kramer tests.
Plasma corticosterone concentration following chronic heterotypic stress
In our previous study, acute restraint stress significantly increased plasma corticosterone concentration from 100 to over 1300 ng/ml. In contrast, there was no significant increase observed following chronic homotypic stress (Zheng et al. Citation2009).
Our current study showed that plasma corticosterone concentration was increased (645 ± 127 ng/ml; n = 6) following chronic heterotypic stress, compared to that of non-stressed rats (127 ± 36 ng/ml; n = 6, P < 0.01 by Student's t-test).
In situ hybridization for OXT and CRF mRNAs in the PVN
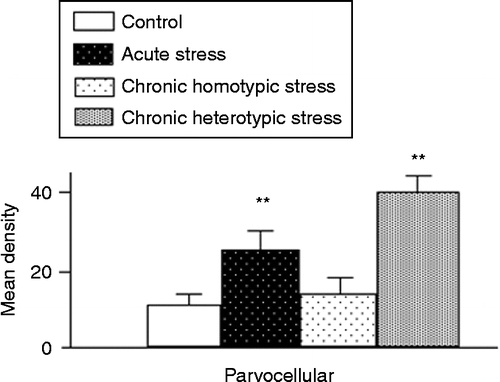
Following acute and chronic heterotypic stress, the MD of CRF mRNA expression was significantly increased in the parvocellular subdivisions including anterior, dorsal, medial, lateral and periventricular parvocellular of the PVN. However, there was no significant increase in CRF mRNA expression observed in the parvocellular PVN following chronic homotypic stress (n = 4, P < 0.01 by one-way ANOVA followed by Tukey–Kramer test; ).
Figure 3. In situ hybridization autoradiographs for CRF mRNA (a) and OXT mRNA (b) in the PVN in control, acute stress, chronic homotypic stress, and chronic heterotypic stress rats. 3V; 3rd ventricle; scale bar 200 μm.
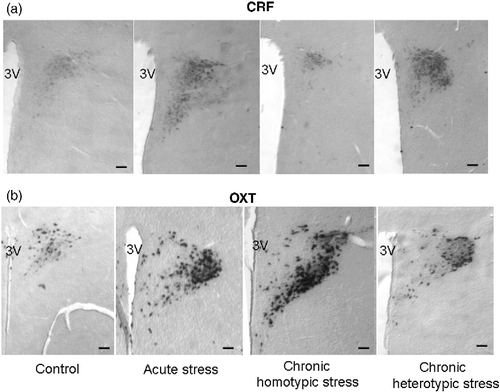
Figure 4. The MD of CRF mRNA-containing cells in control, acute stress, chronic homotypic and chronic heterotypic stress rats in the parvocellular subdivision of the PVN. Values are mean ± SEM, n = 4 rats per group. **P < 0.01 compared with control by one-way ANOVA followed by Tukey–Kramer tests.
Within the parvocellular subdivisions of the PVN, OXT mRNA expression seemed to be increased following chronic homotypic stress; however, this was not statistically significant (). There were no significant differences in the MD for OXT mRNA expression in the anterior magnocellular (AM) subdivision of the PVN following any type of stress ().
Figure 5. The MD of OXT mRNA-containing cells in sub-divisions of the PVN in control, acute stress, chronic homotypic and chronic heterotypic stress rats. (a) The parvocellular subdivision; (b) AM; (c) PM subdivision of the PVN. Data are mean ± SEM, n = 4 rats per group, *P < 0.05, **P < 0.01 compared with control, #P < 0.05 compared with acute and heterotypic stress by one-way ANOVA followed by Tukey–Kramer.
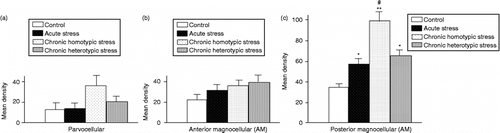
The MD of OXT mRNA-containing cells in the posterior magnocellular (PM) subdivisions of the PVN ( − 1.7 mm from bregma) was remarkably increased following acute stress, chronic homotypic stress, and chronic heterotypic stress (). The increase in OXT mRNA expression in the PM subdivision was much more pronounced following chronic homotypic stress, compared to acute and chronic heterotypic stress (n = 4, P < 0.05 by one-way ANOVA followed by Tukey–Kramer tests; ).
Discussion
Similar to the upper GI tract, an adaptation mechanism seems to develop to reverse colonic dysmotility following chronic homotypic stress in rats. The sensitivity to central CRF in mediating accelerated colonic transit is not altered following chronic homotypic stress, because central administration of CRF was able to accelerate colonic transit following chronic homotypic stress (Masere et al. Citation2009). We have recently demonstrated that increased CRF mRNA expression in the PVN following acute restraint stress was no longer observed following chronic homotypic stress in rats (Zheng et al. Citation2010). These results indicate that attenuation of HPA axis activity following chronic homotypic stress is due to reduced CRF expression in the hypothalamus.
Our current study showed that the restored, normal, colonic transit following chronic homotypic stress was reversed by ICV injection of an OXT antagonist. We also showed that OXT mRNA expression is upregulated, while CRF mRNA expression is downregulated in the PVN following chronic homotypic stress. There was a significant negative correlation observed between CRF mRNA and OXT mRNA expression following acute stress and chronic homotypic stress (Zheng et al. Citation2010). These findings suggest that central OXT is involved in mediating restored, normalized, colonic motility following chronic homotypic stress. Thus, it is highly likely that chronic homotypic stress upregulates OXT expression in the hypothalamus, resulting in attenuation of CRF expression and hence of activity in the HPA pathway.
In contrast to chronic homotypic stress, accelerated colonic transit and increased CRF mRNA expression in the PVN were observed following chronic heterotypic stress. This indicates that adaptation of colonic motility may not develop in response to chronic heterotypic stress in rats.
A CRFR1 antagonist, but not a CRFR2 antagonist, almost completely abolished the acceleration of colonic transit following chronic heterotypic stress, indicating that accelerated colonic transit induced by chronic heterotypic stress is mediated via central CRFR1.
It has been demonstrated that the anxiolytic and stress-attenuating effects of OXT are mediated by its inhibitory effect on CRF mRNA expression. We previously showed that increased CRF mRNA expression in response to acute restraint stress was completely abolished by ICV injection of OXT in rats (Zheng et al. Citation2010). However, the inhibitory mechanism mediating OXT actions on CRF mRNA expression remains to be investigated. As the majority of neuronal responses to OXT are excitatory (Inenaga and Yamashita Citation1986), the inhibitory effect of OXT may not be directly on CRF neurons.
Gamma-aminobutyric acid (GABA) is the major inhibitory amino acid transmitter of the mammalian central nervous system. GABAergic neurons are located in the immediate surroundings of the PVN (peri-PVN), especially at the dorsal part of the PVN (Roland and Sawchenko Citation1993). These GABA-projecting neurons into the PVN are known to inhibit CRF expression via GABAA receptors (Bali and Kovacs Citation2003; Cullinan et al. Citation2008). GABAA receptors are expressed on the hypophysiotropic CRF neurons in the PVN (Cullinan Citation2000). In the central amygdala, OXT enhances GABAergic transmission (Huber et al. Citation2005). We have recently shown that the inhibitory effect of OXT on the increase of CRF mRNA expression in response to acute restraint stress is abolished by ICV injection of a selective GABAA receptor antagonist, bicuculline methiodide (BMI). ICV injection of BMI significantly increased CRF mRNA expression following chronic homotypic stress in rats (Bülbül et al. Citation2011). These findings indicate that the inhibitory effect of OXT on CRF mRNA expression is mediated via GABAA receptors at the hypothalamus.
It has been shown that OXT injection into the dorsal motor nucleus of the vagus elicits a reduction in gastric motor activity in anesthetized rats (Rogers and Hermann Citation1987). However, little is known about the central effects of OXT on GI motility in conscious rats. We have previously shown that restraint stress augments postprandial gastric contractions and impairs the coordination between antral and pyloric motility, resulting in delayed gastric emptying in conscious rats (Nakade et al. Citation2006). ICV administration of OXT, which was ineffective on gastric emptying in normal conditions, significantly improved delayed gastric emptying induced by acute restraint stress (Zheng et al. Citation2010). Similarly, ICV administration of OXT (up to 10 μg) was ineffective on gastric motility in normal conditions, while it significantly attenuated augmented gastric motility induced by acute restraint stress in freely moving conscious rats (Bulbul et al. Citation2010).
In contrast, others demonstrated that ICV administration of OXT (10 μg) inhibited postprandial gastric motility under non-stressed conditions in rats (Flanagan et al. Citation1992). As the rats were placed into polypropylene tubes during the motility recording (Flanagan et al. Citation1992), this procedure might have caused a stress response, resulting in the augmentation of gastric motility. Thus, augmented gastric motility induced by stress may be antagonized by OXT. We propose that central OXT does not play a major role in regulating GI motility in non-stressed conditions. In contrast, central OXT attenuates augmented gastric motility (Babygirija et al. Citation2010a), delayed gastric emptying (Babygirija et al. Citation2010a), and accelerated colonic transit in stressful conditions.
At the PVN level, OXT is produced in the magnocellular and parvocellular neurons, while CRF is produced in the parvocellular neurons (Sawchenko and Swanson Citation1982). Synaptic associations between CRF and magnocellular OXT neurons of the PVN have also been demonstrated (Hisano et al. Citation1992). Forced swimming stress increases OXT mRNA expression in the magnocellular neurons, but not the parvocellular neurons, of the PVN in rats (Wotjak et al. Citation2001). Restraint stress induces c-fos expression in OXTergic magnocellular neurons in the SON and PVN in rats (Miyata et al. Citation1995).
The SON consists exclusively of magnocellular neurons, while the PVN consists of both magnocellular and parvocellular neurons. The magnocellular neurons are part of the hypothalamic-neurohypophysial system, while the parvocellular neurons constitute the central part of the HPA axis and some project to the autonomic preganglionic neurons at the brain stem (Herman et al. Citation2008). Thus, acute and chronic heterotypic stress stimulates CRF release from the parvocellular neurons, resulting in acceleration of colonic transit via autonomic parasympathetic neurons (Nakade et al. Citation2007a) as well as activation of the HPA axis. It is, therefore, conceivable that the inhibitory action of OXT on CRF mRNA expression is independent of the HPA axis.
We have recently shown that the number of OXT-immunoreactive cells in the PM and medial parvocellular subdivisions of the PVN was remarkably increased following chronic homotypic stress, compared to that of acute stress and chronic heterotypic stress. However, there were no significant changes observed in OXT-immunoreactive cells in the other parvocellular subdivisions of the PVN, bed nucleus of stria terminalis and the amygdala (Zheng et al. Citation2010). Our current in situ hybridization study demonstrated that the cytochemical changes in OXT synthesis (translation) were well correlated with its gene expression (transcription). Further studies are needed to investigate whether the projection of OXTergic magnocellular and/or parvocellular neurons to GABAergic neurons of the peri-PVN is altered following chronic stress.
Recent in vitro study showed that hyperactivity of the colonic smooth muscle in response to acetylcholine is observed following chronic heterotypic stress in rats. The elevated plasma levels of norepinephrine enhance the gene expression of l-type Ca2+ channels in colonic circular smooth muscle cells (Choudhury et al. Citation2009). This suggests that in addition to the hypothalamus, functional changes were also developed in the peripheral tissues in response to chronic heterotypic stress.
Functional GI symptoms are common in the general population, with a reported prevalence of 25–40%. Functional GI symptoms are responsible for up to 33% of consultations with gastroenterologists in the UK and 40% in the USA. Epidemiological studies suggest considerable overlap between FD and IBS. About half of the FD patients fulfill the Rome II criteria for IBS (Corsetti et al. Citation2004). This overlap may be explained by the evidence that stress responses of the upper and lower GI tract are both regulated by the hypothalamic circuits considered here. Thus, both FD and IBS associated stress may be treatable once hypothalamic OXT expression is upregulated.
Although peripheral CRFR1 antagonists have been developed to treat IBS patients, the efficacy of the antagonists remains controversial. We propose that the restoration of GI motility in both the upper and lower GI tract develops through the mechanism of upregulation of endogenous OXT expression in the hypothalamus following chronic homotypic stress. Following chronic heterotypic stress, exogenous centrally applied OXT also restored GI motility via attenuating CRF expression. Our findings may contribute to a better understanding of the mechanism and treatment of stress-associated functional GI disorders.
Declaration of interest: The authors report no conflicts of interests. The authors alone are responsible for content and writing of the paper.
References
- Argiolas A, Melis MR, Vargiu L, Gessa GL. 1987. d(CH2)5Tyr(Me)-[Orn8] vasotocin, a potent oxytocin antagonist, antagonizes penile erection and yawning induced by oxytocin and apomorphine, but not by ACTH-(1–24). Eur J Pharmacol. 134:221–224.
- Babygirija R, Zheng J, Bulbul M, Cerjak D, Ludwig K, Takahashi T. Sustained delayed gastric emptying during repeated restraint stress in oxytocin knockout mice. J Neuroendocrinol. 2010a; 22:1181–1186.
- Babygirija R, Zheng J, Ludwig K, Takahashi T. Central oxytocin is involved in restoring impaired gastric motility following chronic repeated stress in mice. Am J Physiol Regul Integr Comp Physiol. 2010b; 298:R157–R165.
- Babygirija R, Bülbül M, Cerjak D, Ludwig K, Takahashi T. 2011. Sustained acceleration of colonic transit following chronic homotypic stress in oxytocin knockout mice. Neurosci Lett. 495:77–81.
- Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. 2001. CNS region-specific oxytocin receptor expression: Importance in regulation of anxiety and sex behavior. J Neurosci. 21:2546–2552.
- Bali B, Kovacs KJ. 2003. GABAergic control of neuropeptide gene expression in parvocellular neurons of the hypothalamic paraventricular nucleus. Eur J Neurosci. 18:1518–1526.
- Bülbül M, Babygirija R, Ludwig K, Takahashi T. 2010. Central oxytocin attenuates augmented gastric postprandial motility induced by restraint stress in rats. Neurosci Lett. 479:302–306.
- Bülbül M, Babygirija R, Cerjak D, Yoshimoto S, Ludwig K, Takahashi T. 2011. Hypothalamic oxytocin attenuates CRF expression via GABAA receptors in rats. Brain Res. 1387:37–45.
- Chang L, Heitkemper MM. 2002. Gender differences in irritable bowel syndrome. Gastroenterology. 123:1686–1701.
- Choudhury BK, Shi XZ, Sarna SK. 2009. Norepinephrine mediates the transcriptional effects of heterotypic chronic stress on colonic motor function. Am J Physiol Gastrointest Liver Physiol. 296:G1238–G1247.
- Corsetti M, Caenepeel P, Fischler B, Janssens J, Tack J. 2004. Impact of coexisting irritable bowel syndrome on symptoms and pathophysiological mechanisms in functional dyspepsia. Am J Gastroenterol. 99:1152–1159.
- Cullinan WE. 2000. GABA(A) receptor subunit expression within hypophysiotropic CRH neurons: A dual hybridization histochemical study. J Comp Neurol. 419:344–351.
- Cullinan WE, Ziegler DR, Herman JP. 2008. Functional role of local GABAergic influences on the HPA axis. Brain Struct Funct. 213:63–72.
- Drossman DA, Camilleri M, Mayer EA, Whitehead WE. 2002. AGA technical review on irritable bowel syndrome. Gastroenterology. 123:2108–2131.
- Flanagan LM, Olson BR, Sved AF, Verbalis JG, Stricker EM. 1992. Gastric motility in conscious rats given oxytocin and an oxytocin antagonist centrally. Brain Res. 578:256–260.
- Fujiwara K, Maruyama M, Usui K, Sakai T, Matsumoto H, Hinuma S, Kitada C, Inoue K. 2003. Appearance of prolactin-releasing peptide-producing neurons in the area postrema of adrenalectomized rats. Neurosci Lett. 338:127–130.
- Gomez F, Manalo S, Dallman MF. 2004. Androgen-sensitive changes in regulation of restraint-induced adrenocorticotropin secretion between early and late puberty in male rats. Endocrinology. 145:59–70.
- Herman JP, Flak J, Jankord R. 2008. Chronic stress plasticity in the hypothalamic paraventricular nucleus. Prog Brain Res. 170:353–364.
- Hisano S, Li S, Kagotani Y, Daikoku S. 1992. Synaptic associations between oxytocin-containing magnocellular neurons and neurons containing corticotropin-releasing factor in the rat magnocellular paraventricular nucleus. Brain Res. 576:311–318.
- Huber D, Veinante P, Stoop R. 2005. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 308:245–248.
- Inenaga K, Yamashita H. 1986. Excitation of neurones in the rat paraventricular nucleus in vitro by vasopressin and oxytocin. J Physiol. 370:165–180.
- Ishiguchi T, Amano T, Matsubayashi H, Tada H, Fujita M, Takahashi T. 2001. Centrally administered neuropeptide Y delays gastric emptying via Y2 receptors in rats. Am J Physiol Regul Integr Comp Physiol. 281:R1522–R1530.
- Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. 2004. Partner's stress status influences social buffering effects in rats. Behav Neurosci. 118:798–804.
- Martinez V, Tache Y. 2001. Role of CRF receptor 1 in central CRF-induced stimulation of colonic propulsion in rats. Brain Res. 893:29–35.
- Masere C, Nakade Y, Zheng J, Babygirija R, Ludwig K, Takahashi T. 2009. Chronic restraint stress has no more stimulatory effects on colonic motility in rats. Neurosci Lett. 453:147–150.
- Matsunaga M, Konagaya T, Nogimori T, Yoneda M, Kasugai K, Ohira H, Kaneko H. 2009. Inhibitory effect of oxytocin on accelerated colonic motility induced by water-avoidance stress in rats. Neurogastroenterol Motil. 21:e856–e859.
- McCarthy MM, McDonald CH, Brooks PJ, Goldman D. 1996. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol Behav. 60:1209–1215.
- Melis MR, Spano MS, Succu S, Argiolas A. 1999. The oxytocin antagonist d(CH2)5Tyr(Me)2–Orn8–vasotocin reduces non-contact penile erections in male rats. Neurosci Lett. 265:171–174.
- Million M, Wang L, Martinez V, Tache Y. 2000. Differential Fos expression in the paraventricular nucleus of the hypothalamus, sacral parasympathetic nucleus and colonic motor response to water avoidance stress in Fischer and Lewis rats. Brain Res. 877:345–353.
- Miyata S, Itoh T, Lin SH, Ishiyama M, Nakashima T, Kiyohara T. 1995. Temporal changes of c-fos expression in oxytocinergic magnocellular neuroendocrine cells of the rat hypothalamus with restraint stress. Brain Res Bull. 37:391–395.
- Monnikes H, Schmidt BG, Raybould HE, Tache Y. 1992. CRF in the paraventricular nucleus mediates gastric and colonic motor response to restraint stress. Am J Physiol. 262:G137–G143.
- Nakade Y, Tsuchida D, Fukuda H, Iwa M, Pappas TN, Takahashi T. 2005. Restraint stress delays solid gastric emptying via a central CRF and peripheral sympathetic neuron in rats. Am J Physiol Regul Integr Comp Physiol. 288:R427–R432.
- Nakade Y, Tsuchida D, Fukuda H, Iwa M, Pappas TN, Takahashi T. 2006. Restraint stress augments postprandial gastric contractions but impairs antropyloric coordination in conscious rats. Am J Physiol Regul Integr Comp Physiol. 290:R616–R624.
- Nakade Y, Fukuda H, Iwa M, Tsukamoto K, Yanagi H, Yamamura T, Mantyh C, Pappas TN, Takahashi T. Restraint stress stimulates colonic motility via central corticotropin-releasing factor and peripheral 5-HT3 receptors in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2007a; 292:G1037–G1044.
- Nakade Y, Mantyh C, Pappas TN, Takahashi T. Fecal pellet output does not always correlate with colonic transit in response to restraint stress and corticotropin-releasing factor in rats. J Gastroenterol. 2007b; 42:279–282.
- Neumann ID. 2002. Involvement of the brain oxytocin system in stress coping: Interactions with the hypothalamo–pituitary–adrenal axis. Prog Brain Res. 139:147–162.
- Petersson M, Uvnas-Moberg K. 2007. Effects of an acute stressor on blood pressure and heart rate in rats pretreated with intracerebroventricular oxytocin injections. Psychoneuroendocrinology. 32:959–965.
- Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S. 2006. Anxiolytic-like activity of oxytocin in male mice: Behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl). 185:218–225.
- Rogers RC, Hermann GE. 1987. Oxytocin, oxytocin antagonist, TRH, and hypothalamic paraventricular nucleus stimulation effects on gastric motility. Peptides. 8:505–513.
- Roland BL, Sawchenko PE. 1993. Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 332:123–143.
- Sawchenko PE, Swanson LW. 1982. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 205:260–272.
- Tache Y, Maeda-Hagiwara M, Turkelson CM. 1987. Central nervous system action of corticotropin-releasing factor to inhibit gastric emptying in rats. Am J Physiol. 253:G241–G245.
- Talley NJ. 1994. Why do functional gastrointestinal disorders come and go?. Dig Dis Sci. 39:673–677.
- Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine J, Muller-Lissner SA. 1999. Functional bowel disorders and functional abdominal pain. Gut. 45 Suppl. 2: II43–II47.
- Tong WD, Ridolfi TJ, Kosinski L, Ludwig K, Takahashi T. 2010. Effects of autonomic nerve stimulation on colorectal motility in rats. Neurogastroenterol Motil. 22:688–693.
- Waldherr M, Neumann ID. 2007. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc Natl Acad Sci USA. 104:16681–16684.
- Windle RJ, Shanks N, Lightman SL, Ingram CD. 1997. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 138:2829–2834.
- Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. 2004. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J Neurosci. 24:2974–2982.
- Wotjak CT, Naruo T, Muraoka S, Simchen R, Landgraf R, Engelmann M. 2001. Forced swimming stimulates the expression of vasopressin and oxytocin in magnocellular neurons of the rat hypothalamic paraventricular nucleus. Eur J Neurosci. 13:2273–2281.
- Zheng J, Dobner A, Babygirija R, Ludwig K, Takahashi T. 2009. Effects of repeated restraint stress on gastric motility in rats. Am J Physiol Regul Integr Comp Physiol. 296:R1358–R1365.
- Zheng J, Babygirija R, Bulbul M, Cerjak D, Ludwig K, Takahashi T. 2010. Hypothalamic oxytocin mediates adaptation mechanism against chronic stress in rats. Am J Physiol Gastrointest Liver Physiol. 299:G946–G953.