Abstract
This study compared cortisol responses to a standardized psychosocial stressor during a major depressive episode (MDE) and again during remission in adolescents and young adults. Twenty-six individuals with no personal or family history of a major psychiatric disorder (NC) and 24 individuals with a diagnosis of major depressive disorder (MDD) at Time 1 participated in the study. The MDD group showed robust cortisol responses during their index episode and after recovery. In contrast, the NC group showed habituation to the repeated psychosocial stressor, as evident in a flatter cortisol response profile at Time 2. Within the MDD group, net peak cortisol during the first stress test was positively associated with the duration of the index MDE and negatively associated with the total duration of all MDEs. Whereas summary indices of cortisol responses were relatively stable across repeated stress tasks within the MDD group, this was not the case for NC. Results demonstrate that cortisol responses fail to habituate to repeated psychosocial stress during recovery from an MDE and could reflect a trait-like marker of risk for recurrence.
Introduction
Disrupted hypothalamic–pituitary–adrenal (HPA) function often characterizes individuals with major depressive disorder (MDD), and has been implicated in the development and course of this debilitating and recurrent disorder (Holsboer, Citation2000). One critical task for researchers is to determine whether and how depressed individuals differ from non-depressed individuals in their HPA responses to pharmacologic and psychosocial challenges. Pharmacologically challenging studies comparing depressed and non-depressed individuals have found impaired HPA negative response to the dexamethasone suppression test (DST), and a combination of blunted adrenocorticotropin hormone (ACTH) levels and elevated cortisol levels following corticotropin-releasing-hormone (CRH) challenge (Birmaher et al., Citation1996; Kaufman et al., Citation2001; Lopez-Duran et al., Citation2009; Ribeiro et al., Citation1993). Psychosocial stress protocols allow researchers to gauge endogenous activity of the entire HPA system and complement pharmacologic/neuroendocrine challenges, which do not recruit suprahypothalamic circuits involved in the HPA response (Rao et al., Citation2008). A meta-analysis of psychosocial challenge studies in adults found blunted afternoon cortisol reactivity (i.e. similar peak cortisol levels in the context of higher baseline cortisol) and delayed cortisol recovery during a major depressive episode (MDE) compared to non-depressed controls (Burke et al., Citation2005).
A second critical task for researchers is to distinguish whether differences in HPA function between depressed and non-depressed individuals are relatively stable (and possibly pre-existing) trait-like risk markers or if they are state-dependent features that resolve after recovery from an MDE. Disruptions of the stress response may persist beyond recovery and increase vulnerability to subsequent episodes (Siever & Davis, Citation1985). Research examining HPA negative feedback among remitted-depressed individuals suggests a normalization of cortisol non-suppression after recovery (Holsboer et al., Citation1982). However, it remains unclear whether cortisol responses to psychosocial stress, which appear to be altered in depressed individuals, can be characterized as state-like or trait-like risk markers. The present study addresses this important question prospectively in a sample of depressed individuals assessed during an MDE and again after recovery. Based on evidence that enhanced HPA response to a psychosocial stress task may be limited to depressed individuals with comorbid anxiety disorders, analyses examined the independent effects of depression and anxiety (Young et al., Citation2004). The psychosocial stress protocol employed in this study has three key advantages that distinguish it from the majority of studies in this field: (1) accurate assessment of basal (pre-stress) cortisol levels using a 2.5 hour acclimation phase; (2) detailed post-stress assessment allowing examination of both linear and quadratic within-individual change in cortisol levels; and (3) assessment during the evening when the HPA system is more quiescent and subtle changes in cortisol responses might be detectable (Crowley et al., Citation2007).
Method
Participants
This study is part of a larger ongoing programmatic investigation of the onset and course of depression in adolescents and young adults. Participants were 50 adolescents and young adults divided into two groups: individuals with no lifetime personal or family history of psychiatric disorder at both time points [normal controls (NC); n = 26], and individuals with current MDD at Time 1 (n = 24). The depressed participants met criteria for MDD for a minimum duration of 4 weeks. Participants with a lifetime history of mania, hypomania, schizophrenia, schizoaffective disorder or autism, or with a family history of bipolar disorder, were excluded from the study. All participants were medically healthy and free from psychotropic medication (for a minimum of 8 weeks; most participants were medication-naïve and none received treatment for the index depressive episode at the time of recruitment) and alcohol or illicit drug use, as determined by physical examination, laboratory investigations and urine drug screens. The MDD and ND groups were matched on age, sex and time interval between stress tasks. All procedures were approved by the institutional review board.
Measures
Depression
The diagnosis of MDD and other psychiatric disorders at each time point was based on semi-structured interviews: the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL; Kaufman et al., Citation1997) for adolescents and the Structured Clinical Interview for DSM-IV (SCID-I; First et al., Citation1997) for young adults. The K-SADS-PL was administered to both the adolescent and parent, and summary scores were tabulated. Self-reported depressive symptoms at each time point were assessed with the Beck Depression Inventory (BDI; Beck et al., Citation1961).
Family history of psychopathology
History of psychiatric disorders in family members was determined by a semi-structured interview: the Family History-Research Diagnostic Criteria (FH-RDC; Andreasen et al., Citation1977). The adolescent or young adult’s mother was interviewed regarding lifetime psychiatric disorders in all first-degree relatives (including self, spouse, and all offspring).
Psychosocial stressor
A standardized psychosocial stress protocol, the Trier Social Stress Test (TSST), was used to induce HPA response (Buske-Kirschbaum et al., Citation1997; Kirschbaum et al., Citation1993) that involved a 5-minute public speaking task (following a 5-minute preparation period) and a 5-minute mental arithmetic task performed in front of an audience (Rao et al., Citation2008). Instructions for the speech task and the starting number for the serial subtraction task were altered for the second assessment to avoid the possibility that participants would give a rehearsed speech or memorize the correct sequence of numbers. Baseline saliva samples were collected at 30-minute intervals for 2 hours prior to the stress task (five samples) following a 30-minute acclimation to the laboratory. Post-stress saliva samples were collected immediately after the task and at 10-minute intervals for 60 minutes (seven samples). All stress protocols were conducted in the late afternoon/early evening to control for delayed circadian phase in adolescents (Crowley et al., Citation2007). The second TSST was administered at the first 6-month follow-up visit when the participant with MDE met criteria for recovery (i.e. 12 weeks with no clinically significant symptoms). Age- and duration-matched controls were then selected for the repeated TSST. Following the first TSST, an experimenter informed participants in a neutral manner that they performed well given the circumstances. Participants were fully debriefed regarding the experimental deception after the second TSST.
Cortisol
Cortisol levels were determined in duplicate using a commercially available enzyme immunoassay kit (Enzyme-Linked ImmunoSorbent Assay, ALPCO diagnostics, Salem, NH). Pre-stress cortisol was computed as the mean of the fourth and fifth pre-stress samples. Net peak cortisol was computed as each individual’s pre-stress value subtracted from his/her peak post-stress cortisol level. Area under the curve with respect to increase (AUCi) can be conceptualized as an index of the sensitivity of the cortisol response to a stressor, and may be negative if levels decline below the baseline value. AUCi cortisol was computed according to procedures outlined by Pruessner et al. (Citation2003). Recovery from peak was computed as each individual’s recovery cortisol (mean of final two post-stress samples) subtracted from his/her peak cortisol. Recovery from baseline (pre-stress) was computed as each individual’s recovery cortisol (mean of final two post-stress samples) subtracted from his/her pre-stress value.
Longitudinal follow-up
The KSADS-PL and Longitudinal Interval Follow-up Evaluation (LIFE; Shapiro & Keller, Citation1979) were administered at 6-month intervals to determine the longitudinal course of depression. Recovery from an MDE was determined by a rating of ≤2 on the Psychiatric Status Rating component of the LIFE and a Hamilton Depression Rating Scale (Hamilton, Citation1960) of <6 for a minimum of 3 months prior to the follow-up stress protocol. NC were required to be free from psychopathology at each follow-up in order to be included in the repeated TSST assessment.
Data analytic plan
Cortisol data were log-transformed to correct for skew. To examine within- and between-individual change simultaneously, we specified a multilevel model (MLM) using hierarchical linear models (HLM6; Raudenbush et al., Citation2004) consisting of a within-person (i.e. level 1) sub-model describing cortisol responses to each TSST, a level 2 submodel describing the influence of TSST session, and a between-person (i.e. level 3) submodel describing how cortisol responses to each TSST varied between MDD and NC groups (Bryk & Raudenbush, Citation1992; Singer & Willett, Citation2003). We examined quadratic change trajectories for cortisol levels in response to the TSST based on evidence from other studies assessing cortisol responses to psychosocial stress tasks (Laurent & Powers, Citation2006). In the quadratic change model presented below, π0 represents the cortisol trajectory’s intercept at the start of the TSST, π1 represents the instantaneous rate of change in cortisol levels (i.e. slope) at the start of cortisol sampling and π2 represents how this rate of change changes over the course of the TSST (i.e. curvature) (Singer & Willett, Citation2003). For example, an individual may exhibit increasing cortisol levels at the start of sampling (i.e. a positive value for π1), but this rate of increase could slow over time such that cortisol levels reach a peak and eventually decline (i.e. a negative value for π2).
MLM was used to test the cross-level 3-way interaction of MDD status, TSST session and cortisol sample time predicting cortisol responses to the TSST. Variables included in interactions were centered. Simple slope analyses were conducted on significant interactions (Aiken & West, Citation1991). Follow-up analyses examined the relative impact of MDD and anxiety disorders on cortisol responses to each TSST.
Next, multiple regression models were used to examine predictors of the following cortisol summary indices at Time 1 and Time 2: net peak, AUCi, recovery from peak and recovery from baseline/pre-stress. Initial analyses examined MDD status and depressive symptoms as predictors of cortisol summary indices in the entire sample. Subsequent analyses including only the MDD group examined the main effect of depression characteristics (i.e. age at first MDD onset, duration of index MDE, number of MDEs, total duration of MDEs) on cortisol summary indices. Finally, the analyses examined stability of cortisol indices within MDD and NC groups from Time 1 to Time 2 using bivariate correlations.
Results
presents the means and standard deviations of all study variables for the MDD and NC groups. The NC were significantly older than the MDD group (mean age difference 2.5 years). The MDD group had significantly higher BDI scores during their episode (Time 1) and remission (Time 2) compared to the NC group. Although the groups differed significantly on BDI scores at Time 2, the BDI values in participants with depression were in the non-clinical range during remission. Depressive symptoms at Time 1 were not significantly correlated with any cortisol measures at Time 1. Depressive symptoms at Time 1 were significantly correlated with net peak cortisol at Time 2 (r = 0.47, p = 0.001), AUCi cortisol at Time 2 (r = 0.48, p = 0.001, and recovery from peak cortisol at Time 2 (r = −0.37, p = 0.013). Age was not significantly correlated with net peak cortisol (r = 0.02, p = 0.875), AUCi cortisol (r = 0.01, p = 0.943), recovery from peak cortisol (r = −0.02, p = 0.876) or recovery from baseline cortisol (r = 0.01, p = 0.959) at Time 1, nor was it significantly correlated with net peak cortisol (r = 0.04, p = 0.775), AUCi cortisol (r = −0.01, p = 0.946), recovery from peak cortisol (r = −0.03, p = 0.831) or recovery from baseline cortisol (r = 0.03, p = 0.863) at Time 2. No sex differences were found in net peak cortisol (t = 1.40, p = 0.169), AUCi cortisol (t = 1.55, p = 0.129), recovery from peak cortisol (t = 0.48, p = 0.633) or recovery from baseline cortisol (t = 1.14, p = 0.260) at Time 1, nor were sex differences found in net peak cortisol (t = 0.79, p = 0.434), AUCi cortisol (t = 0.34, p = 0.738), recovery from peak cortisol (t = 0.81, p = 0.421) or recovery from baseline cortisol (t = 0.31, p = 0.762) at Time 2. Therefore, neither sex nor age was included as a covariate in multiple regression models.
Table 1. Demographic and clinical characteristics of the sample.
Multilevel models predicting within-individual change in cortisol responses across repeated TSSTs
Cortisol levels for the NC group during the first and second sessions are presented in and for the MDD group during the first and second sessions in (raw cortisol levels in ng/ml are presented for ease of interpretation). Preliminary analyses without substantive predictors confirmed our hypothesis that cortisol responses to the TSST would be characterized by quadratic change. The interaction of MDD status, TSST session and cortisol sample times was tested for a model including both instantaneous rate of change in cortisol levels at the start of the TSST (π1) and how this rate of change occurred over the course of the TSST (π2). Level 2 predictors examined whether differences in instantaneous rate of change (β11) and curvature (β21) existed between TSST sessions. Finally, the cross-level 3-way interactions of MDD, TSST and instantaneous rate of change (ϒ111) and MDD, TSST and curvature (ϒ211) were assessed. The 3-level MLM model was as follows:
Figure 1. Mean cortisol levels ( ± SEM) to repeated standardized laboratory stressors (TSST) for normal controls (NC, n = 26). Pre-stress 1 = 30 minutes prior to TSST (1.5 hours of acclimation); pre-stress 2 = immediately prior to TSST (2 hours acclimation).
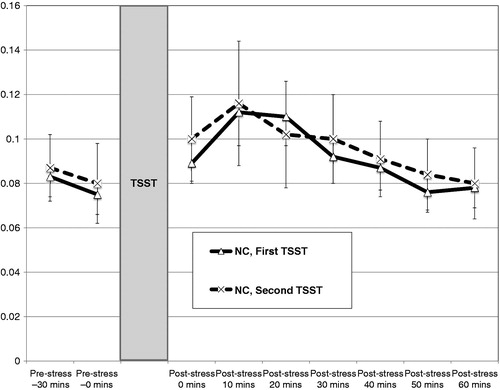
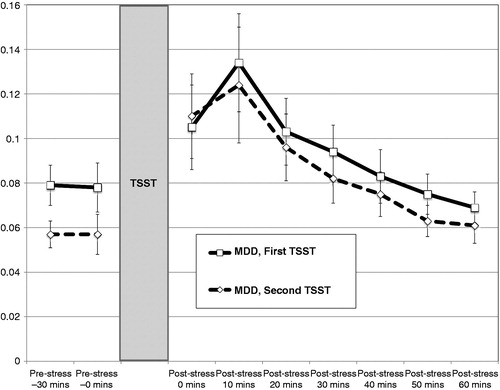
Figure 2. Mean cortisol levels (± SEM) to repeated standardized laboratory stressors (TSST) for individuals with major depression at Time 1 (MDD, n = 24). Pre-stress 1 = 30 minutes prior to TSST (1.5 hours acclimation); pre-stress 2 = immediately prior to TSST (2 hours of acclimation). All individuals with MDD had recovered from their index episode prior to the second TSST.
Level 1 Model:
Level 2 Model:
Level 3 Model:
Results of this 3-level MLM model are presented in . The interaction of MDD status, TSST session and instantaneous rate of change (Time) was significant (B = 0.10, SE = 0.05, p = 0.044) (). Simple slope analyses revealed that within-individual cortisol levels did not significantly change for the NC group at the start of the first (B = 0.05, SE = 0.03, p = 0.094) or second (B = 0.02, SE = 0.03, p = 0.569) TSSTs. For the MDD group, within-individual cortisol levels increased significantly at the start of the second TSST (B = 0.12, SE = 0.04, p = 0.002), but not the first TSST (B = 0.06, SE = 0.03, p = 0.095). The interaction of MDD status, TSST session and quadratic change (Time2) was also significant (B = − 0.01, SE = 0.01, p = 0.037). Simple slope analyses revealed significant deceleration in cortisol levels for the NC group during the first TSST (B = −0.01, SE = 0.003, p = 0.020) but not the second TSST (B = −0.003, SE = 0.002, p = 0.180). For the MDD group, a significant deceleration in cortisol levels (i.e. slowing rate of change over time) was observed during the first TSST (B = −0.01, SE = 0.004, p = 0.033) and the second TSST (B = −0.02, SE = 0.005, p = 0.002). When MLM was used to examine cortisol responses for each TSST separately, results indicated no significant group differences in cortisol instantaneous rate of change (B = 0.005, SE = 0.04, p = 0.899) or quadratic change (B = −0.001, SE = 0.004, p = 0.779) at Time 1; however, at Time 2, the recovered MDD group exhibited more rapid increases in cortisol levels at the start of the TSST (B = 0.10, SE = 0.04, p = 0.006) and faster deceleration in cortisol levels over the course of the TSST (B = −0.01, SE = 0.004, p = 0.003) compared to the NC group.
Figure 3. Interaction of group status (MDD, NC), TSST session (First, Second), and cortisol sampling time predicting instantaneous rate of change in cortisol levels at the start of sampling. *p = 0.002.
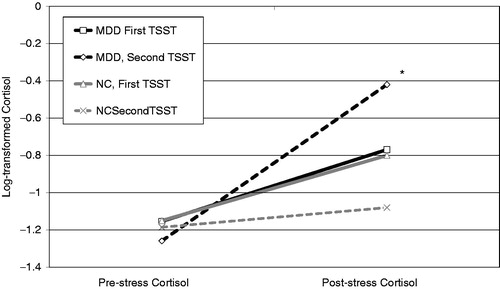
Table 2. Multilevel model predicting change in cortisol responses across repeated TSSTs.
Follow-up analyses examined the relative impact of MDD and anxiety disorders on cortisol responses to each TSST. At Time 1, anxiety disorders were significantly associated with instantaneous rate of change in cortisol levels at the start of the TSST (B = −0.15, SE = 0.067, p = 0.024) and with quadratic change (B = 0.02, SE = 0.007, p = 0.013). That is, individuals with anxiety disorders exhibited declining cortisol levels at the start of saliva sampling, but their cortisol levels began to increase more and more rapidly as the task continued. At Time 1, current MDD was not associated with instantaneous rate of change in cortisol levels (B = 0.04, SE = 0.040, p = 0.366) or with quadratic change (B = −0.005, SE = 0.004, p = 0.258). At Time 2 a different pattern emerged: recent depression was associated with increasing cortisol levels at the start of saliva sampling (B = 0.10, SE = 0.040, p = 0.014); however, the rate of increase in cortisol levels declined significantly over the course of the task (B = −0.01, SE = 0.004, p = 0.010). At Time 2, anxiety disorders were no longer associated with instantaneous rate of change (B = 0.03, SE = 0.067, p = 0.682) or quadratic change (B = −0.005, SE = 0.007, p = 0.531) in cortisol levels.
Multiple regression models predicting cortisol summary indices for the first and second TSSTs
Within the overall sample at Time 1, neither MDD status nor depressive symptoms were significantly associated with cortisol summary indices. Within the MDD group at Time 1, duration of index MDE was positively associated with net peak cortisol (B = 0.01, SE = 0.01, p = 0.035) and total duration of all MDEs was negatively associated with net peak cortisol (B = −0.01, SE = 0.01, p = 0.044). Within the overall sample at Time 2, longer duration between the first and second TSST was positively associated with net peak cortisol (B = 0.001, SE = 0.00, p = 0.020) and negatively associated with recovery from peak cortisol (B = −0.001, SE = 0.00, p = 0.020). Within the MDD group at Time 2, none of the depression characteristics were significantly associated with summary cortisol indices.
Stability of cortisol responses across repeated TSSTs
Within the MDD group across repeated TSSTs, net peak cortisol was positively correlated (r = 0.54, p = 0.006), recovery from baseline cortisol was positively correlated (r = 0.55, p = 0.005) and AUCi was positively correlated at the level of a non-significant trend (r = 0.40, p = 0.055); recovery from peak cortisol was not significantly correlated (r = 0.19, p = 0.371). Within the NC group across repeated TSSTs, none of the summary cortisol indices were significantly correlated: net peak (r = 0.05, p = 0.803); AUCi (r = 0.11, p = 0.583); recovery from baseline (r = 0.27, p = 0.192) and recovery from peak (r = −0.03, p = 0.868). MLM was used to further examine stability of cortisol responses across TSST sessions for each group. For the MDD group, results indicated no significant change across TSSTs in cortisol instantaneous rate of change (B = 0.07, SE = 0.04, p = 0.101) or quadratic change (B = −0.01, SE = 0.004, p = 0.089). Similarly, the NC group did not show significant change across TSSTs in cortisol instantaneous rate of change (B = −0.04, SE = 0.03, p = 0.250) or quadratic change (B = 0.004, SE = 0.003, p = 0.239).
Discussion
Multilevel models revealed robust cortisol responses in depressed individuals during their MDE as well as after recovery. Among depressed individuals, cortisol levels exhibited quadratic change over the course of both TSSTs, as evident in initial increases and a progressive slowing rate of change that produced a single peak and subsequent decline in cortisol levels over the recovery period. In contrast, cortisol levels in normal controls only exhibited quadratic change during their first TSST and were “flat” compared to the recovered MDD group at Time 2. This relatively flat cortisol response to the second TSST in the NC group is consistent with prior research in healthy individuals showing diminishing cortisol responses to social evaluation stressors administered repeatedly over daily (Kirschbaum et al., Citation1995) and four-week intervals (Schommer et al., Citation2003). However, it should be noted that neither instantaneous rate of change nor quadratic change in cortisol levels differed significantly across TSSTs for the NC group. Depressed individuals in the current study did not exhibit habituation when they were confronted again with the psychosocial stressor during recovery. Instead, the positive and significant instantaneous rate of change in cortisol levels observed for depressed individuals during the second, but not the first, TSST could represent a sensitization to the psychosocial stress task.
Summary indices of cortisol responses to the psychosocial stress task administered to depressed individuals during aN MDE and again after recovery were highly correlated and could reflect trait-like markers of risk for depression. Higher levels of depressive symptoms during the MDE predicted greater cortisol responses after recovery. Moreover, cortisol responses during recovery were greater for depressed individuals with a more chronic recent MDE. Taken together, these findings suggest that risk for depression can be indexed by cortisol responses to stressors between depressive episodes.
Greater cortisol reactivity to lower levels of psychosocial stress in remitted- and never-depressed individuals has been linked to more rapid increases in depressive symptoms during prospective follow-up (Morris et al., Citation2012b). In addition, features of the TSST cortisol response have been shown to interact with depression history to predict changes in depressive symptoms prospectively, such that the rate of increase in depressive symptoms for individuals with higher anticipatory cortisol levels or greater cortisol reactivity at baseline was stronger for those with more prior MDEs (Morris et al., Citation2012b). In contrast, another study found that risk for depression relapse was linked to more prior MDEs and lower pre-stress cortisol levels (Chopra et al., Citation2008). Future research should examine neurobiological substrates of both habituation and stress sensitization to determine whether these play a role in the changing relations between stressful life events and depressive symptoms across successive recurrences (Morris et al., Citation2010; Petrowski et al., Citation2012).
Net peak cortisol was positively associated with the duration of their ongoing MDE, yet negatively associated with the total duration of all their MDEs. How can these divergent findings for recent versus lifetime depression history be explained? A growing literature on HPA function and life stress highlights a pattern of diminishing HPA activity over time (Miller et al., Citation2007; Morris et al., Citation2012a). For example, meta-analyses have found that diurnal cortisol secretion decreases as more time elapses following the onset of chronic stress (Miller et al., Citation2007); the authors suggest that “when a chronic stressor first arises there is an initial activation of the HPA axis […] as time passes, this activity diminishes, and cortisol secretion rebounds to below normal” (pp. 31–32). Net peak cortisol responses to the TSST in the present study appear to reflect a similar temporal pattern of decreasing reactivity for those with greater lifetime MDE exposure. Whereas declining daily cortisol output is associated with negative mental and physical health outcomes (Heim et al., Citation2000), it remains unclear whether diminishing cortisol stress reactivity also plays a role in the risk for developing these conditions.
In the present study, anxiety disorders were associated with more rapid acceleration in cortisol levels for currently depressed individuals, but were not associated with cortisol responses during recovery from the depressive episode. A previous study examining the impact of comorbidity on HPA reactivity found enhanced ACTH responses to psychosocial stress in depressed individuals with comorbid anxiety disorders (Young et al., Citation2004). The authors of that study reported that anxiety levels in response to the challenge were not linked to ACTH levels and were not detected among individuals with “pure” MDD or anxiety disorders, leading them to speculate that enhanced ACTH response is driven by the interaction of depressive and anxiety disorders (Young et al., Citation2004). The present study extends these findings by showing that anxiety disorders enhanced cortisol reactivity in currently depressed individuals only; during recovery, history of depression was linked to cortisol reactivity and comorbid anxiety disorders no longer altered HPA function.
Limitations of the present study provide directions for future research. Despite the modest sample sizes, methodological features such as the collection of seven post-stress cortisol samples and the use of MLM analytic strategies were used to maximize statistical power. The follow-up TSST was administered approximately four months after the initial stress task, once depressed individuals had fully recovered. Duration of the inter-TSST interval was associated with Time 2 cortisol outcomes (i.e. net peak cortisol and recovery from peak cortisol). However, concerns about the potentially confounding influence of inter-TSST interval on analyses examining group differences were minimized by matching depressed and normal controls on inter-TSST duration. In fact, depressed individuals with a longer episode had higher cortisol responses during recovery despite the longer time elapsed from the initial assessment. Another potential limitation is that the present study included a wide age range encompassing adolescents and young adults. Although neither age nor sex were significantly associated with cortisol outcomes, previous evidence for sex and maturational differences in HPA function (Kudielka et al., Citation2009) suggest that the absence of these effects in the current study should be interpreted with caution given the modest sample sizes. In addition, menstrual cycle phase across repeated TSST sessions was not held constant for female participants due to concerns regarding the delay of treatment at Time 1; prior research has demonstrated higher salivary cortisol responses during the luteal phase (Kirschbaum et al., Citation1999). Finally, analyses examining the impact of comorbid anxiety disorders in depressed individuals were based on a small number of participants and require replication in larger samples.
In conclusion, the present findings revealed that depressed individuals exhibited more robust cortisol responses during recovery, whereas normal controls showed habituation to the stressor, suggesting that HPA responses to psychosocial stress may reflect a more trait-like correlate of depression. It remains to be seen whether sensitization to social evaluation stress in recovered depressed individuals contributes to increased risk for recurrence. A critical avenue for future research remains to determine the implications of HPA hyper- versus hypo-reactivity for depression-risk and response to psychotherapeutic and/or pharmacologic treatment.
Declaration of interest
The authors report no conflicts of interest.
Matthew C. Morris was supported in part by an RCTR/MeTRC grant (U54 RR026140/MD007593), and an independent grant (R01 MH068391) from the National Institutes of Health. Uma Rao was supported in part by the grants from the National Institutes of Health (R01 DA014037, R01 DA015131, R01 DA017804, R01 DA017805, R01 MH062464, R01 MH068391, G12 RR003032/MD007586, UL1 RR024975/TR000445 and U54 RR026140/MD007593), and by the Sarah M. and Charles E. Seay Chair in Child Psychiatry at UT Southwestern Medical Center and the Endowed Chair in Brain and Behavior Research at Meharry Medical College. These funding agencies had no further role in the study design, data collection, analysis or interpretation of data, writing of the report, or the decision to submit the paper for publication.
Acknowledgements
The authors would like to thank all the volunteers who participated in this study.
References
- Aiken LS, West SG. (1991). Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. (1977). The family history method using diagnostic criteria. Arch Gen Psychiatry 34:1229–35
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. (1961). An inventory for measuring depression. Arch Gen Psychiatry 40:1228–31
- Birmaher B, Dahl RE, Perel J, Williamson DE, Nelson B, Stull S, Kaufman J, et al. (1996). Corticotropin-releasing hormone challenge in prepubertal major depression. Biol Psychiatry 39:267–77
- Bryk AS, Raudenbush SW. (1992). Hierarchical linear models: applications and data analysis methods. Thousand Oaks: Sage
- Burke HM, Davis SC, Otte C, Mohr DC. (2005). Depression and psychological response to stress: a meta-analysis. Psychoneuroendocrinology 30:846–56
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. (1997). Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom Med 59:419–26
- Chopra KK, Segal ZV, Buis T, Kennedy SH, Levitan RD. (2008). Investigating associations between cortisol and cognitive reactivity to sad mood provocation and the prediction of relapse in remitted major depression. Asian J Psychiatry 1:33–6
- Crowley SJ, Acebo C, Carskadon MA. (2007). Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med 8:602–12
- First MB, Spitzer RL, Gibbon M, Williams JBW. (1997). User’s guide for the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) -- Clinician Version. Washington, DC: American Psychiatric Press
- Hamilton M. (1960). A rating scale for depression. J Neurol Neurosurg Psychiatry 25:56–62
- Heim C, Ehlert U, Hellhammer D. (2000). The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology 25:1–35
- Holsboer F, Liebl R, Hofschuster E. (1982). Repeated dexamethasone suppression test during depressive illness – normalization of test result compared with clinical improvement. J Affect Disord 4:93–101
- Holsboer F. (2000). The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 23:477–501
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. (1997). Schedule for Affective Disorders and Schizophrenia for School-aged Children-Present and Lifetime versions (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–8
- Kaufman J, Martin A, King RA, Charney D. (2001). Are child-, adolescent-, and adult-onset depression one and the same disorder? Biol Psychiatry 49:980–1001
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. (1999). Impact of gender, menstrual cycle phase, and oral conraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychom Med 61:154–62
- Kirschbaum C, Pirke KM, Hellhammer DH. (1993). The Trier Social Stress Test – a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28:76–81
- Kirschbaum C, Prüssner JC, Stone AA, Federenko I, Gaab J, Lintz D, Schommer N, Hellhammer DH. (1995). Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom Med 57:468–74
- Kudielka BM, Hellhammer DH, Wüst S. (2009). Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology 34:2–18
- Laurent HK, Powers SI. (2006). Social-cognitive predictors of hypothalamic-pituitary-adrenal reactivity to interpersonal conflict in emerging adult couples. J Soc Pers Relat 23:703–20
- Lopez-Duran NL, Kovacs M, George CJ. (2009). Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: a meta-analysis. Psychoneuroendocrinology 34:1272–83
- Miller GE, Chen E, Zhou ES. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull 133:25–45
- Morris MC, Ciesla JA, Garber J. (2010). A prospective study of stress autonomy versus stress sensitization in adolescents at varied risk for depression. J Abnorm Psychol 119:341–54
- Morris MC, Compas BE, Garber J. (2012a). Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin Psychol Rev 32:310–15
- Morris MC, Rao U, Garber J. (2012b). Cortisol responses to psychosocial stress predict depression trajectories: social-evaluative threat and prior depressive episodes as moderators. J Affect Disord 143:223–30
- Petrowski K, Wintermann G-B, Siepmann M. (2012). Cortisol response to repeated psychosocial stress. Appl Psychophysiol Biofeedback 37:103–7
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28:916–31
- Rao U, Hammen C, Ortiz LR, Chen LA, Poland RE. (2008). Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biol Psychiatry 64:521–6
- Raudenbush SW, Bryk AS, Congdon R. (2004). HLM 6 for Windows. Lincolnwood, IL: Scientific Software International, Inc
- Ribeiro S, Tandon R, Grunhaus L, Greden J. (1993). The DST as a predictor of outcome in depression: a meta-analysis. Am J Psychiatry 150:1618–29
- Schommer NC, Hellhammer DH, Kirschbaum C. (2003). Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary system to repeated psychosocial stress. Psychosom Med 65:450–60
- Shapiro R, Keller M. (1979). Longitudinal interval follow-up evaluation (LIFE). Boston, MA: Massachusetts General Hospital
- Siever LJ, Davis KL. (1985). Toward a dysregulation hypothesis of depression – overview. Am J Psychiatry 142:1017–31
- Singer JD, Willett JB. (2003). Applied longitudinal data analysis: modeling change and event occurrence. New York: Oxford University Press
- Young EA, Abelson JL, Cameron OG. (2004). Effect of comorbid anxiety disorders on the hypothalamic–pituitary–adrenal axis response to a social stressor in major depression. Biol Psychiatry 56:113–20
