Abstract
Deficits in executive functioning and working memory associated with frontal lobe dysfunction are prominent in depression and work-related long-term sick leave (LTSL). This study used functional magnetic resonance imaging (fMRI) to investigate potential differences in brain activation patterns in these conditions. In addition, the function of the hypothalamic–pituitary–adrenal (HPA) axis was examined and compared between groups. Since there is a clear overrepresentation of women in these diagnostic groups, and to ensure a more homogenous sample population, only women were included. To examine the neural correlates of relevant cognitive processes in patients on sick leave >90 days due to work-related LTSL, recently diagnosed patients with major depression Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV criteria, untreated), and healthy controls (n = 10, each group), a 2-back working memory task and a visual long-term memory task were administered during fMRI scanning. HPA axis functioning was investigated using a diurnal curve of saliva cortisol and a dexamethasone suppression test. Task performance was comparable among the three groups. Multivariate image analysis revealed that both memory tasks engaged a similar brain network in all three groups, including the prefrontal and parietal cortex. During the 2-back task, LTSL patients had significant frontal hypoactivation compared to controls and patients with depression. Saliva cortisol measurements showed a flattening of the diurnal rythmicity in LTSL patients compared to patients with depression and healthy contols. Taken together, these findings indicate that work stress-related LTSL and major depression are dissociable in terms of frontal activation and diurnal cortisol rhythmicity.
Introduction
Stress has emerged as an important factor in psychiatric diagnoses. A dysregulation of stress systems is implicated in major diagnostic categories, including mood and anxiety disorders, potentially reflecting psychological responses to work-related and personal stressors (McEwen Citation2003; Wahlberg et al. Citation2009). Psychosocial factors and individual differences in coping style, emotional state, cognition, and appraisal have major impact on stress regulation (Berntson and Cacioppo Citation2000; CitationCacioppo 2002). The brain is the central mediator of physiological adjustments and behavioral responses to behavioral challenges (McEwen Citation2006). Hormonal systems, notably the hypothalamic–pituitary–adrenal (HPA) axis as well as immune and metabolic systems are involved in the process of protecting the organism from and adapting to challenges. This process, referred to as allostasis, is an essential component of maintaining homeostasis (McEwen Citation2003). One example of allostasis is improved memory and immune function with moderate increases in cortisol, which helps overcome challenging situations. Persistently high concentrations of cortisol may in contrast have adverse effects, including inhibiting the formation of new neurons (neurogenesis) in the hippocampal region of the brain, possibly leading to cognitive deficits and dysregulation of the HPA axis (Alderson and Novack Citation2002; Lupien et al. Citation2005). The close relationship between stress, allostatic load, and its impact on cognitive and endocrine function is well established in psychiatric research today (Lupien et al. Citation2007). Notably, specific alterations of HPA axis and cognitive function in different diagnostic categories of psychiatric diseases, such as major depression and post traumatic stress disorder (PTSD), are well documented (Lupien et al. Citation2009). In Sweden, more than 50% of patients who were on long-term sick leave (LTSL) reported work-related stress as the main cause of disease (The Swedish National Social Insurance Board Citation2003).
Research efforts in the field of work-related stress are complicated by the lack of consensus about diagnostic criteria. Depression is the category of use in the field of stress-related disorders, despite the fact that several scientific papers indicate that depression and work-related stress do not share patterns of physiological and psychological changes (Rydmark et al. Citation2006; Wahlberg et al. Citation2009). The concept of burnout is initially a description of the frustration and emotional detachment seen in social and health workers (Mason Citation1975; Maslach et al. Citation2001). Originally, the term was not meant as a classification of disease, but rather a description of the process of exhaustion or adaptation to an overwhelming work situation. The Swedish National Board of Health and Welfare recently recommended a specific International statistical classification of diseases and related health problems (ICD)-10 code (i.e. exhaustion syndrome F43.8) to classify the closely related terms: clinical burnout, vital exhaustion, and mental fatigue. As proposed byÅsberg and co-workers, burnout should not be considered a necessary or sufficient condition for developing stress-related disease. Rather, it is a process or coping strategy to endure challenging situations at work that may lead to disease or exhaustion syndrome (Asberg et al. Citation2010). Since there is no international consensus on this issue, we have used the term work-related LTSL in this study.
The symptoms of individuals on work-related LTSL overlap with core symptoms of major depression (Wahlberg et al. Citation2009), which has prompted examinations of whether individuals on LTSL due to work stress and patients with major depression share HPA axis pathophysiology (Rydmark et al. Citation2006). Though major depression has been associated with up-regulated reactivity of the HPA axis, patients with stress were found to exhibit a marked decrease in the reactivity of the HPA axis (Wahlberg et al. Citation2009). In the same study, no support was obtained for a reduction in hippocampal volume, and cognitive impairment was more indicative of a disruption of the frontocortical system rather than hippocampus dysfunction. We and others have reported a similar pattern of neurocognitive alterations in stressed patients, with evidence of frontal cognitive deficits (Sandstrom et al. Citation2005) and intact hippocampal volume (Sandstrom et al. Citation2011). In contrast, major depression has repeatedly been associated with lowered performance on hippocampal-dependent explicit/episodic memory tasks (Airaksinen et al. Citation2004) and reduced hippocampal volume (Videbech and Ravnkilde Citation2004).
Collectively, these findings suggest differences between major depression and chronic stress. In particular, individuals on LTSL due to work stress tend to exhibit frontal, rather than hippocampal, dysfunction. This view is in line with a notion of the stress–brain link that implicates many cortical and subcortical regions other than the hippocampus, including the frontal cortex (Lupien and Lepage Citation2001). Converging evidence indicates that not only affective but also cognitive regions in the frontal lobes are dysfunctional in major depression (Steele et al. Citation2007), and patients suffering from major depression exhibit a pattern of cognitive dysfunction that extends beyond the domain of explicit/episodic memory to executive, frontal lobe-sensitive tasks (Stordal et al. Citation2004; Dahlin et al. Citation2008). Furthermore, imaging studies have suggested an increased activity in the subgenual anterior cingulate cortex (sACC) in patients with depression, which is decreased by successful treatment (Mayberg et al. 2003, Citation2005).
Direct, within-study, comparisons of patients on LTSL due to work stress and patients with major depression are critical for addressing the questions of whether these two categories reflect a shared or distinct pathophysiology. To date, such studies are rare and, to the best of our knowledge, no prior study has compared the functional brain responses of patients from each category while engaged in memory tasks.
The main objective of this study was to compare functional magnetic resonance imaging (fMRI) patterns and diurnal cortisol across three groups: (i) controls, (ii) acute unmedicated patients with unipolar major depression, and (iii) patients on LTSL due to work stress. During fMRI scanning, the participants alternated between a baseline task, an n-back working memory protocol known to reliably elicit frontal activity (Dahlin et al. Citation2008), and the continuous visual memory task (CVMT) that has been related to hippocampal activity (Yassa and Stark Citation2008). First, we assessed similarities and differences in task-related fMRI activity among groups using a multivariate partial least squares (PLS) analysis (McIntosh and Lobaugh Citation2004; Salami et al. Citation2010), and then conducted univariate contrasts guided by the multivariate findings. We predicted group differences in engagement of prefrontal cortex, such that the stress group would under-recruit some frontal regions. In addition, the diurnal rhythm of saliva cortisol and the cortisol response to the dexamethasone suppression test (DST) was investigated using saliva samples. We predicted that depressed patients would have higher cortisol levels, especially after dexamethasone (DEX).
Methods and materials
Subjects
A total of 30 females participated in the study, 10 in each group: work stress-related LTSL, unipolar major depression, and healthy controls (). The depressed patients were recruited from the psychiatric clinic at Norrland's University Hospital. The stress patients were recruited from the stress clinic at the same hospital and were part of a larger study (Stenlund et al. Citation2009), which aimed at exploring work-related long-term stress.
Table I. Descriptives.
Exclusion criteria were left-handedness or ambidextrousness, co-morbid psychiatric disorders, known abuse of alcohol or drugs, endocrinological diseases, and neurological disorders. Subjects who were not menstruating were also excluded. All participants were native Swedish speakers. For the stress group, additional exclusion criteria were other diseases that could result in fatigue and/or stress-related symptoms, other diseases that cause future sick leave, other diseases or treatments that can interfere with active participation, post-traumatic stress disorder, unemployment for more than 2 years prior to participation, speech and language difficulties, and a need for individual therapy.
The patients with depression and the controls were all medication-naïve. In the work stress-related LTSL group, four patients were taking selective serotonin reuptake inhibitors. None of the stress patients were taking tricyclic anti-depressants.
The study was approved by the Ethics Committee for Medical Research at Norrland's University Hospital. All patients provided informed written consent after having the procedure and objective of the study carefully explained to them and after the opportunity to ask questions. Subjects were not paid for their participation.
Clinical assessments
The depressed patients were assessed by a clinically experienced psychologist (RS). The interviews reviewed clinical and demographic information, and the obtained data were used to confirm or reject a diagnosis of unipolar major depressive disorder according to DSM-IV. Depression severity was measured with the Montgomery–Åsberg depression rating scale (MADRS) (Montgomery and Asberg Citation1979; Svanborg and Asberg Citation1994). To be included in the depression group, participants had to have a minimum score of 20, which reflects moderate to severe depression. All of the depressed patients were either on sick leave or otherwise incapable to work due to the severity of their symptoms.
The stress patients were subjected to medical and psychological examinations to confirm the diagnosis of burnout (Schaufeli and Enzmann Citation1998). The patients were required to have been on continuous sick leave for burnout and stress-related disorder ≥ 25% of their working hours for at least 3 months and to have an average score of ≥ 4.6 on the Shirom Melamed burnout questionnaire (Kushnir and Melamed Citation1992; Melamed et al. Citation1992, Citation1999).
All subjects were assessed with the MADRS, the Hamilton anxiety scale (HAS), the perceived stress questionnaire (PSQ), and the burnout inventory (BI) on the day of the fMRI examination. Group characteristics are presented in .
All self-rating scales and measurements of psychiatric health used in this work are well known, validated, and reliable with excellent Cronbach's α. MADRS is a widely used questionnaire to measure severity of depression. It is sensitive to change and therefore common in treatment studies. MADRS Cronbach's α varies between 0.80 and 0.90 (Montgomery and Asberg Citation1979). The HAS is considered a gold standard questionnaire designed to assess anxiety symptoms regardless of diagnosis. It is sensitive to change and is frequently used in clinical trials. Cronbach's α ranging between 0.77 and 0.99 has been reported in different studies (Hamilton Citation1959). PSQ is a self-assessment-based instrument for recording subjective perceived stress (Cronbach's α>0.90) (Levenstein et al. Citation1993). The BI is designed to assess components of the burnout syndrome: emotional exhaustion, depersonalization, and reduced personal accomplishment. Its reported internal consistency/Cronbach's α ranges between 0.71 and 0.90.
fMRI procedure
fMRI was used to assess brain responses while the subjects performed two cognitive tasks: the n-back task and a visual long-term memory task. Additionally, during the fMRI session, a low-demand baseline condition was modeled as a block design to achieve similar sensorimotor activation as the experimental tasks.
The n-back task is a commonly used working memory task in fMRI studies. Subjects are presented a series of stimuli and required to respond if the current stimulus matches the stimulus displayed n steps earlier in the sequence. In our version, single words were displayed and the subjects were asked to indicate whether the displayed word was the same as or different from the one displayed two words earlier (2-back).
The visual long-term memory task consisted of a set of black and white abstract and non-verbal pictures drawn from the CVMT (Larrabee et al. Citation1992). The pictures were displayed one at a time and the subjects were told to respond “yes” or “no” as to whether they recognized the picture from earlier presentations (i.e. a continuous recognition test).
For both tasks, responses were given by pressing one of two buttons using the right or left index finger. In the baseline condition, the letters “xxxxx” were displayed on the computer screen either to the left or to the right side, and the subjects were told to press the button on the corresponding side. All stimuli (2-back words, pictures, or letters) were displayed for 2.5 s, followed by a 0.5 s fixation cross, and all blocks consisted of 16 stimuli. The blocked task paradigm alternated between the baseline condition, the 2-back condition, and the visual memory condition, and this was repeated in five sessions in a sequential order. Stimuli were presented in the same order to all subjects during all sessions. Behavioral performance was recorded for reaction times and response accuracy. Prior to fMRI scanning, subjects were instructed and underwent a trial version of the task to ensure that they had understood the instructions.
Data collection was performed using a 1.5 T Philips Intera scanner (Philips Medical Systems, the Netherlands). Functional T2*-weighted images were collected with a single-shot, gradient echo EPI sequence used for blood oxygen level-dependent imaging. The sequence had the following parameters: echo time 50 ms, repetition time 3000 ms, flip angle 90°, field of view 22 × 22 cm, 64 × 64 matrix, and slice thickness 3.9 mm. Thirty-three slices were acquired every 3.0 s. To eliminate signals arising from progressive saturation, five dummy scans were performed prior to image acquisition. Cushions and headphones in the scanner were used to reduce head movement, dampen scanner noise, and communicate with the subjects. The stimuli were presented on a semi-transparent screen, which the subjects viewed through a tilted mirror attached to the head coil. Presentation and reaction times were handled by a PC running E-Prime 1.0 (Psychology Software Tools, PA, USA). Responses were collected with fiber optic response boxes, one in each hand (Lumitouch reply system, Lightwave Medical Industries, Canada). After the functional imaging, high-resolution T1- and T2-weighted structural images were acquired.
Routine analyses
Subjects and controls were screened in a fasting state at 08:00 h with routine venous blood sampling (5 ml) for each individual for later analyses of full blood count, liver and kidney function tests, and thyroid hormones at the clinical chemistry laboratory (University Hospital of Umeå, Sweden).
Cortisol analyses
HPA axis tests were performed within 10 days after cessation of a menstrual bleeding (i.e. during the follicular phase of the menstrual cycle). Study subjects were supplied with marked test tube and instructed to spit in the tubes at 07:00, 11:00, 16:00, 19:00, and 23:00 h. This was followed by a low-dose DST in which subjects ingested 0.25 mg DEX (Decadron® MSD) after the 23:00 h saliva sample. On the following morning, a saliva sample was collected at 07:00 h for estimations of cortisol. No food, tobacco, or tooth brushing was allowed during the hour preceding sampling. Saliva cortisol was analyzed at the clinical chemistry laboratory, University Hospital of Umeå, by radioimmunoassay from Spectra, Orion Diagnostica, Finland, according to the manufacturer's procedure for salivary cortisol. The coefficient of variances (CVs) were < 12% for saliva cortisol with an analytical detection limit of 0.8 nmol/l, according to the manufacturer.
Data and statistical analyses
Pre-processing of fMRI data
The fMRI images were sent to a PC and converted to Analyze format. The images were then pre-processed and analyzed using SPM2 (Wellcome Department of Cognitive Neurology, UK) implemented in Matlab 7.1 (Mathworks, Inc., MA, USA). During the pre-processing steps, the images were corrected for slice timing to adjust for acquisition time differences between slices and subsequently realigned to the first volume to account for subjects movement. The images were then normalized to a standard anatomic space defined by the MNI Atlas (SPM2) and finally spatially smoothed using an 8.0-mm full-width at half-maximum isotropic Gaussian filter kernel.
Multivariate analysis of fMRI data
PLS reflects time-varying distributed patterns in the brain (as a function of a task) without any assumption about how conditions collate to form a pattern and shape of the hemodynamic response function (HRF). A detailed description of how task PLS can be applied to fMRI data can be found in earlier studies (Salami et al. Citation2010). In short, a data matrix is constructed to facilitate the operation of PLS on the entire data. This matrix is subjected to mean centering, followed by singular value decomposition (SVD), to produce orthogonal latent variables (LVs). Each LV contains voxel/design saliencies and brain scores. The latter reflects the strength of the contribution of each subject to the LV, whereas the former reflects brain activation related to the experimental design. The significance of each LV, indicating whether the overall pattern is different from randomness, was assessed by a permutation test (McIntosh and Lobaugh Citation2004). Using PLS, it is feasible to extract the commonality as well as the differences of brain activations across different groups. Here, the critical interest was to investigate whether there were any group differences in brain activity in relation to the three tasks.
Behavioral PLS analyzes the relationship between the behavioral measures (e.g. reaction times) and the functional brain activity of groups. The procedure is roughly the same as the one described above for task PLS, but instead of mean centering, a correlation analysis is conducted between the data and behavioral measures. As such behavioral saliencies were obtained from SVD indicating task-dependent differences in the brain–behavior correlation. A similar pattern of scores depicting experimental differences in behavior can be obtained by conducting a correlation between the brain scores and each behavioral measure. The confidence interval (CI) around a brain–behavior correlation reveals its reliability.
Univariate analysis of fMRI data
Each condition (2-back, visual long-term memory task, and baseline) was modeled as a fixed response (boxcar) waveform convolved with the HRF. Single-subject statistical contrasts (the 2-back task vs. baseline and the visual recognition task vs. baseline) were generated (in SPM5) using a voxel-wise general linear model. Contrast images for each subject at each session were taken into the second-level random effect analysis (one-sample t-test) to identify regions activated across the groups for each model. The reported activations were significant at a voxel level of p < 0.001, uncorrected for multiple comparisons, with an extent of >5 contiguous voxels.
Other data
Data are shown as mean ± 1 standard deviation or means ± SEM (standard error of mean), as indicated in the text. Non-parametric statistics (Kruskal–Wallis) with Bonferroni corrections was used to test for group differences. Repeated measures analysis (ANOVA) for the 07:00 and 11:00 h assessments of saliva cortisol was used to assess putative group by time interactions. A p value < 0.05 was considered statistically significant. SPSS v14.0 was used for the univariate statistical analyses.
Results
Behavioral variables
The average MADRS, PSQ, and burnout scores were highest in the depression group (). All measurements of psychological health in the depression group were significantly different from the control group as well as the work-related LTSL group. All depressed subjects and five LTSL subjects had MADRS scores above the depression limit of 20, whereas none of the controls had MADRS scores over 20. A significant difference in the HAS was found between controls and the patient groups, but no significant difference was found between the depressed patients and individuals on LTSL. Patients with depression were significantly younger than controls and individuals on LTSL. The PSQ and MADRS scores also significantly differed among groups (). Burnout scores and HAS did not differ between patient groups but separated controls from the two patient groups.
Overall, all groups performed the memory tasks with a high level of accuracy during fMRI. The patient groups, particularly the stress group, tended to have slower reaction times than controls. This slowing was particularly marked for the n-back working memory task, but did not reach statistical significance ().
Table II. Behavioral data.
Brain imaging
The multivariate PLS analysis revealed two significant patterns (p < 0.0001, ) that were highly similar across the three groups, suggesting a high degree of similarity in the functional brain patterns of patients and controls. However, some group differences were also observed.
Figure 1. Brain scores and singular images for two significant LVs. Reliable voxels in the singular images are highlighted according to their bootstrap ratio (BSR>3.29; p < 0.001), which is the ratio of voxel saliencies (red and blue represent positive and negative saliencies, respectively) over estimated standard error. For LV1, the most reliable regions reflecting the main effect of memory tasks vs. baseline were left middle occipital (x, y, z: − 16, − 103, 8; BSR = 8.99; 509 voxels) and right cuneus (x, y, z: 14, − 101, 12; BSR = 6.82; 489 voxels). For LV2, the most reliable regions in the positive direction, expressing the effect of 2-back vs. visual memory, were left inferior occipital (x, y, z: − 16, − 94, − 6; BSR = 8.48; 2652 voxels) and right middle frontal (x, y, z: 34, 56, 14; BSR = 8.42; 2076 voxels) cortex, whereas the most reliable regions in the negative direction, indicating the effect of visual memory vs. 2-back, were middle occipital cortex (x, y, z: 30, − 86, 19; BSR = − 14.05; 13,002 voxels). Note that the pattern as a whole is more important than one specific region alone.
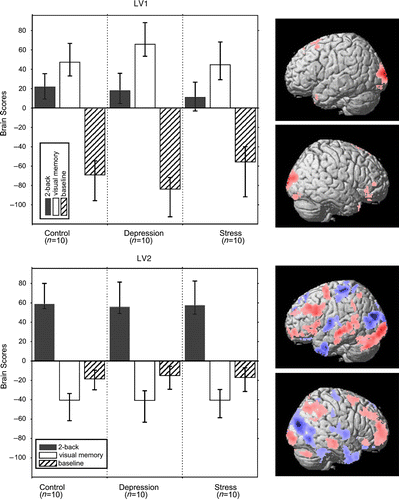
The first LV accounted for 46% of the cross-block covariance and delineated a main effect of memory task (i.e. n-back/CVMT vs. baseline; ). The effect was most pronounced for the visual CVMT, and occipital regions were found to contribute to the effect, reflecting a stronger engagement of visual areas during the processing of complex pictures compared to strings of Xs. For controls and depressed patients, the n-back task also significantly contributed to the effect, but this was not the case for the stress patients (the CI for the n-back task in the LTSL group crossed zero; ). Prefrontal regions, mainly in the right hemisphere, contributed to the effect expressed by LV1 (, top row), thus indicating lesser involvement of prefrontal regions in the n-back for stress patients. This interpretation was examined in more detail in the univariate analyses reported below.
The second pattern (, bottom row), which accounted for 29.5% of the cross-block covariance, mainly reflected a difference between the two cognitive tasks (n-back vs. CVMT). During the n-back task, a well-characterized frontoparietal network was activated (, bottom row; red), whereas dorsal occipital and temporal polar regions were differentially more engaged during CVMT, and to some degree during the baseline task (, bottom row; blue). This effect was similarly expressed across all three groups.
The univariate SPM analyses involved group comparisons of n-back vs. baseline and CVMT vs. baseline. On the basis of the multivariate analysis, we expected weak or no group differences for CVMT, but some differences for n-back. This prediction was confirmed by lack of significant group differences for CVMT along with a significant group difference for n-back. The latter difference was expressed in the left frontal cortex during n-back when the controls and depressed patients were combined into one group and compared with the LTSL group (). A difference was also seen in the right dorsolateral prefrontal cortex, which was engaged by controls and patients with major depression but not by the stress patients ().
Figure 2. (A) Brain regions with significant activation differences during n-back vs. baseline. The reported activations were significant at a voxel level of p < 0.001, uncorrected for multiple comparisons, with an extent of >5 contiguous voxels. Reduced activation was found in the left ventrolateral prefrontal cortex (vlPFC, local maxima x, y, z = − 32, 34, 8) for stress group compared to depressed patients and controls. Blue bars show the mean activity levels in 2-back for controls, depression, and work-related stress on the left, center, and right, respectively. Error bars indicate SEM and ± 95% CI. (B) Brain regions with significant activation differences during n-back vs. baseline, showing relatively less activation in the right dorsolateral prefrontal cortex (dlPFC, local maxima x, y, z = 26, 32, 24) for the work stress-related LTSL group compared to depressed subjects and controls. Red bars show mean activity levels during 2-back for controls, depression, and work-related stress on the left, center, and right, respectively. Error bars indicate SEM and ± 95% CI. The singular images are overlaid on a standard MRI template.
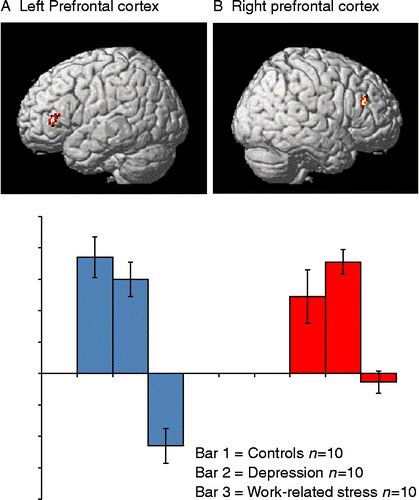
Taken together, the fMRI results revealed pronounced similarities in activation patterns across the groups, indicating largely preserved functional networks in the patients. However, during the n-back task, the LTSL subjects tended to under-recruit regions in the prefrontal cortex.
Diurnal cortisol rhythm
The diurnal salivary cortisol concentration and DST did not reveal any overall differences between groups (). However, the diurnal curve of the LTSL group had a tendency toward flattening. This effect was pronounced between the 07:00 am and 11:00 am recordings, as reflected by a significant group (controls vs. stress patients) by time (07:00 vs. 11:00 h) interaction in an ANOVA [F (15) = 4.65; p = 0.03].
Figure 3. Diurnal saliva cortisol levels, including saliva cortisol after DEX suppression. Data are mean ± SEM. The diurnal curve of the LTSL patients had a non-significant tendency to flatten. This impression was supported by a repeated measurement ANOVA showing a significant group by time interaction in the morning [F (15) = 4.65; p = 0.03].
![Figure 3. Diurnal saliva cortisol levels, including saliva cortisol after DEX suppression. Data are mean ± SEM. The diurnal curve of the LTSL patients had a non-significant tendency to flatten. This impression was supported by a repeated measurement ANOVA showing a significant group by time interaction in the morning [F (15) = 4.65; p = 0.03].](/cms/asset/fb971ce0-1e9c-4e13-a185-e03c60e5416d/ists_a_646347_f0003_b.gif)
Brain–cognition correlations
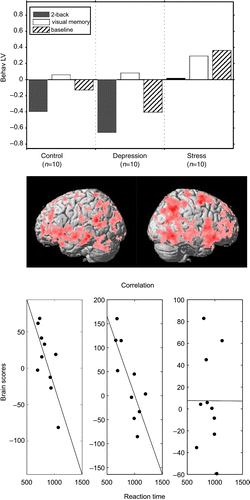
Due to the observation of altered brain activity in LTSL patients during n-back (), we analyzed individual differences in response times during n-back in relation to brain activity (behavioral PLS). The results of this analysis converged with the preceding analyses, by showing a difference in controls and depressed patients relative to stress patients (). Specifically, during n-back, response time was negatively correlated with activity in a widespread cortical network for controls and depressed patients, such that individuals who responded faster had relatively stronger brain activity. No such relation was observed for the LTSL patients.
Figure 4. Behavioral pattern, singular image, and correlation overview for the only significant behavioral PLS LV1 (p < 0.005). The top row demonstrates the behavioral LV that expressed a contrast, indicating task-dependent group differences in the brain–behavior correlation. The second row shows the brain regions (overlaid on a standard MRI template) that negatively correlated with behavioral measures. The most reliable regions in the positive and negative directions were right middle frontal (x, y, z: 46, − 2, 56; BSR = 9.59; 1531 voxels) and left fusiform (x, y, z: − 36, − 56, − 8; BSR = − 4.38; 11 voxels) cortex, respectively. The bottom row shows the correlation overview in scatter plots, reflecting the correlation between brain scores and reaction times for the 2-back task within LV1. The correlation was significant (r = − 0.73; r = − 0.80) for the first two columns (control and depression groups), but was roughly zero for the third column (stress group).
Discussion
The main objective of this study was to compare memory-related brain activity patterns and diurnal cortisol between acute unmedicated patients with unipolar major depression and patients on LTSL due to work stress. Of main interest was to examine whether LTSL patients under-recruited parts of frontal cortex during cognitive processing.
The fMRI results showed pronounced similarities in activation patterns across the groups, suggesting largely intact functional networks. This was further substantiated by intact cognitive performance. However, during the more demanding cognitive task (n-back), the LTSL subjects showed a reduction in prefrontal cortex activation. Specifically, a group difference was observed in the right dorsolateral and left ventrolateral prefrontal cortex. In addition, response times during n-back correlated with brain activity for controls and depressed patients but not for the LTSL subjects, who tended to have a slower response. This pattern of slowed response and reduced frontal activity in patients with stress disorder is consistent with previous findings that patients with stress exhibit reduced performance on cognitively demanding tasks that are assumed to reflect the integrity of frontal lobe functioning (Rydmark et al. Citation2006; Ohman et al. Citation2007; Sandstrom et al. Citation2011). In particular, the findings from the brain–cognition correlation analysis are indicative of alterations of functional working memory networks in LTSL patients. Still, as this observation was found in a more exploratory analysis and was based on a relatively small group of patients, further generalizations must await future replication.
More generally, the present findings are in line with the view that the frontal cortex may be as critical as the hippocampus when it comes to the regulation of stress (Lupien et al. Citation2009). Notably, this is a dual relationship, as the medial prefrontal cortex as well as the hippocampus may be an important target for glucocorticoid actions (Herman et al. Citation2005). Indeed, a recent study has shown that chronic stress or chronic administration of glucocorticoids produces dendritic remodeling in prefrontal pyramidal neurons (Martin and Wellman Citation2011).
The diurnal saliva cortisol curve for the LTSL patients showed a trend toward flattening with higher cortisol levels at 11:00 h, whereas controls and depressed patients had similar diurnal patterns. It remains to be determined if a blunted diurnal cortisol rhythm relates to changes in frontal cortex function. Previous work on the effect of glucocorticoids on memory function showed that they lead to deficits in cognitive tasks sensitive to frontal lobe dysfunction (working memory), but do not impact cognitive functions sensitive to hippocampal damage. Notably, repeated cortisol measurements across several days, including different weekdays, might provide additional insight. Also, estimation of the cortisol awakening response be of interest, since several studies have suggested an altered awakening response in different neuropsychiatric conditions (Kudielka and Wust Citation2010). We did not find any difference in DEX suppression between depressed subjects and controls. This is of interest and suggests that short-term exposure to a depressive state may be less related to failure of “shut-off” of the HPA axis. Indeed, the total length of depression periods may be an important predictor of hippocampal dysfunction/volume which may relate to dysfunctional feedback function in the HPA axis. The number of participants in this study was also insufficient to reveal subgroups of depressed patients with different HPA axis activity. It has also been suggested that age of onset for depression may influence hippocampal function/volume, but this is a matter of controversy (MacQueen and Frodl Citation2011).
The healthy subjects and depressed patients showed marked similarities in brain activation patterns, despite the fact that depressed subjects indicated poorer health in all measures of subjective well-being. Notably, the depressed group did not show the increased sACC activity that has been reported earlier by Mayberg et al. (Citation1999) and Mayberg (Citation2003). These discrepancies may be related to the fact that the depressed patients in this study were recently diagnosed. Repeated episodes of major depression, as well as the severity of episodes, are known to correlate with structural and functional changes in the brain, possibly reflecting hypersecretion of glucocorticoids (Sapolsky Citation2000). Taken together, the amount of time a person is sick might be a crucial factor for developing changes in frontal lobe functioning. Importantly, we wanted to avoid treatment with anxiolytics and other substances known to influence cognitive function, thereby creating another possible confounder. An additional possible confounder is the younger age of the depressed group vs. the LTSL group in our study. Finally, it is possible that the high scores in experienced stress and burnout in our depressed group raise the possibility that some of them are suffering from work-related stress.
In summary, the present findings suggest a difference between patient categories, such that long-term stress relative to acute depression induces changes in functional brain activity, notably in areas within the frontal cortex, as well as a flattening of the diurnal cortisol curve. These findings need to be validated in future studies with larger samples, also including men. Future studies should also explore whether functional and cognitive dysfunction, as shown in this study, is reversible by various interventions.
Acknowledgments
AS and RS contributed equally to this article. This study was financially supported by grants from the Research and Development Units, Jämtland and Västerbotten County Councils,Åke Wiberg's foundation, and the European Union (Eurocores).
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper. The funding sources had no influence over the design, implementation, or analysis of the study. None of the authors has any financial interest related to the present data.
References
- Airaksinen E, Larsson M, Lundberg I, Forsell Y. 2004. Cognitive functions in depressive disorders: Evidence from a population-based study. Psychol Med. 34:83–91.
- Alderson AL, Novack TA. 2002. Neurophysiological and clinical aspects of glucocorticoids and memory: A review. J Clin Exp Neuropsychol. 24:335–355.
- Asberg M, Grape T, Krakau I, Nygren A, Rohde M, Wahlberg A, Wahrborg P. 2010. Stress as the cause of mental illness. Lakartidningen. 107:1307–1310.
- Berntson GG, Cacioppo JT. 2000. Psychobiology and social psychology: Past, present and future. Pers Soc Psychol Rev. 4 1: 3–15.
- Cacioppo JT. 2000. Social neuroscience: Understanding the pieces fosters understanding the whole and vice versa. Am Psychol. 57 11: 819–831.
- Dahlin E, Neely AS, Larsson A, Backman L, Nyberg L. 2008. Transfer of learning after updating training mediated by the striatum. Science. 320:1510–1512.
- Hamilton M. 1959. The assessment of anxiety states by rating. Br J Med Psychol. 32:50–55.
- Herman JP, Ostrander NM, Mueller NK, Figueiredo H. 2005. Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 29 8: 1201–1213.
- Kudielka BM, Wust S. 2010. Human models in acute and chronic stress: Assessing determinants of individual hypothalamus–pituitary–adrenal axis activity and reactivity. Stress. 13:1–14.
- Kushnir T, Melamed S. 1992. The Gulf War and its impact on burnout and well-being of working civilians. Psychol Med. 22:987–995.
- Larrabee GJ, Trahan DE, Curtiss G. 1992. Construct validity of the continuous visual memory test. Arch Clin Neuropsychol. 7:395–405.
- Levenstein S, Prantera C, Varvo V, Scribano ML, Berto E, Luzi C, Andreoli A. 1993. Development of the perceived stress questionnaire: A new tool for psychosomatic research. J Psychosom Res. 37:19–32.
- Lupien SJ, Lepage M. 2001. Stress, memory, and the hippocampus: Can't live with it, can't live without it. Behav Brain Res. 127:137–158.
- Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, Schramek T, Tu MT. 2005. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 30:225–242.
- Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. 2007. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn. 65:209–237.
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. 2009. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 10:434–445.
- MacQueen G, Frodl T. 2011. The hippocampus in major depression: Evidence for the convergence of the bench and bedside in psychiatric research?. Mol Psychiatry. 16:252–264.
- Martin KP, Wellman CL. 2011. NMDA receptor blockade alters stress-induced dendritic remodeling in medial prefrontal cortex. Cereb Cortex. 21 10: 2366–2373 doi: bhr021 [pii]10.1093/cercor/bhr021.
- Maslach C, Schaufeli WB, Leiter MP. 2001. Job burnout. Annu Rev Psychol. 52:397–422.
- Mason JW. 1975. A historical view of the stress field. J Human Stress. 1:22–36.
- Mayberg HS. 2003. Modulating dysfunctional limbic-cortical circuits in depression: Towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 65:193–207.
- Mayberg HS, Liotti M, Brannan SK, McGinnis BS, Mahurin RK, Jerabek PA, Silva A, Tekell JL, Martin CC, Lancaster JL, Fox PT. 1999. Reciprocal lilmbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry. 156:675–682.
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. 2005. Deep brain stimulation for treatment-resistant depression. Neuron. 45:651–660.
- McEwen BS. 2003. Mood disorders and allostatic load. Biol Psychiatry. 54:200–207.
- McEwen BS. 2006. Protective and damaging effects of stress mediators: Central role of the brain. Dialogues Clin Neurosci. 8:367–381.
- McIntosh AR, Lobaugh NJ. 2004. Partial least squares analysis of neuroimaging data: Applications and advances. Neuroimage. 23 Suppl 1: 250–263.
- Melamed S, Kushnir T, Shirom A. 1992. Burnout and risk factors for cardiovascular diseases. Behav Med. 18:53–60.
- Melamed S, Ugarten U, Shirom A, Kahana L, Lerman Y, Froom P. 1999. Chronic burnout, somatic arousal and elevated salivary cortisol levels. J Psychosom Res. 46:591–598.
- Montgomery SA, Asberg M. 1979. A new depression scale designed to be sensitive to change. Br J Psychiatry. 134:382–389.
- Ohman L, Nordin S, Bergdahl J, Slunga Birgander L, Stigsdotter Neely A. 2007. Cognitive function in outpatients with perceived chronic stress. Scand J Work Environ Health. 33:223–232.
- Rydmark I, Wahlberg K, Ghatan PH, Modell S, Nygren A, Ingvar M, Heilig M. 2006. Neuroendocrine, cognitive and structural imaging characteristics of women on longterm sickleave with job stress-induced depression. Biol Psychiatry. 60:867–873.
- Salami A, Eriksson J, Kompus K, Habib R, Kauppi K, Nyberg L. 2010. Characterizing the neural correlates of modality-specific and modality-independent accessibility and availability signals in memory using partial-least squares. Neuroimage. 52:686–698.
- Sandstrom A, Rhodin IN, Lundberg M, Olsson T, Nyberg L. 2005. Impaired cognitive performance in patients with chronic burnout syndrome. Biol Psychol. 69:271–279.
- Sandstrom A, Peterson J, Sandstrom E, Lundberg M, Nystrom IL, Nyberg L, Olsson T. 2011. Cognitive deficits in relation to personality type and hypothalamic-pituitary-adrenal (HPA) axis dysfunction in women with stress-related exhaustion. Scand J Psychol. 52:71–82.
- Sapolsky RM. 2000. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 57:925–935.
- Schaufeli W, Enzmann D. 1998. The burnout companion to study & practice: A critical analysis. London: Taylor & Francis.
- Steele JD, Currie J, Lawrie SM, Reid I. 2007. Prefrontal cortical functional abnormality in major depressive disorder: A stereotactic meta-analysis. J Affect Disord. 101:1–11.
- Stenlund T, Birgander LS, Lindahl B, Nilsson L, Ahlgren C. 2009. Effects of Qigong in patients with burnout: A randomized controlled trial. J Rehabil Med. 41:761–767.
- Stordal KI, Lundervold AJ, Egeland J, Mykletun A, Asbjornsen A, Landro NI, Lund A. 2004. Impairment across executive functions in recurrent major depression. Nord J Psychiatry. 58:41–47.
- Svanborg P, Asberg M. 1994. A new self-rating scale for depression and anxiety states based on the comprehensive psychopathological rating scale. Acta Psychiatr Scand. 89:21–28.
- The Swedish National Social Insurance Board. 2003. Exhaustionsyndromes. Stockholm: The Swedish National Social Insurance Board.
- Videbech P, Ravnkilde B. 2004. Hippocampal volume and depression: A meta-analysis of MRI studies. Am J Psychiatry. 161:1957–1966.
- Wahlberg K, Ghatan PH, Modell S, Nygren A, Ingvar M, Asberg M, Heilig M. 2009. Suppressed neuroendocrine stress response in depressed women on job-stress-related long-term sick leave: A stable marker potentially suggestive of preexisting vulnerability. Biol Psychiatry. 65:742–747.
- Yassa MA, Stark CE. 2008. Multiple signals of recognition memory in the medial temporal lobe. Hippocampus. 18:945–954.