Abstract
Stress exposure is known to be a risk factor for alcohol use and anxiety disorders. Comorbid chronic stress and alcohol dependence may lead to a complicated and potentially severe treatment profile. To gain an understanding of the interaction between chronic psychosocial stress and drug exposure, we studied the effects of concomitant chronic stress exposure on alcohol reward using two-bottle choice and ethanol-conditioned place preference (CPP). The study consisted of exposure of the chronic subordinate colony (CSC) mice “intruders” to an aggressive “resident” mouse for 19 consecutive days. Control mice were single housed (SHC). Ethanol consumption using two-bottle choice paradigm and ethanol CPP acquisition was assessed at the end of this time period. As expected, CSC exposure increased anxiety-like behavior and reduced weight gain as compared to SHC controls. Importantly, in the two-bottle choice procedure, CSC mice showed higher alcohol intake than SHC. When testing their response to ethanol-induced CPP, CSC mice achieved higher preference for the ethanol-paired chamber. In fact, CSC exposure increased ethanol-CPP acquisition. Taken together, these data demonstrate the long-term consequences of chronic psychosocial stress on alcohol intake in male mice, suggesting chronic stress as a risk factor for developing alcohol consumption and/or anxiety disorders.
Introduction
Drug addiction and psychiatric disorders exert an enormous financial and medical impact on society in terms of health complications, family disintegration, loss of employment, crime, and suicide. Furthermore, alcoholism is highly associated with stress-induced psychopathologies such as anxiety, depression, or post-traumatic stress disorder (PTSD) (Varlinskaya & Spear, Citation2010). Moreover, stress exposure during adulthood stimulates cocaine, amphetamine, heroin, and nicotine-induced behavioral changes (Walter et al., Citation2010; Zhao et al., Citation2009). In addition, several studies have shown positive correlation between increased alcohol consumption and stress exposure in humans (Nesic & Duka, Citation2006; Stevens et al., Citation2008) as well as in laboratory animals (Chester et al., Citation2008; Gomez et al., Citation2012). However, attempts to model the effects of stress on ethanol intake have yielded contrasting results. For example, after chronic but not acute stress exposure (foot shock), adolescent mice acquired significant ethanol-induced conditioned place preference (CPP) (2 g/kg alcohol) which did not produce CPP under non-stress conditions (Song et al., Citation2007). Also, exposure to a mild stressor produced an increase in ethanol consumption (Minnick et al., Citation1995). In rats, increases in ethanol intake and preference are observed after 1-day and 7-day chronic overcrowding stressed groups (Nagaraja & Jeganathan, Citation2003). In contrast, social defeat stress exposure significantly decreased alcohol intake in home cage drinking and rate of alcohol reinforcements in operant conditioning in rats (van Erp et al., Citation2001). Also in mice, other results showed that swim stress produced a significant decrease in ethanol consumption (Boyce-Rustay et al., Citation2008).
Chronic subordinate colony (CSC) housing is a well-established model of adult chronic psychosocial stress in rodents. Following CSC procedure, mice show marked alterations in neuronal activation of numerous brain regions (amygdala, hippocampus and limbic areas) (Singewald et al., Citation2009). These regions were highly associated with addiction in general and alcoholism in particular (Sanna et al., Citation2002). At a hormonal level, a reduced hypothalamic vasopressin mRNA was found after 19 days of CSC exposure (Reber & Neumann, Citation2008), a neuropeptide very recently linked to alcohol self-administration in rats (Edwards et al., Citation2012). Together, these data raise questions about the incentive motivation for alcohol in mice submitted to CSC, especially since these animals also show an altered hypothalamic-pituitary-adrenocortical (HPA) axis (Reber et al., Citation2006). This axis is activated in response to ethanol drinking (Armario, Citation2010; Hofmann et al., Citation2007; Mohn et al., Citation2011).
To examine lasting effects of chronic psychosocial stress exposure on ethanol-induced behavior, the present study assessed voluntary alcohol consumption in a two-bottle choice paradigm and ethanol-elicited acquisition of CPP in mice. Chronic social stress-induced anxiety-like behavior was assessed in elevated plus-maze [(EPM) entries and time spent in the open arms]. The purpose of the present study was to assess the lasting effects of prolonged social stress exposure on ethanol reward in adult mice.
Materials and methods
Animals
Male C57BL/6 mice weighing 20–25 g (experimental mice) or Tuck-Ordinary “TO” (resident mice) weighing 35–40 g were housed in standard Plexiglas observation cages (35 × 23 × 19 cm) before the experimental procedure started. All mice were bred in the local central animal facility of the College of Medical and Health Sciences and were kept under standard laboratory conditions (12/12 h light-dark cycle, lights off at 6 pm, ∼22 °C). Bedding was produced locally and autoclaved before use, and mice had free access to tap water and standard mouse chow diet obtained from the National Feed and Flour Production and Marketing Company LLC (Abu Dhabi, UAE). The experimental procedures were approved by the local Research Ethics Committee (Protocol No A01-12).
Drugs
The tastants saccharin (Catalog number 109185) and quinine hemisulfate salt monohydrate (catalog number 22642) were obtained from Sigma-Aldrich (MO, USA). Absolute ethanol (catalog number 131086) was purchased from Panreac Quimica SAU (Barcelona, Spain). For the two-bottle drinking choice paradigm, ethanol and the tastants were diluted in tap water. In contrast, for the CPP experiment ethanol was diluted in isotonic saline (NaCl 0.9%) and used for i.p. injections (1.5 g/kg). The volume of injection was adjusted to body weight.
CSC housing stress procedure
CSC was conducted as previously described (Reber et al., Citation2007; Schmidt et al., Citation2010; Singewald et al., Citation2009; Uschold-Schmidt et al., Citation2012). TO outbred mice were purchased from Harlan Olac (Bicester, UK) and bred in the local central animal facility of the College of Medicine and Health Sciences. The TO males display high levels of intraspecies and interspecies aggression (Brain & Al-Maliki, Citation1979; Brain & Evans, Citation1974). They have shown an intermediate aggression in putative test of aggression, compared to other strains (Jones & Brain, Citation1987). Briefly, four experimental subordinate “intruder” mice were introduced into home cage of larger dominant TO male mouse “resident” that had been housed with female mice for 10 days to enhance its territoriality and aggression. The CSC procedure lasted for 19 consecutive days. Males that started to injure their opponents by harmful bites were not used. Being too aggressive, two resident TO mice were discarded and one experimental (subordinate) mouse was not included in the CPP study as it died during conditioning due to injuries. To avoid habituation, the four intruder mice were transferred to a new cage with a novel resident on days 8 and 15. In all colonies, the larger male mouse established a dominant status by chasing and attacking all four experimental mice, as described in a previous report with rats (Stefanski et al., Citation2001). In parallel, single housed control (SHC) mice remained undisturbed in their home cages except for change of bedding once a week if needed.
EPM test
To assess the effect of CSC on anxiety-like behavior, mice were tested on the EPM between 8 and 11 am for 5 min on day 20. In the evening of the last CSC day (19th), all cages were transferred to the behavioral testing room. The CSC mice were never single-housed before undergoing the EPM procedure. The EPM setup was described previously (Bahi et al., Citation2009). Each mouse was placed on the central platform facing a closed arm. The maze was cleaned thoroughly before each test. The number of entries into the open and closed arms and the time spent on the respective arms were monitored to allow calculation of the percentage of time spent on open arms, and the percentage of entries into the open arms. The maze was cleaned with water and a dry paper towel between mice.
Open-field behavior
To assess the effect of CSC on locomotor activity, mice were tested in the open-field arena between 1 and 3 pm on day 20 (after completion of the EPM test). The open field was a 32 × 32 cm white Plexiglas arena, surrounded by 20-cm high walls. The floor of the arena was marked into 64 equal squares by black lines and the central 16 squares were defined as the center area. Mice were placed at the center of the arena, and allowed to explore freely for 10 min. Line-crossing (defined as at least three paws in a square) was used as a measure of locomotor activity. The time spent at the center of the arena was quantified and used as a measure of anxiety. The floor and the walls of the arena were cleaned with a sponge wetted with water and dried with a paper towel after each test.
Alcohol exposure: Two-bottle free-choice drinking paradigm
The experimental timeline is depicted in . On day 20 and after assessing the open-field behavior, mice were singly housed and given access to escalating concentrations of ethanol solutions. Oral alcohol self-administration and preference were studied using a two-bottle choice paradigm as described previously (Bahi et al., Citation2012). Briefly, mice were presented with two 10 ml graduated pipettes with stainless steel drinking spouts, which were securely held through the wire mesh cage lid. On day 1, one pipette was filled with 2.5% of ethanol solution. The concentration of ethanol was raised every third day, increasing from 2.5% to 5%, 10% and finally to 20% (v/v) ethanol in tap water.
Figure 1. Experimental timeline. The timelines show sequence and duration of experimental protocols of the effect of CSC on (A) voluntary ethanol intake and preference and (B) ethanol-induced conditioned-lace preference.
The positions of water and ethanol tubes were alternated each day to avoid place preference. Ethanol solutions were made by diluting 95% ethanol with the appropriate volume of water (i.e. volume-by-volume). Fluid intake in milliliters was converted to grams with the assumption for all solutions that 1 mL = 0.789 g. Mice were weighed every third day when the ethanol concentration was raised. For each concentration, the average ethanol consumption per day was obtained and used for the analysis. To obtain an accurate measure of ethanol consumption, grams of ethanol consumed per kilogram of body weight per 24 h were calculated for each mouse. Ethanol preference [(ethanol intake/ethanol + water intake) × 100] was also determined. Intake from both bottles was summed to obtain total fluid intake per body weight. An experimental timeline is shown in .
To control for any differences in sweet or bitter tastant preferences, intake of saccharin and quinine solutions was assessed after completion of the alcohol preference tests. After the final day of ethanol consumption, the mice were given water for 1 week. The mice were then once again offered 10 ml graduated pipettes of water and a tastant containing either a sweet (saccharin) or bitter (quinine) as before (3 days, escalating concentrations), and the tube position was alternated daily to avoid side preference. Saccharin was obtained from Sigma and prepared as 0.04% and 0.08%, solutions and quinine hemisulfate (Sigma) was prepared as 20 and 40 μM solutions. As before, the mice were weighed every day and the intake of each solution was determined daily. There was a 1-week water-only period between the saccharin and quinine tastants. An experimental timeline is shown in .
Ethanol-induced CPP
An unbiased CPP paradigm was used to study this effect of ethanol in both CSC and SHC. The method has been previously described in detail (Bahi & Dreyer, Citation2011; Bahi, Citation2012). Briefly, the experimental chambers used in our study were rectangular boxes divided into two compartments by an internal wall with a door in it. Both compartments were in the same size but were visually and tactilely distinct. The experimental procedure involved 1 habituation trial, 12 conditioning trials, and 1 test trial. In the habituation trial, mice received an injection of isotonic saline (10 ml/kg, i.p.) immediately before being placed in the middle of the chambers for 15 min. During this phase, the guillotine door is opened, so the mice can move freely in the box. The time spent in each box was recorded.
After this habituation phase, mice were divided into two groups: a SHC (n = 11) and chronically stressed colony housed mice (CSC; n = 14). After 19 days, the EPM and open-field tests were performed (day 20) and the second phase of the CPP procedure started on day 21. In this experiment, we assessed the effect of chronic psychosocial stress on ethanol CPP-acquisition as depicted in .
In the conditioning phase, mice were single-housed on day 20 and randomly assigned into two groups: saline and ethanol. Briefly, each mouse received an injection of either ethanol (1.5 g/kg, i.p.) or saline (10 ml/kg, i.p.), and restricted to its designated conditioning chamber for 30 min. For the 15-min test session, all mice received an injection of saline (10 ml/kg, i.p.) and were then put into the center of the box. Mice were allowed to explore the entire box. The exploration time spent in the ethanol-paired compartment was monitored. A place preference score was calculated for each mouse as the difference between post-conditioning and pre-conditioning time spent in the ethanol-paired compartment. One mouse was not included in the CPP analysis as it was injured and removed from the study [SHC n = 11; CSC n = 13].
Determination of blood ethanol metabolism
After completion of the EtOH-CPP study, SHC and CSC mice were used to test the effect of differences in body weight on blood ethanol levels when given an equal amount of ethanol (g) per kg of body weight. Mice were treated with a 1.5 g/kg dose of ethanol (i.p.; 20% v/v in saline), and blood ethanol concentration (BEC) was determined at 30, 60 and 120 min after injection using an alcohol dehydrogenase assay as described earlier (Bahi et al., Citation2012).
Statistical analysis
For statistical comparisons, the software package SPSS (version 19.0) was used. Data were expressed as mean ± SEM. The analysis of the effect of housing on body weight gain, the EPM, and the open-field behavior was analyzed using one-way measure of variance (ANOVA). The effects of housing on weight, ethanol consumption, taste consumption, CPP, and BEC were analyzed using a mixed repeated measure two-way ANOVA with housing (CSC or SHC) as the between-subjects factor. In cases of a significant main effect, post hoc comparisons were performed with Bonferroni's test. The criterion for statistical significance was p < 0.05.
Results
Chronic psychosocial stress-induced changes in body weight and anxiety behavior
Body weight
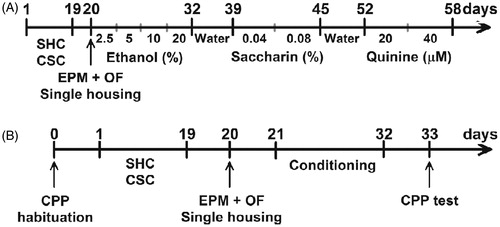
Weekly analysis of cumulative body weight gain showed that exposure to chronic psychosocial stress decreased the rate of body weight gain, consistent with other chronic stress paradigms (Bielajew et al., Citation2002). As expected, ANOVA with repeated measures revealed significant effects of time [F(3,141) = 247.871; p < 0.001; ] and housing [F(1,47) = 21.183; p < 0.001]. The CSC (n = 27) male mice gained less weight than the SHC controls (n = 22). In addition, the time × housing interaction [F(3,141) = 29.948; p < 0.005] was significant. As shown in , there was a significant statistical difference in body weight gain between the two groups [F(1,47) = 58.510; p < 0.001; one-way ANOVA].
Figure 2. Chronic psychosocial stress effects on body weight gain. (A) The daily defeated group CSC male mice gained significantly less body weight compared to SHC. (B) Chronic stressed mice showed attenuated weight after 19 days CSC. Data represent mean ± SEM. The number of animals per group was 22 SHC and 27 CSC mice. **p < 0.005 versus SHC control mice.
Elevated plus-maze
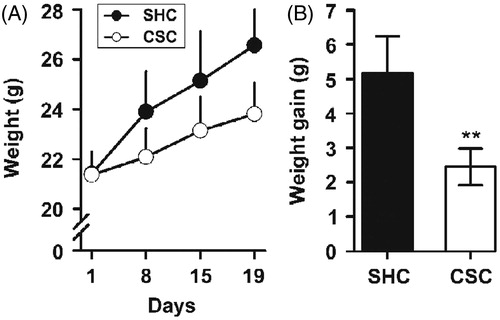
The primary objective of this experiment was to determine the effect of chronic psychosocial stress on anxiety-like behavior in mice. For this purpose, we used the EPM test. A number of studies report stress-induced anxiety-like behavior in rodents (Qin et al., Citation2011), but relatively few studies have assessed the effects of chronic psychosocial stress on anxiety behavior. We found that CSC (n = 27) male mice exhibited significantly more anxiety-like behavior in the plus-maze than their SHC male littermates (n = 22). In fact, CSC mice displayed a significant reduced percentage of open arms entries [F(1,47) = 28.526; p < 0.001; ] and spent less time [F(1,47) = 21.27 8; p < 0.001; ] in the open arms of the maze. In addition, CSC mice displayed lower number ofentries in the open arms of the maze [F(1,47) = 38.056; p < 0.001; ]. More importantly, data analysis did not reveal any main effects of stress exposure on the number of entries in the closed arms [F(1,47) = 0.053; p = 0.819; ], which is often used as an index of locomotor activity.
Figure 3. Anxious behavior of CSC male mice. Behavior of CSC mice in the EPM test: CSC mice showed (A) reduced percentage entries of open arms (OA) visits, (B) spent less time in the OA of the maze, and (C) have less number of entries in the OA. (D) Both SHC and CSC groups have the same number of entries in the closed arms (CA), indicating that stress procedure did not affect spontaneous locomotor activity. Data represent mean ± SEM. The number of animals per group was 22 SHC and 27 CSC mice. *p < 0.0001 versus SHC control mice.
Open field
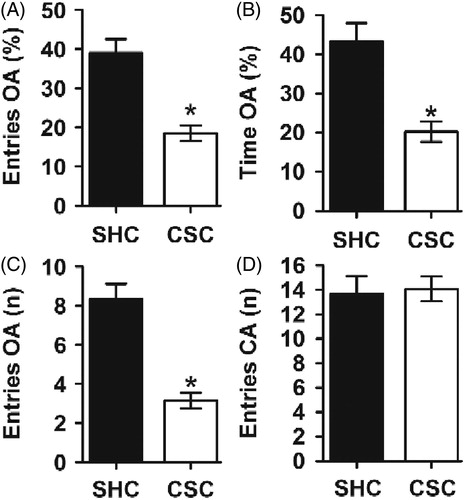
Results for open-field behavior are shown in . There were no significant differences between groups in the number of line crossings performed [F(1,47) = 0.232; p = 0.632; ], indicating that CSC mice showed no alterations in spontaneous locomotion. However, there was a significant difference between SHC (n = 22) and CSC mice (n = 27) in time spent at the center of the arena. CSC spent less time at the center than SHC mice [F(1,47) = 9.929; p = 0.003; ] compared to control animals, indicative of increased anxiety-like behavior.
Figure 4. Open-field test. (A) There was no significant difference between the SHC and CSC mice in general locomotor activity as measured by total line crossings. In contrast, (B) CSC mice spent less time in the center of the arena, indicating an increased anxietylike behavior of these mice compared to the SHC. Data represent mean ± SEM. The number of animals per group was 22 SHC and 27 CSC mice. *p < 0.0001 versus SHC control mice.
Chronic psychosocial stress-induced changes in ethanol intake
Ethanol consumption and preference in the two-bottle choice paradigm
We first asked whether chronic psychosocial stress changes alcohol consumption and preference using a two-bottle free-choice paradigm in which mice could drink either water or an ascending series of ethanol concentrations (2.5, 5, 10, and 20%). shows ethanol consumption, ethanol preference, and total fluid intake for male SHC (n = 11) and CSC (n = 13) mice over the 2 weeks of the experiment.
Figure 5. Chronic psychosocial stress effects on ethanol consumption and preference. (A) Average of ethanol intake (ml/24 h) across all days showed increased ethanol intake in CSC mice compared to SHC controls. (B) CSC mice displayed more ethanol intake than SHC expressed as ml per mouse. (C) Average of daily ethanol consumption expressed as grams per kg of body weight. (D) Daily ethanol intake of ethanol expressed as g/kg of body weight across the four ethanol solutions. (E) No difference in total fluid intake (water þ ethanol) was observed between CSC and SHC male mice throughout the experiment. (F) CSC mice consumed less water than SHC control animals. Data represent mean ± SEM. The number of animals per group was 11 SHC and 13 CSC mice. *p < 0.05 versus SHC control mice.
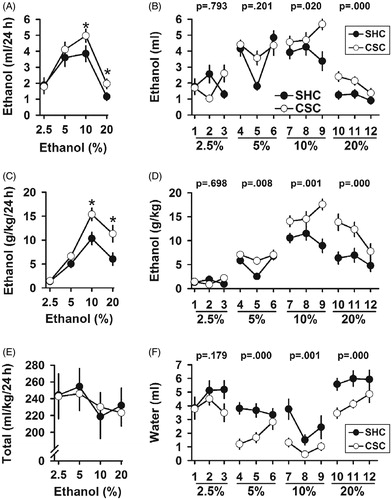
Ethanol consumption
As shown in , a repeated two-way ANOVA measure with stress as between-subjects variables and alcohol concentration as a within-subjects variable of daily ethanol intake revealed a main effect of both factors [main effect of stress: F(1,66) = 11.161; p < 0.001; main effect of ethanol concentration: F(3,198) = 79.698; p < 0.0001]. Post hoc tests of these main effects revealed that chronically stressed mice (CSC) consumed significantly more ethanol than did control SHC mice. In addition to these main effects, there was a significant interaction between stress and ethanol concentration [F(3,198) = 2.678; p < 0.048]. The stress procedure clearly produced a reduction in body weight. Thus, reduced body weight might inflate an apparent increase in alcohol consumption since the data are presented in relation to body weight. Given the differences in body weight, alcohol intake was also reported as daily (ml) intake before averaging over several days for a given concentration. As shown in , two-way ANOVA with ethanol and stress as the between subject factors revealed a main effect of ethanol concentration [F(3,88) = 61.412; p = 0.000], as well as a main effect of stress [F(1,88) = 10.365; p = 0.002]. No interaction was found between the two factors. However, tests of within-subjects effects revealed a significant three-way interaction between ethanol, stress, and time [F(6,176) = 4.903; p < 0.000]. We also analyzed ethanol consumption as g/kg/24 h and similar results were found [main effect of stress: F(1,66) = 45.754; p < 0.001; main effect of ethanol concentration: F(3,198) = 124.711; p < 0.001] (). Post hoc tests of these main effects revealed that ethanol consumption was higher in CSC mice compared to their SHC controls. Alcohol consumption was normally distributed over the course of the experiment due to changes in alcohol concentration. There was a significant interaction between stress and ethanol concentration [F(3,198) = 8.892; p < 0.001]. Furthermore, we expressed the alcohol consumption as daily intake (g/kg) before averaging and data are shown in . Daily ethanol intake expressed as g/kg of body weight revealed a main effect of both factors [main effect of ethanol concentration: F(3,88) = 120.647; p < 0.000; main effect of stress: F(1,88) = 47.303; p < 0.000]. The interaction between ethanol and stress was significant [F(3,88) = 8.602; p < 0.000]. Interestingly, tests of within-subjects effects also revealed a significant three-way interaction between ethanol, stress, and time [F(6,176) = 2.694; p = 0.016]. Post hoc tests revealed that the impact of stress was present at the 5%, 10%, and 20% alcohol concentrations.
Total fluid intake
To examine possible changes in total fluid intake resulting from the chronic psycho-social stress procedure, data for ethanol and water volumes were averaged for each ethanol concentration. The two-way repeated measures ANOVA revealed no significant impact of stress [F(1,66) = 0.059; p = 0.810] or alcohol concentration [F(3,198) = 1.981; p = 0.118] on total fluid intake (). Moreover, there was no significant interaction between stress and ethanol concentration available [F(3,198) = 0.285; p = 0.836]. Post hoc analysis revealed that SHC and CSC mice drank similar amount of total fluid. Given the large body weight differences, we also reported water consumption across the four- ethanol concentration. As depicted in , CSC mice consumed less water than SHC controls. Post hoc tests revealed that the impact of stress was present at the 5%, 10%, and 20% alcohol concentrations.
Ethanol preference
A repeated two-way ANOVA measure with stress as between-subjects variable and alcohol concentration as a within-subjects variable revealed a significant effect of stress [F(1,66) = 34.137; p < 0.001] and alcohol concentration [F(3,198) = 95.871; p < 0.001] on alcohol preference (data not shown). Post hoc analysis of these effects revealed that CSC mice had increased ethanol preference relative to SHC male mice. In addition, there was a significant interaction between stress and alcohol concentration [F(3,198) = 4.970; p = 0.002]. Pairwise comparisons indicated that, as for ethanol consumption, ethanol preference in CSC mice was significantly higher when mice were presented 5%, 10%, and 20% ethanol solutions (data not shown).
Non-alcohol preference drinking: Saccharin consumption & preference
Increased ethanol intake in CSC mice could be the result of an impaired sense of taste. Therefore, we evaluated the consumption and preference for a sweet solution (saccharin) and a bitter solution (quinine), to determine whether any difference existed in general taste preferences and whether increased ethanol consumption in the CSC group was unrelated to the pharmacological effects of ethanol.
Saccharin consumption
As shown in , there were no differences in saccharin consumption between the two groups (SHC vs. CSC) in [F(1,66) = 0.708; p = 0.403]. Moreover, the interaction between stress and saccharin concentration was not significant [F(1,66) = 0.001; p = 0.995]. In contrast, a significant effect of concentration was apparent [F(1,66) = 123.47; p < 0.001], which reflected a general tendency for both SHC and CSC mice towards higher intake of more concentrated saccharin solutions. In fact, both CSC and SHC male mice drank more saccharin from the higher concentration (0.08%). Planned comparisons did not show significant differences between the two groups within each saccharin concentration.
Figure 6. Chronic psychosocial stress effects on saccharin consumption and preference. (A) Daily saccharin consumption (g/kg) across all days of the experiment showed similar saccharin intake in CSC mice compared to SHC controls, but both groups consumed more saccharin from the higher concentration. (B) CSC and SHC mice showed similar saccharin preference but tend to prefer the higher concentration. (C) No difference in total fluid intake was observed between CSC and SHC male mice throughout the experiment. Data represent mean ± SEM. The number of animals per group was 11 SHC and 13 CSC mice. *p < 0.005 versus saccharin lower concentration (closed bars: SHC; open bars: CSC).
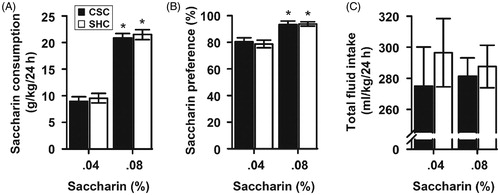
Saccharin preference
As shown in , both CSC and SHC mice had equal preference for saccharin at both concentrations. As for saccharin consumption, stress had no effect on saccharin preference [F(1,66) = 0.169; p = 0.683]. In contrast, there was a significant effect of concentration [F(1,66) = 103.625; p < 0.001] on saccharin preference reflecting a general tendency toward higher preference for more concentrated sweet solutions (0.08% compared to 0.04%). In addition, the interaction between stress and saccharin concentration was not statistically significant [F(1,66) = 0.698; p = 0.406].
Total fluid intake
As depicted in , there was no difference in total fluid intake between SHC and CSC mice [main effect of stress: F(1,66) = 0.613; p = 0.343; main effect of saccharin concentration: F(1,66) = 0.004; p = 0.949; stress × saccharin concentration interaction: F(1,66) = 0.709; p = 0.403].
Non-alcohol preference drinking: Quinine consumption and preference
One week after the saccharin drinking study, the same mice were tested for quinine (bitter) consumption and preference in a two-bottle choice paradigm using two ascending concentrations of quinine (20 and 40 uM).
Quinine consumption
As depicted in , there was no difference between the two groups (SHC versus CSC) in quinine consumption [F(1,66) = 2.508; p = 0.118]. The interaction between stress and quinine concentration was not significant [F(1,66) = 0.073; p = 0.788]. In contrast, there was a significant effect of concentration [F(1,66) = 15.653; p < 0.001], which reflected a general tendency for both SHC and CSC mice towards higher intake of more concentrated quinine solutions. In fact, both CSC and SHC male mice drank more quinine from the higher concentration (40 μM). Post hoc comparisons did not show significant differences between the two groups within each quinine concentration.
Figure 7. Chronic psychosocial stress effects on quinine consumption and preference. (A) Daily quinine consumption (g/kg) across all days of the experiment showed similar quinine intake in CSC mice compared to SHC controls, but both groups consumed more quinine from the higher concentration. (B) CSC and SHC mice showed similar quinine preference. (C) No difference in total fluid intake was observed between CSC and SHC male mice throughout the experiment. Data represent mean ± SEM. The number of animals per groups was 11 SHC and 13 CSC mice. *p < 0.005 versus quinine lower concentration (closed bars: SHC; open bars: CSC).
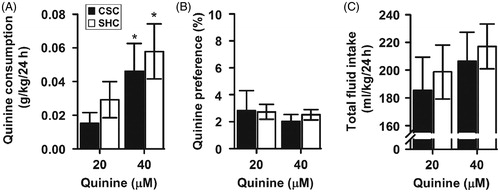
Quinine preference
As shown in , both CSC and SHC mice had equal preference for quinine at both concentrations. In fact, stress had no effect on quinine preference and there was no difference between the two groups [F(1,66) = 0.062; p = 0.804]. Also, there was no significant effect of concentration [F(1,66) = 0.3 64; p = 0.548] on quinine preference. More importantly, the interaction between stress and quinine concentration was not significant [F(1,66) = 0.123; p = 0.727].
Total fluid intake
As depicted in , there was no difference in the total fluid intake between SHC and CSC mice [main effect of stress: F(1,66) = 0.512; p = 0.477; main effect of quinine concentration: F(1,66) = 2.765; p = 0.101; stress × quinine concentration interaction: F(1,66) = 0.062; p = 0.804].
CSC mice develop greater ethanol-induced CPP
CPP was used to study the rewarding properties of ethanol. After conditioning with saline in both conditioning compartments (saline-saline control), neither SHC (n = 11) nor CSC (n = 13) mice showed any innate preference for a particular compartment and spent approximately equal times in both compartments of the CPP cage [F(1,22) = 0.080; p = 0.780] suggesting the absence of biased preference for color/floor texture. Importantly, and as depicted in , neither SHC nor CSC males developed any significant preference for any compartment in either post or preconditioning sessions [F(1,22) = 0.003; p = 0.959]. In contrast, when etha-nol (1.5 g/kg) was used for conditioning, a two-way ANOVA analysis revealed that CSC mice (n = 14) show greater preference for the ethanol-paired compartments as compared to SHC controls (n = 11) (). There were significant main effectsofstress[F(1,45) = 6.649; p = 0.013] and ethanol [F(1,45) = 32.300; p < 0.001] and a stress × ethanol interaction [F(1,45) = 6.298; p = 0.016].
Figure 8. Effect of CSC on ethanol-induced CPP and BEC. (A) When conditioned with ethanol, CSC mice showed higher preference for the ethanol paired box but no difference was found when saline was used for conditioning in both compartments. Data represent mean ± SEM. *p < 0.01, **p < 0.001 versus ethanol conditioning. #p < 0.01 versus SHC mice. Saline/SHC, n = 11; saline/CSC, n = 13; ethanol/SHC, n = 11; ethanol/CSC, n = 14. (B) BEC 30, 60, and 120 min after an i.p. injection of 1.5 g/kg of ethanol in SHC and CSC mice, presented as mean ± SEM.
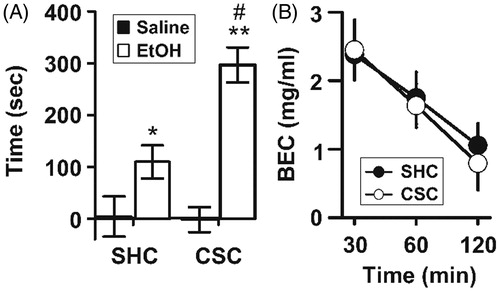
It is possible that the difference in ethanol rewarding properties seen in SHC and CSC mice might be a result of altered ethanol metabolism in these mice. In other words, we needed to ensure that the phenotypic differences observed above are not due to a change in ethanol metabolism. To test this possibility, BEC of the SHC and CSC mice was measured after the injection of a single dose of 1.5 g/kg ethanol as in the EtOH-CPP studies. Then we measured BEC at time points ranging from 30 min to 2 h after ethanol administration. There were no differences between SHC and CSC mice in BEC at any time after injection. As shown , BEC is similar in the CSC andSHC mice[main effect of time F(2,28) = 57.172; p < 0.0001, whereas there was no effect of stress F(1,14) = 0.015; p = 0.903 and no interaction F(2,28) = 0.135; p = 0.875].
Discussion
Metabolic and behavioral consequences of CSC housing in male mice
The present results show that chronic psychosocial stress exposure during adulthood increases alcohol-conditioned reward and drinking in mice. The current results are in agreement with previous studies showing that prolonged periods of stress result in higher anxiety levels, despite use of different methodologies and animal species. When tested in hierarchical dominance/submissive relationships, submissive mice had decreased number of entries into the open arms and decreased time spent in the exploration of the open arms of the plus maze (Denmark et al., Citation2010), suggesting that submissive mice exhibit anxiety-like responses, which predicts that they may be more sensitive to stressful stimuli. Longer period of stress has been shown to produce similar effects. In fact, after 7 weeks of chronic stress, animals display an increased anxiety-like behavior in the EPM and the novelty-suppressed feeding test (Sterlemann et al., Citation2008). Using DBA/2OlaHsd mice, chronic social defeat leads to increased avoidance behavior and reduction in directed and general exploration, an anxiogenic phenotype that is reversed by the corticotropin-releasing hormone receptor 1 (CRHR1)-antagonist R121919/NBI 30775 (Erhardt et al., Citation2009). Subordinate 129SvEv mice also show increased anxiety in the EPM and social avoidance of an unfamiliar male mouse (Dadomo et al., Citation2011). Finally, increased anxiety-like behavior is also observed in male (Nephew & Bridges, Citation2011; Rygula et al., Citation2008) and female (Mathews et al., Citation2008a,Citationb; McCormick et al., Citation2008) rats subjected to chronic social stress.
Chronic psychosocial stress reduced body weight gain in subordinate CSC mice. These data confirm previous reports that chronic stress results in significant body weight loss in subordinates, which is one of the most pronounced and reliable consequences of subordination stress (Tamashiro et al., Citation2004). The body weight decrease observed in intruder mice results from loss of both adipose and lean tissue, with preferential retention of visceral adiposity, suggesting that the latter results from subordination stress (Tamashiro et al., Citation2007). Taken together, results demonstrated remarkable stress vulnerability for subordinates and establish the validity to use this chronic psychosocial stress as a model for obesity and anxiety-related disorders.
Chronic psychosocial stress-induced alcohol consumption in mice
The goal of our study was to investigate the effect of chronic psychosocial stress on alcohol vulnerability. Our results reveal that alcohol consumption and preference were increased by chronic stress. We confirm in the present study that chronic stress exacerbates alcohol intake and preference in mice. In humans, stressful events and stressful conditions are all correlated with indicators of alcoholism (Linsky et al., Citation1987). Also, an acute psychosocial stressor (the Trier Social Stress Test) increases alcohol consumption in non-treatment-seeking alcoholics (Thomas et al., Citation2011). In contrast, stress increases sedative effects of ethanol and did not increase desire for more alcohol (de Wit et al., Citation2003).
These findings are consistent with those of a previous study showing both increases and decreases in ethanol intake, depending on the type of stressor, the history of prior stress and/or ethanol exposure, sex, genetic background of the animals, and other variables. In fact, moderately and severely fight-stressed submissive mice showed increased consumption of alcohol solution (Hilakivi-Clarke & Lister, Citation1992; Faber et al., Citation2006). Social stress duration affected ethanol intake in mice. Compared with a single defeat, five consecutive daily defeat sessions had a slow onset effect in increasing alcohol preference and consumption (Croft et al., Citation2005). In rats, subordinate male ethanol consumption is significantly higher than that of dominants, suggesting that the social stress of subordination may be a factor in alcohol consumption (Blanchard et al., Citation1987). Other reports showed that the experience of mild, repeated social defeat stress can preferentially increase alcohol drinking in Long-Evans rats (Caldwell & Riccio, Citation2010). In non-human primates, chronic stress of social subordination was correlated with increased alcohol drinking (McKenzie-Quirk & Miczek, Citation2008). Thus, these social stress-induced alcohol drinking does parallel the common human situation in which stressful events can lead to excessive alcohol intake and alcoholism (Blanchard et al., Citation1993).
The increased intake and preference for ethanol observed in CSC mice supports the initial hypothesis. This difference did not involve any impairment in total liquid intake between the two groups. Therefore, other factors known to contribute to ethanol appetitive behaviors were examined. It is possible that the palatability of ethanol differs between SHC and CSC mice; however, in the current studies, we observed no differences in the consumption and preference of saccharin or quinine solutions, suggesting that there were no differences in taste preferences between the two groups.
Experience of chronic social stress was often linked to increase in alcohol voluntary consumption, but experimental approaches using animal models of ethanol self-administration have had widely varying outcomes. In fact, in both the home cage drinking and operant conditioning groups of male Long-Evans rats, social stress significantly decreased alcohol intake or rate of alcohol reinforcements, respectively (van Erp & Miczek, Citation2001). The same group has also shown that short social stress episodes reduced daily ethanol intake (van Erp et al., Citation2001), suggesting that these findings do not support the hypothesis for enhanced alcohol intake following social stress exposure. In contrast, when dependent rats previously trained to self-administer ethanol received chronic daily intermittent foot shock (non-social stressor), they did not show any difference in ethanol consumption (Dayas et al., Citation2004).
Previous studies that reported either a suppression of drinking (van Erp & Miczek, Citation2001; van Erp et al., Citation2001) or no change (Bowers et al., Citation1997) used protocols with ethanol access in the presence of stress-related environmental cues, intermittent stress exposures, or tested drinking at different temporal parameters from those used in the present experiment. Furthermore, acute exposure to social defeat decreased ethanol self-administration (12% w/v, 1 h day), lowered rates of responding during extinction, and did not reinstate alcohol-seeking behavior in rats (Funk et al., Citation2005). One explanation is that reduced or equal consumption involved fear-conditioned suppression of reward-seeking behavior. Also, the reduction of ethanol intake may correspond to anhedonia. Our findings indicate the importance of choosing adequate stress and temporal parameters (severity and stress duration) to avoid anhedonia.
Chronic psychosocial stress-induced alcohol conditioned reward in mice
The CPP test was used to assess the effect of stress on ethanol preference. In the present study, we demonstrated that chronic social stress may facilitate the acquisition of alcohol CPP. We found that, compared with SHC mice, CSC mice had an increased response to ethanol-rewarding properties in the CPP test. This finding supports clinical demonstrations that traumatic experiences, such as maltreatment, are associated with enhanced risk of alcohol and substance abuse disorders (De Bellis, Citation2002). Our findings are consistent with previous studies demonstrating that stress exposure might lead to alcohol vulnerability. In fact, it has been reported that inescapable tail-shock enhanced the CPP response to oxycodone, but not to ethanol (Der-Avakian et al., Citation2007). In addition, when adolescent mice were exposed to chronic (1 week) but not acute (1 day) foot-shock stress before CPP training, mice were more sensitive to the rewarding properties of ethanol. In fact, 2 g/kg ethanol elicited significant place preference only in stress-exposed mice (Song et al., Citation2007). Furthermore, C57Bl/6 J mice exposed to repeated forced swim stress 5 min prior to daily place conditioning with ethanol (0.8 g/kg) displayed a robust potentiation of ethanol-CPP compared to unstressed mice (Sperling et al., Citation2010). These results indicate that CSC mice were more sensitive than SHC mice to the rewarding properties of ethanol.
One potential factor that might explain the difference in ethanol intake and preference observed in this study is that the BECs differ between the SHC and CSC groups. To test this possibility, we measured BEC in both groups following injection of an acute dose of ethanol (1.5 g/kg) that produced differential stress-dependent place preference. The results show no differences in BEC between the stress groups. In fact, the increased ethanol intake in CSC mice cannot be explained by a change in ethanol pharmacokinetics. These data indicate dissociation between EtOH-CPP and BEC, suggesting that the stress-dependent differential preference for ethanol observed in the current study cannot be associated to differential BEC.
Overall, these data demonstrate that behavioral correlates linked to social status and social stress in mice are paralleled by significant increments in the rewarding efficacy of alcohol. In addition, the present studies do not diminish the extensive literature linking social stress to the rewarding effects of alcohol, but clearly show that additional investigations are needed to fully characterize the effects of chronic social stress on ethanol reward-related behaviors. Therefore, determining the influence of chronic social stress on addictive behaviors is a crucial, yet challenging, and complex task.
Declaration of interest
The author has received funds from the United Arab Emirates University (salary and research funding). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The author has no financial interests that might be perceived to influence the results or the discussion reported in this article. The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
| Abbreviations: | ||
| BEC: | = | blood ethanol concentration |
| CPP: | = | conditioned-place preference |
| CSC: | = | chronic subordinate colony |
| EPM: | = | elevated plus-maze |
| OF: | = | open field |
| SHC: | = | single house colony |
| TO mice: | = | tuck-ordinary mice |
Acknowledgements
The author would like to acknowledge Mr Mohamed Elwasila and Mr Mohamed Shafiullah for their technical assistance.
References
- Armario A. (2010). Activation of the hypothalamic-pituitary-adrenal axis by addictive drugs: Different pathways, common outcome. Trends Pharmacol Sci 31:318–25
- Bahi A. (2012). The selective metabotropic glutamate receptor 7 allosteric agonist AMN082 prevents reinstatement of extinguished ethanol-induced conditioned place preference in mice. Pharmacol Biochem Behav 101:193–200
- Bahi A, Dreyer JL. (2011). Involvement of tissue plasminogen activator “tPA” in ethanol-induced locomotor sensitization and conditioned-place preference. Behav Brain Res 226:250–8
- Bahi A, Fizia K, Dietz M, Gasparini F, Flor PJ. (2012). Pharmacological modulation of mGluR7 with AMN082 and MMPIP exerts specific influences on alcohol consumption and preference in rats. Addict Biol 17:235–47
- Bahi A, Mineur YS, Picciotto MR. (2009). Blockade of protein phosphatase 2B activity in the amygdala increases anxiety- and depression-like behaviors in mice. Biol Psychiatry 66:1139–46
- Bielajew C, Konkle AT, Merali Z. (2002). The effects of chronic mild stress on male Sprague-Dawley and Long Evans rats: I. Biochemical and physiological analyses. Behav Brain Res 136:583–92
- Blanchard RJ, Hori K, Tom P, Blanchard DC. (1987). Social structure and ethanol consumption in the laboratory rat. Pharmacol Biochem Behav 28:437–42
- Blanchard RJ, Yudko EB, Blanchard DC. (1993). Alcohol, aggression and the stress of subordination. J Stud Alcohol Suppl 11:146–55
- Bowers WJ, Sabongui AG, Amit Z. (1997). The role of ethanol availability on stress-induced increases in ethanol consumption. Alcohol 14:551–6
- Boyce-Rustay JM, Janos AL, Holmes A. (2008). Effects of chronic swim stress on EtOH-related behaviors in C57BL/6J, DBA/2J and BALB/cByJ mice. Behav Brain Res 186:133–7
- Brain PF, Al-Maliki S. (1979). Effects of lithium chloride injections on rank-related fighting, maternal aggression and locust-killing responses in naive and experienced ‘TO' strain mice. Pharmacol Biochem Behav 10:663–9
- Brain PF, Evans CM. (1974). Influences of two naturally occurring androgens on the attack directed by “trained fighter” TO strain mice towards castrated mice of three different strains. ICRS J Int Res Commun 2:1672
- Caldwell EE, Riccio DC. (2010). Alcohol self-administration in rats: Modulation by temporal parameters related to repeated mild social defeat stress. Alcohol 44:265–74
- Chester JA, Barrenha GD, Hughes ML, Keuneke KJ. (2008). Age and sex-dependent effects of footshock stress on subsequent alcohol drinking and acoustic startle behavior in mice selectively bred for high-alcohol preference. Alcohol Clin Exp Res 32:1782–94
- Croft AP, Brooks SP, Cole J, Little HJ. (2005). Social defeat increases alcohol preference of C57BL/10 strain mice; effect prevented by a CCKB antagonist. Psychopharmacology (Berl) 183:163–70
- Dadomo H, Sanghez V, Di Cristo L, Lori A, Ceresini G, Malinge I, Parmigiani S, et al. (2011). Vulnerability to chronic subordination stress-induced depression-like disorders in adult 129SvEv male mice. Prog Neuropsychopharmacol Biol Psychiatry 35:1461–71
- Dayas CV, Martin-Fardon R, Thorsell A, Weiss F. (2004). Chronic footshock, but not a physiological stressor, suppresses the alcohol deprivation effect in dependent rats. Alcohol Alcohol 39:190–6
- De Bellis MD. (2002). Developmental traumatology: A contributory mechanism for alcohol and substance use disorders. Psycho-neuroendocrinology 27:155–70
- Denmark A, Tien D, Wong K, Chung A, Cachat J, Goodspeed J, Grimes C, et al. (2010). The effects of chronic social defeat stress on mouse self-grooming behavior and its patterning. Behav Brain Res 208:553–9
- Der-Avakian A, Bland ST, Rozeske RR, Tamblyn JP, Hutchinson MR, Watkins LR, Maier SF. (2007). The effects of a single exposure to uncontrollable stress on the subsequent conditioned place preference responses to oxycodone, cocaine, and ethanol in rats. Psychopharmacology (Berl) 191:909–17
- de Wit H, Soderpalm AH, Nikolayev L, Young E. (2003). Effects of acute social stress on alcohol consumption in healthy subjects. Alcohol Clin Exp Res 27:1270–7
- Edwards S, Guerrero M, Ghoneim OM, Roberts E, Koob GF. (2012). Evidence that vasopressin V1b receptors mediate the transition to excessive drinking in ethanol-dependent rats. Addict Biol 17:76–85
- Erhardt A, Muller MB, Rodel A, Welt T, Ohl F, Holsboer F, Keck ME. (2009). Consequences of chronic social stress on behaviour and vasopressin gene expression in the PVN of DBA/2OlaHsd mice-influence of treatment with the CRHR1-antagonist R121919/NBI 30775. J Psychopharmacol 23:31–9
- Faber F, Gembardt F, Sun X, Mizutani S, Siems WE, Walther T. (2006). Lack of angiotensin II conversion to angiotensin III increases water but not alcohol consumption in aminopeptidase A-deficient mice. Regul Pept 136:130–7
- Funk D, Harding S, Juzytsch W, Le AD. (2005). Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 183:341–9
- Gomez JL, Lewis MJ, Luine VN. (2012). The interaction of chronic restraint stress and voluntary alcohol intake: Effects on spatial memory in male rats. Alcohol 46:499–504
- Hilakivi-Clarke L, Lister RG. (1992). Social status and voluntary alcohol consumption in mice: Interaction with stress. Psycho-pharmacology (Berl) 108:276–82
- Hofmann CE, Ellis L, Yu WK, Weinberg J. (2007). Hypothalamic-pituitary-adrenal responses to 5-HT1A and 5-HT2A/C agonists are differentially altered in female and male rats prenatally exposed to ethanol. Alcohol Clin Exp Res 31:345–55
- Jones SE, Brain PF. (1987). Performances of inbred and outbred laboratory mice in putative tests of aggression. Behav Genet 17:87–96
- Linsky AS, Colby JP Jr, Straus MA. (1987). Social stress, normative constraints and alcohol problems in American states. Soc Sci Med 24:875–83
- Mathews IZ, Mills RG, McCormick CM. (2008a). Chronic social stress in adolescence influenced both amphetamine conditioned place preference and locomotor sensitization. Dev Psychobiol 50:451–9
- Mathews IZ, Wilton A, Styles A, McCormick CM. (2008b). Increased depressive behaviour in females and heightened corticosterone release in males to swim stress after adolescent social stress in rats. Behav Brain Res 190:33–40
- McCormick CM, Smith C, Mathews IZ. (2008). Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res 187:228–38
- McKenzie-Quirk SD, Miczek KA. (2008). Social rank and social separation as determinants of alcohol drinking in squirrel monkeys. Psychopharmacology (Berl) 201:137–45
- Minnick SA, Miller SL, Wehner JM. (1995). The effects of acute stress on ethanol absorption in LS and SS mice. Alcohol 12:257–63
- Mohn CE, Fernandez-Solari J, De Laurentiis A, Bornstein SR, Ehrhart-Bornstein M, Rettori V. (2011). Adrenal gland responses to lipopolysaccharide after stress and ethanol administration in male rats. Stress 14:216–26
- Nagaraja HS, Jeganathan PS. (2003). Effect of acute and chronic conditions ofover-crowding on free choice ethanol intake in rats. Indian J Physiol Pharmacol 47:325–31
- Nephew BC, Bridges RS. (2011). Effects of chronic social stress during lactation on maternal behavior and growth in rats. Stress 14:677–84
- Nesic J, Duka T. (2006). Gender specific effects of a mild stressor on alcohol cue reactivity in heavy social drinkers. Pharmacol Biochem Behav 83:239–48
- Qin M, Xia Z, Huang T, Smith CB. (2011). Effects of chronic immobilization stress on anxiety-like behavior and basolateral amygdala morphology in Fmr1 knockout mice. Neuroscience 194:282–90
- Reber SO, Birkeneder L, Veenema AH, Obermeier F, Falk W, Straub RH, Neumann ID. (2007). Adrenal insufficiency and colonic inflammation after a novel chronic psycho-social stress paradigm in mice: Implications and mechanisms. Endocrinology 148:670–82
- Reber SO, Neumann ID. (2008). Defensive behavioral strategies and enhanced state anxiety during chronic subordinate colony housing are accompanied by reduced hypothalamic vasopressin, but not oxytocin, expression. AnnN YAcad Sci 1148:184–95
- Reber SO, Obermeier F, Straub RH, Falk W, Neumann ID. (2006). Chronic intermittent psychosocial stress (social defeat/overcrowding) in mice increases the severity of an acute DSS-induced colitis and impairs regeneration. Endocrinology 147:4968–76
- Rygula R, Abumaria N, Havemann-Reinecke U, Ruther E, Hiemke C, Zernig G, Fuchs E, Flugge G. (2008). Pharmacological validation of a chronic social stress model of depression in rats: Effects of reboxetine, haloperidol and diazepam. Behav Pharmacol 19:183–96
- Sanna PP, Simpson C, Lutjens R, Koob G. (2002). ERK regulation in chronic ethanol exposure and withdrawal. Brain Res 948:186–91
- Schmidt D, Reber SO, Botteron C, Barth T, Peterlik D, Uschold N, Mannel DN, Lechner A. (2010). Chronic psychosocial stress promotes systemic immune activation and the development of inflammatory Th cell responses. Brain Behav Immun 24:1097–104
- Singewald GM, Nguyen NK, Neumann ID, Singewald N, Reber SO. (2009). Effect of chronic psychosocial stress-induced by subordinate colony (CSC) housing on brain neuronal activity patterns in mice. Stress 12:58–69
- Song M, Wang XY, Zhao M, Wang XY, Zhai HF, Lu L. (2007). Role ofstress in acquisition of alcohol-conditioned place preference in adolescent and adult mice. Alcohol Clin Exp Res 31:2001–5
- Sperling RE, Gomes SM, Sypek EI, Carey AN, McLaughlin JP. (2010). Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology (Berl) 210:199–209
- Stefanski V, Knopf G, Schulz S. (2001). Long-term colony housing in Long Evans rats: Immunological, hormonal, and behavioral consequences. J Neuroimmunol 114:122–30
- Sterlemann V, Ganea K, Liebl C, Harbich D, Alam S, Holsboer F, Muller MB, Schmidt MV. (2008). Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: Implications for stress-related disorders. Horm Behav 53:386–94
- Stevens S, Gerlach AL, Rist F. (2008). Effects ofalcohol on ratings of emotional facial expressions in social phobics. J Anxiety Disord 22:940–8
- Tamashiro KL, Hegeman MA, Nguyen MM, Melhorn SJ, Ma LY, Woods SC, Sakai RR. (2007). Dynamic body weight and body composition changes in response to subordination stress. Physiol Behav 91:440–8
- Tamashiro KL, Nguyen MM, Fujikawa T, Xu T, Yun Ma L, Woods SC, Sakai RR. (2004). Metabolic and endocrine consequences of social stress in a visible burrow system. Physiol Behav 80:683–93
- Thomas SE, Bacon AK, Randall PK, Brady KT, See RE. (2011). An acute psychosocial stressor increases drinking in non-treatment-seeking alcoholics. Psychopharmacology (Berl) 218:19–28
- Uschold-Schmidt N, Nyuyki KD, Fuchsl AM, Neumann ID, Reber SO. (2012). Chronic psychosocial stress results in sensitization of the HPA axis to acute heterotypic stressors despite a reduction of adrenal in vitro ACTH responsiveness. Psychoneuroendocrinology 37:1676–87
- van Erp AM, Miczek KA. (2001). Persistent suppression of ethanol self-administration by brief social stress in rats and increased startle response as index of withdrawal. Physiol Behav 73:301–11
- van Erp AM, Tachi N, Miczek KA. (2001). Short or continuous social stress: Suppression of continuously available ethanol intake in subordinate rats. Behav Pharmacol 12:335–42
- Varlinskaya EI, Spear LP. (2010). Sensitization to social anxiolytic effects of ethanol in adolescent and adult Sprague-Dawley rats after repeated ethanol exposure. Alcohol 44:99–110
- Walter M, Wiesbeck GA, Riecher-Rossler A, Borgwardt SJ. (2010). Neural effects of heroin-relation to anxiety stress. Prog Neuropsychopharmacol Biol Psychiatry 34:816–17
- Zhao LY, Zhang XL, Shi J, Epstein DH, Lu L. (2009). Psychosocial stress after reactivation of drug-related memory impairs later recall in abstinent heroin addicts. Psychopharmacology (Berl) 203:599–608