Abstract
Knowledge about children's stress reactivity and its correlates is mostly based on one stress task, making it hard to assess the generalizability of the results. The development of an additional stress paradigm for children, that also limits stress exposure and test time, could greatly advance this field of research. Research in adults may provide a starting point for the development of such an additional stress paradigm, as changes in salivary cortisol and alpha-amylase (sAA) over a 1-h pre-stress period in the laboratory correlated strongly with subsequent reactivity to stress task (Balodis et al., 2010, Psychoneuroendocrinology 35:1363–73). The present study examined whether such strong correlations could be replicated in 9- to 11-year-old children. Cortisol and sAA samples were collected from 158 children (83 girls) during a 2.5-h visit to the laboratory. This visit included a 1-h pre-stress period in which children performed some non-stressful tasks and relaxed before taking part in a psychosocial stress task (TSST-C). A higher cortisol arrival index was significantly and weakly correlated with a higher AUCg but unrelated to cortisol reactivity to the stressor. A higher sAA arrival index was significantly and moderately related to lower stress reactivity and to a lower AUCi. Children's personality and emotion regulation variables were unrelated to the cortisol and sAA arrival indices. The results of this study do not provide a basis for the development of an additional stress paradigm for children. Further replications in children and adults are needed to clarify the potential meaning of an arrival index.
Introduction
Knowledge about children's physiological stress reactivity and its correlates is important, as previous research has indicated that repeated and long-lasting activation of the stress-system has adverse effects (e.g. Charmandari et al., Citation2005; McEwen, Citation2008; Sapolsky, Citation1998). At the moment, most knowledge on this topic is based on one stress task, the Trier Social Stress Test for Children (TSST-C; Buske-Kirschbaum et al., Citation1997). This makes it unclear how results generalize to other stressful situations. In addition, research in this area is associated with ethical and practical considerations regarding the exposure to a stress task, and the amount of time necessary to execute existing stress tasks. Developing an additional stress task that also reduces the impact of these issues could significantly increase the number of studies in this area, and thereby increase the pace at which important knowledge is gained.
A paper by Balodis et al. (Citation2010) could provide a starting point for the development of such an additional paradigm. These researchers examined the relation between changes in salivary cortisol and alpha-amylase (sAA) level during a 1-h pre-stress period in the lab, termed the “arrival index”, and cortisol and sAA reactivity in response to Trier Social Stress Test (TSST; Kirschbaum et al., Citation1993) in 50 healthy young adults. Correlation analyses between the arrival index and commonly used measures reflecting reactivity to the stressor showed Pearson correlations of up to r = 0.76 for cortisol and r = 0.86 for sAA. This implies that changes in biomarker concentrations during the first hour in the lab share a large amount of variance – 58% for cortisol and 74% for sAA – with reactivity to a subsequently administered stress task. As a result, replicating the results presented above in a sample of children could lie at the foundation of the development of an additional stress paradigm for middle childhood that shares important aspects – like unpredictability and uncontrollability – with current procedures, while at the same time being sufficiently different to prevent redundancy. That is, such a stress paradigm would measure anticipatory stress, constitute of a very simple procedure, and reduce ethical considerations about purposefully exposing children to a stressful situation.
In the present study, we therefore replicated the analyses conducted by Balodis et al. (Citation2010) with data that we had collected using a similar experimental timeline in a sample of 9- to 11-year-old children. Thus, the current study examined the correlation between the arrival index and different measures of physiological stress reactivity for both cortisol and sAA in children. In their discussion of the findings, Balodis et al. (Citation2010) also suggested that individual differences in the arrival index might reflect individual differences in personality and trait mood but the data necessary to investigate this were not available in their sample. As our data included information on the children's personality characteristics and trait use of emotion regulation strategies, we also explored whether these variables were related to the arrival index in the current sample.
Methods
Participants
Parents and children were invited through 31 primary schools in Nijmegen and surrounding areas (The Netherlands) to participate in a study on different aspects of responses to stress and their consequences for cognitive functioning. Exclusion criteria were a diagnosis of a developmental disorder, the use of psychotropic or centrally acting corticosteroid medication, and stuttering. Recruitment (for details see de Veld et al., Citation2012) resulted in 165 participants. Two children were excluded because during data collection it was discovered they met one of the exclusion criteria. Furthermore, five children were excluded from the present analysis because they did not complete the entire data collection protocol. Thus, the sample for the current study consisted of 158 children (83 girls; Mage = 10.61 years, SD = 0.52). The majority of the participants was Caucasian (94%, most others were of African descent), and had at least one parent with a college or university degree (79%).
The study was approved by the ethics committee of the Faculty of Social Sciences of the Radboud University Nijmegen. All parents provided written informed consent prior to their child's participation.
Procedure
A week before the stress test, all children completed questionnaires and memory tasks during a home visit (not relevant for the current study). At the end of the home visit, parents were handed the questionnaire to rate their child's personality (see “Instruments and measures”), and were requested to bring the completed questionnaire along to the lab visit 1 week later.
The lab visit took place after school, in the afternoon (Med = 15.45 h, IQR = 14.34–16.03 h), in the laboratory of the Behavioural Science Institute of the Radboud University Nijmegen. On arrival, children were led to a separate room, where the experimenter explained that they would be asked to do some tasks and fill out several questionnaires. After this introduction, children provided a saliva sample (S1; within 5 min after arrival), filled out a short questionnaire, performed a memory task, and filled out a trait emotion regulation questionnaire (see “Instruments and measures”). This was followed by a 30-min relaxation period during which children listened to relaxing music, and could read a magazine or make puzzles. Right after relaxation they filled in a short questionnaire and provided a second saliva sample (S2). After this, children were taken to an adjacent room where an adapted and extended Trier Social Stress Test for Children (TSST-C; Buske-Kirschbaum et al., Citation1997) was initiated to induce stress. The TSST-C comprises a public speaking task in which children are asked to provide an ending to a story read out loud by the experimenter, and a mental arithmetic task. Both tasks are performed in front of a jury of two confederates in white lab coats. In the present study, children picked a most and least preferred present out of six small items (e.g. an inflatable ball or toilet brush) right before entering the TSST-C room (Jones et al., Citation2006), and were told that a favorable judgment by the jury would earn them their favorite present, whereas in case of an unfavorable judgment they would get the least preferred present. After the TSST-C, children were seated in front of the TSST-C jury. There they performed a working memory task, supplied a saliva sample (S3), filled out a short questionnaire and performed an additional memory task. Although the main purpose of the memory task was to assess memory for use in a different study, performance of such tasks in front of a jury can be considered stressful, and the task was therefore considered a part of the stress task for the purpose of the current study. This entire procedure took ∼34 min. Afterwards, the children went back to the first room. There they provided another saliva sample (S4), filled in several questionnaires, provided a fifth saliva sample (S5), completed another questionnaire, received positive feedback on their performance during the stress task and completed a short questionnaire. Then, a 25-min post-stress relaxation period was initiated. Ten minutes into this relaxation period, a saliva sample was obtained (S6). After relaxation, children completed several questionnaires, performed a memory task, provided a last saliva sample (S7), completed a last questionnaire and were debriefed. The entire procedure took ∼2.5 h.
Instruments and measures
Cortisol and sAA
To obtain reliable cortisol and sAA measures, participants were asked to only drink water in the 2 h before arrival in the lab, to limit physical exercise in the hour prior to arrival, and to abstain from meals at least 45 min before arrival. Children did not eat during the procedure.
Seven saliva samples (S1–S7) were obtained throughout the course of the procedure, at −57, −2, 26, 36, 42, 58 and 80 min from the onset of the stressor. Participants swallowed all saliva in order to empty their mouths, and collected all subsequently secreted saliva in their mouths for min, after which they used a short straw to spit the saliva into a small tube. This procedure was repeated until at least 0.25 ml of saliva was collected, with a maximum total collection time of 5 min. Samples were kept frozen at −20 °C until their shipment to the analysis lab.
Cortisol concentrations were determined at the Endocrinology Laboratory of the University Medical Center Utrecht, using an in house competitive radio-immunoassay employing a polyclonal anticortisol–antibody (K7348). [1,2-3H(N)]-hydrocortisone (Amersham TRK407) was used as a tracer. The lower limit of detection was 1 nmol/l and inter-assay and intra-assay variations were <10%.
sAA concentrations were determined from the same saliva samples as were used to determine cortisol concentrations. The analyses were performed at the Endocrinology Laboratory of the University Medical Center Utrecht. Alpha amylase was measured on the DxI analyzer (Beckman Coulter Inc., Fullerton, CA). Saliva samples were diluted 500× with 0.2% BSA in 0.01 M Phosphate buffer pH 7.0. Interassay variation was <2.2%.
All physiological data were screened for outliers, which were defined within each assessment point as values >3 SD above the mean. All outliers were winsorized by replacing their values with the value of 3 SD above the mean (Tukey, Citation1977).
The arrival index was defined as the percent change in cortisol and sAA concentrations from S1 to S2, calculated as S2 minus S1 divided by S1 and multiplied by 100. A positive score indicates an increase in biomarker concentration from S1 to S2, with higher scores indicating stronger increases from arrival to pre-stress, whereas a negative score indicates a decrease, with lower scores indicating stronger decreases.
The stress index was defined as the percent change in cortisol and sAA concentrations from S2 to S3, calculated as S3 minus S2 divided by S2 and multiplied by 100. A positive score indicates an increase in biomarker concentration from S2 to S3, with higher scores indicating stronger increases in response to the stressor, whereas a negative score indicates a decrease, with lower scores indicating stronger decreases.
The area under the curve with respect to ground (AUCg), as a measure for total biomarker concentration, and the area under the curve with respect to increase (AUCi), as an additional measure for reactivity, were calculated for S2–S6 for both cortisol and sAA using the formulas described in Pruessner et al. (Citation2003). AUC measures were divided by the protocol duration to correct for slight variations in individual protocol lengths.
Personality
The personality traits Extraversion, Agreeableness, Conscientiousness, Emotional stability and Openness-intellect were assessed using the Big Five Bipolar Rating Scales (B5BBS-25; Mervielde, Citation1992). This questionnaire consists of 25 Dutch bipolar markers, five for each personality trait. The two opposite poles are connected by a 7-point rating scale that is used to indicate which of the two poles is most descriptive of the child. The factor structure of this measure has been found to correspond to the Big Five personality traits, and the measure has been validated for use in school-aged children (Mervielde et al., Citation1995). Cronbach's alpha indices of reliability for the five scales ranged from 0.69 to 0.90 in the current sample. Mean item scores were computed for all five personality traits.
Trait emotion regulation strategies
Children's trait use of emotion regulation strategies was assessed with an adapted version of the Emotion Regulation Questionnaire (ERQ; Gross & John, Citation2003). The ERQ is a 10-item questionnaire assessing the use of both suppression and reappraisal. The four-item suppression scale includes items such as “I keep my emotions to myself”. The reappraisal scale contains six items such as “I control my emotions by changing the way I think about the situation I am in”. Responses are indicated on a 7-point Likert scale (1 = strongly disagree, 7 = strongly agree).
For use in the current study, the Dutch translation of the ERQ (Koole, Citation2004) was adapted for the use in 10-year-old children by simplifying the formulation of the items, and extending the instructions (see de Veld et al., Citation2012). Principal components analysis revealed a two-factor solution, corresponding to the original factor structure reported in adults by Gross & John (Citation2003). Reliability in the current sample was sufficient for both scales (Cronbach's alpha 0.64 for suppression, and 0.68 for reappraisal). Mean item scores for each scale were computed as indices for trait use of reappraisal and suppression.
Statistical analyses
To assess whether there was a significant increase in cortisol and sAA to the stressor, we used repeated measures ANOVA with Time (S1–S7) as a within subject factor. As both cortisol and sAA data for each assessment point were not normally distributed, these data were normalized with log 10 (cortisol) and sqrt (sAA) transformations prior to analysis. In case of a violation of the sphericity assumption, multivariate statistics are reported.
To examine the association between the arrival index and subsequent reactivity measures, we performed three correlation analyses. In the first, we correlated the cortisol arrival index to the cortisol stress index, AUCg and AUCi. In the second, we correlated the sAA arrival index to the sAA stress index, AUCg and AUCi. In the third and final correlation analysis, we correlated the arrival indices for both cortisol and sAA to the emotion regulation strategies and personality traits. As none of the physiological data were normally distributed non-parametric Spearman's rank order correlations were used for all analyses.
Results
Descriptives and preliminary analyses
Descriptives for all relevant variables are presented in . A repeated measures ANOVA on the cortisol data with Time as a within subject factor indicated a significant effect of time, Wilks' Lambda = 0.19, F (6, 152) = 105.02, p < 0.001, multivariate partial eta squared = 0.81. A repeated measures ANOVA on the sAA data with Time as a within subject factor also indicated a significant effect of time for this variable, Wilks’ Lambda = 0.41, F (6, 148) = 36.24, p < 0.001, multivariate partial eta squared = 0.60. For both cortisol and sAA, the significant effect of Time was due to an increase in concentration in response to the stressor ().
Figure 1. Cortisol and sAA responses to the stressor. Duration of the stressor is indicated with the dark gray bars.
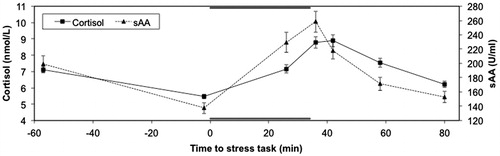
Table 1. Descriptives for the study variables.
Cortisol
All correlations between cortisol measures are presented above the diagonal in . The cortisol arrival index showed a significant positive correlation with the cortisol AUCg (rs = 0.24, n = 158, p < 0.01). Thus, a higher cortisol arrival index was related to a higher cortisol AUCg. The cortisol arrival index was unrelated to the cortisol stress index and cortisol AUCi.
Table 2. Correlations between the arrival index, stress index, AUCg and AUCi, separately for cortisol (above the diagonal) and sAA (below the diagonal).
Salivary alpha-amylase
All correlations between sAA measures are presented below the diagonal in . The sAA arrival index showed a significant negative correlation with both the sAA stress index (rs = −0.43, n = 155, p < 0.001) and sAA AUCi (rs = −0.41, n = 154, p < 0.001). Thus, a higher sAA arrival index was related to a lower sAA stress index and a lower sAA AUCi.
Relation between arrival indices, trait emotion regulation and personality traits
There were no significant correlations between the cortisol and sAA arrival indices on the one hand, and trait emotion regulation use and personality traits on the other ().
Table 3. Correlations between the cortisol and sAA arrival indices, and personality and emotion regulation variables.
Discussion
In the current study, we replicated the analyses conducted by Balodis et al. (Citation2010) in a sample of 9- to 11-year-old children by examining the correlation between the change in salivary biomarkers of stress during the first hour in the laboratory and stress reactivity to a subsequent psychosocial stress task. For cortisol, we found that a higher arrival index was unrelated to cortisol reactivity as reflected in the stress index and the AUCi. A higher cortisol arrival index did show a significant but weak correlation with a higher cortisol AUCg, which is not a measure of cortisol reactivity but rather represents total hormone concentration (Pruessner et al., Citation2003). This correlation can be partly explained by the fact that both measures include the pre-stress sample S2: the arrival index as an end point, and the AUCg as a starting point. That is, a high arrival index signifies a relatively high pre-stress value at S2, and as this value serves as a baseline in computing the AUCg, a relatively high value at S2 will also result in a relatively high value for the AUCg. Because the arrival index was not related to the AUCi and the stress index, these results would be indicating that in the current sample changes in cortisol over the pre-stress period in the laboratory were not related to cortisol reactivity to the stress task.
For sAA, we did find a relation between changes in pre-stress concentrations and subsequent reactivity. For this measure, a higher arrival index was moderately correlated with both a lower stress index and a lower AUCi. As the average response pattern showed a decrease in sAA over the first hour in the laboratory, this indicates that for children whose sAA is high on arrival, and whose levels either remain high or even increase, reactivity to the stressor is lower. This is in line with the law of initial values (Wilder, Citation1962), and could be indicating a ceiling effect. Finally, we also explored whether the arrival indices for sAA and cortisol were related to personality characteristics or trait emotion regulation strategy use. However, this was not the case.
The current results are not in line with the findings of a strong correlation between the change in salivary biomarkers over the first hour in the laboratory and subsequent stress reactivity to a stress task that were reported by Balodis et al. (Citation2010) in a study with adults. There are several potential explanations for the observed differences. One is that the difference in findings might be due to possible differences in the pre-stress part of the protocols used in the two studies, for example with respect to foreknowledge about the upcoming stress task, or the procedure during the pre-stress period. The children participating in the current study were told that they would come to the lab to participate in tasks that are similar to those performed at school, and had already participated in a relatively stress-free control condition 1 week before the laboratory session. Hence, the children might have been rather unsuspecting about the upcoming stress task. In addition, they were engaged in non-stressful tasks and a relaxation period during the pre-stress period. Both of these factors might have led to less elevated baseline samples or less anticipatory stress in the current sample (Nicolson, Citation2008). However, as we neither have information about the foreknowledge that participants in the study by Balodis et al. (Citation2010) had, nor about the content of their pre-stress period, it cannot be said with certainty that these factors contributed to the difference in findings.
The difference between the current findings and those reported by Balodis et al. (Citation2010) might also be due to the different ages of the respective samples. Although cortisol responses to psychosocial stress seem similar in middle childhood and adulthood (Kudielka et al., Citation2004; Yim et al., Citation2010b), there is some evidence that sAA reactivity, in addition to basal levels of cortisol and sAA, may differ across these age groups (Strahler et al., Citation2010; Yim et al., Citation2010a). Such age differences may be indicative of developmental changes in responsivity of the stress system that in turn could result in differential interrelations between physiological stress measures. It is also possible that children and adults differ on psychological factors like appraisals of the laboratory visit, or rumination during the pre-stress period in the laboratory.
In sum, the cortisol arrival index was not related to subsequent cortisol reactivity to stress, and the correlations between the sAA arrival index and measures for subsequent sAA reactivity to stress were much weaker than those found by Balodis et al. (Citation2010). As such, the results of the current study do not provide a basis for the development of an additional laboratory stress induction protocol that can be used in middle childhood. Moreover, the arrival indices of neither cortisol nor sAA were related to personality and trait emotion regulation. This indicates that, at least in children this age, inter-individual differences in the arrival index do not necessarily represent a child characteristic but might instead be related to differences in biomarker concentrations due to external factors, like mode of transportation to the lab, or specific activities during the pre-stress period.
For now, it seems that a pre-stress period employed in stress research with children can only be used as it was originally intended: to make children feel comfortable in the lab in order to minimize differences in baseline physiological activity resulting from pre-arrival activities, thereby allowing for clear assessment of individual differences in physiological responses to the subsequent stressor (Gunnar et al., Citation2009). Nonetheless, given the differences between the results of the current study in children and those found in adults by Balodis et al. (Citation2010), it would be interesting if researchers used the TSST with both children and adults to gain further knowledge on the arrival index. In doing so, it is particularly important to keep the pre-stress procedure consistent between age groups, both with respect to foreknowledge about the upcoming procedure, as well as the content of the pre-stress period. The addition of a saliva sample taken at home could provide information about biomarker levels prior to arrival in the lab. The results of such a study could shed more light on the potential meaning of an arrival index in participants of different ages, and provide valuable knowledge with regard to the development of an additional stress test for children.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Acknowledgements
We thank the children who kindly participated in this study, their parents and all research assistants and students who assisted with data collection.
References
- Balodis IM, Wynne-Edwards KE, Olmstead MC. (2010). The other side of the curve: examining the relationship between pre-stressor physiological responses and stress reactivity. Psychoneuroendocrinology 35:1363–73
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. (1997). Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom Med 59:419–26
- Charmandari E, Tsigos C, Chrousos G. (2005). Endocrinology of the stress response. Annu Rev Physiol 67:259–84
- de Veld DMJ, Riksen-Walraven JM, de Weerth C. (2012). The relation between emotion regulation strategies and physiological stress responses in middle childhood. Psychoneuroendocrinology 37:1309–19
- Gross JJ, John OP. (2003). Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol 85:348–62
- Gunnar MR, Talge NM, Herrera A. (2009). Stressor paradigms in developmental studies: what does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology 34:953–67
- Jones A, Godfrey KM, Wood P, Osmond C, Goulden P, Phillips DIW. (2006). Fetal growth and the adrenocortical response to psychological stress. J Clin Endocrinol Metab 91:1868–71
- Kirschbaum C, Pirke KM, Hellhammer DH. (1993). The ‘Trier Social Stress Test’ – a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28:76–81
- Koole SL. (2004). Volitional shielding of the self: effects of action orientation and external demands on implicit self-evaluation. Soc Cogn 22:100–25
- Kudielka B, Buske-Kirschbaum A, Hellhammer D, Kirschbaum C. (2004). HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology 29:83–98
- McEwen BS. (2008). Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol 583:174–85
- Mervielde I. (1992). The B5BBS-25: a Flemish set of bipolar markers for the “big-five” personality factors. Psychol Belg 32:195–210
- Mervielde I, Buyst V, De Fruyt F. (1995). The validity of the Big-five as a model for teachers' ratings of individual differences among children aged 4–12 years. Pers Individ Differ 18:525–34
- Nicolson NA. (2008). Measurement of cortisol. In: Luecken LJ, Gallo LG, editors. Handbook of physiological research methods in health psychology. Thousand Oaks, CA: Sage Publications, Inc p 37–74
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28:916–31
- Sapolsky RM. (1998). Why zebras don't get ulcers: an updated guide to stress, stress-related diseases, and coping. New York: Freeman
- Strahler J, Mueller A, Rosenloecher F, Kirschbaum C, Rohleder N. (2010). Salivary α-amylase stress reactivity across different age groups. Psychophysiology 47(3):587–95
- Tukey JW. (1977). Exploratory data analysis. Reading, MA: Addison Wesley
- Wilder J. (1962). Basimetric approach (law of initial value) to biological rhythms. Ann NY Acad Sci 98:1211–20
- Yim IS, Granger DA, Quas JA. (2010a). Children's and adults' salivary alpha-amylase responses to a laboratory stressor and to verbal recall of the stressor. Dev Psychobiol 52:598–602
- Yim IS, Quas JA, Cahill L, Hayakawa CM. (2010b). Children's and adults’ salivary cortisol responses to an identical psychosocial laboratory stressor. Psychoneuroendocrinology 35:241–8
