Abstract
Visceral sensitivity is of pathophysiological importance in abdominal pain disorders and can be modulated by inflammation and stress. However, it is unclear whether inflammation and stress alter visceral perception independently of each other or in conjunction through neuroendocrine interactions. Therefore, we compared the short- and long-term effects of experimental colitis and water avoidance stress (WAS), alone or in combination, on visceral sensitivity in female Wistar rats. Colitis was induced by trinitrobenzene sulfonic acid (TNBS) and colonoscopically confirmed. During WAS, rats were placed on a platform surrounded by water for 1 h. Visceral sensitivity was assessed by quantifying the visceromotor responses (VMRs) to colorectal distension. Activation of the hypothalamic–pituitary–adrenal axis was determined by measuring serum corticosterone in a separate protocol. TNBS instillation resulted in overt colitis, associated with significant visceral hypersensitivity during the acute inflammatory phase (3 days post-TNBS; n = 8/group); after colitis had subsided (28 days post-TNBS), hypersensitivity was resolved (n = 4–8/group). Single WAS was associated with increased VMRs of a magnitude comparable to acute TNBS-induced hypersensitivity (n = 8/group). However, after repetitive WAS no significant hypersensitivity was present (n = 8/group). No additive effect of colitis and stress was seen on visceral pain perception (n = 6–8/group). Corticosterone levels were only increased in acute TNBS-colitis, acute WAS and their combination. To conclude, both colitis and stress successfully induced short-term visceral hypersensitivity and activated the hypothalamic–pituitary–adrenal axis, but long-term effects were absent. In addition, our current findings do not support an additive effect of colitis and stress on visceral sensitivity in female Wistar rats.
Introduction
Visceral hypersensitivity is considered to contribute to chronic abdominal pain in gastrointestinal disorders such as inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) (Barbara et al., Citation2011; Vermeulen et al., Citation2014). In IBD, including Crohn’s disease and ulcerative colitis, inflammatory mediators released in the gut wall sensitize primary afferents, contributing to visceral hypersensitivity, whereas in IBS, hypersensitivity is present in the absence of an identifiable organic cause (De Schepper et al., Citation2008a). IBS and IBD are interrelated as up to 57% of IBD patients in remission suffer from IBS-like symptoms (Simren et al., Citation2002). Also, after an episode of severe gastroenteritis, 3–36% of patients develop new-onset IBS (post-infectious IBS, PI-IBS) (Spiller & Garsed, Citation2009). These clinical observations indicate that inflammation induces alterations in sensory signaling that can persist long after the initial insult has resolved.
In addition, traumatic life events such as abuse, neglect or loss of a parent have been independently associated with IBS and chronic life stress is linked to subsequent symptom intensity (Bennett et al., Citation1998; Chitkara et al., Citation2008). The finding that acute mental stress alters visceral sensitivity in both healthy controls and IBS patients further corroborates the importance of psychological factors (Posserud et al., Citation2004). Interestingly, adverse life events preceding or following acute gastroenteritis significantly increase the risk of acquiring PI-IBS (Gwee et al., Citation1999). Also, stress contributes to symptoms and disease activity in IBD patients (Maunder & Levenstein, Citation2008). These studies indicate an interaction between centrally mediated psychological factors and local inflammation, but whether and to what extent this interaction contributes to the development and/or maintenance of visceral hypersensitivity in these patients is unclear.
Several studies have attempted to gain further insight into this process with conflicting results (Supplementary Table S1). Liebregts et al. (Citation2007) subjected female Lewis rats to trinitrobenzene sulfonic acid (TNBS)-induced colitis and/or repeated water avoidance stress (WAS) and found that both inflammation and stress caused long-lasting alterations of visceral sensory function and that visceral hypersensitivity was more pronounced after combined exposure to inflammation and stress. In contrast, Larsson et al. (Citation2009) reported that in female C57BL/6 mice dextran sulfate sodium (DSS)-induced colitis induced visceral hypersensitivity during the acute but not the post-inflammatory phase, whereas repeated WAS, alone or in combination with colitis, did not affect visceral nociception. Finally, Chen et al. (Citation2013) showed that in male Sprague–Dawley rats DSS-colitis did not induce visceral hypersensitivity acutely, but did so only in the late inflammatory phase, and these authors found that heterotypic stress following inflammation exerted synergistic effects on afferent nerve signaling. Hence, it appears that the extent to which inflammation and stress alter visceral perception (independently of each other or in conjunction) is quite dependent upon the type, intensity and duration of the inflammation and stressor, along with the strain and sex of the animals. In the current study we evaluated the effectiveness of both TNBS-colitis and acute or repeated WAS to induce acute or longer-lasting visceral hypersensitivity in female Wistar rats and hypothesized that combining both triggers might exert an additive effect on visceral nociception.
Materials and methods
Animals
Female Wistar rats (200–225 g) were obtained from Charles River Laboratories (Wilmington, MA) and allowed to acclimatize to housing conditions for 1 week before experimentation. Rats were kept under a controlled room temperature (22 ± 2 °C) and humidity (60%) with a 12:12-h light/dark cycle (lights on at 07:00 h). Rats had access to food and water ad libitum and were housed in pairs up to the moment of electrode implantation, after which they were individually housed. All animal experiments were approved by the Committee for Medical Ethics and the use of Experimental Animals at the University of Antwerp (2008-01 and 2010-18) and were in accordance with current European laws concerning animal experimentation. Induction of colitis, colonoscopy, electrode implantation, vaginal smear and euthanasia were all performed under sodium pentobarbital anesthesia (0.6 mg ml−1; 45–100 mg kg−1 intraperitoneally). In contrast, WAS and VMR procedures were performed in fully awake rats.
Induction of colitis
Distal colitis was induced as previously published by our group (De Schepper et al., Citation2008b; Deiteren et al., Citation2014; Vermeulen et al., Citation2013, Citation2011). Briefly, after a 24-h fast and under pentobarbital anesthesia (45 mg kg−1), 0.5 ml of 0.9% saline (control) or 7.5 mg of TNBS dissolved in 40% ethanol (colitis) was instilled intrarectally through a flexible catheter (4.5 cm length). TNBS consistently induces a transmural colitis that peaks after 3–7 days, resolves spontaneously within 2–8 weeks and is clinically, immunologically and histopathologically reminiscent of a flare of Crohn’s disease (Morris et al., Citation1989; te Velde et al., Citation2006; Vermeulen et al., Citation2011).
Exposure to stress
The validated WAS protocol consists of placing the rat on a platform (10 × 8 × 8 cm) fixed to the centre of a tank (49 × 38 × 56 cm) filled with water (21 °C) up to 1 cm below the platform for a period of 1 h (Bradesi et al., Citation2005; Million et al., Citation1999). Sham stress entailed relocating the rat to a cage filled with clean bedding for 1 h. Acute stress was induced by a single 1-h WAS session, whereas repetitive stress consisted of daily 1-h WAS exposures during 7–10 consecutive days, depending on the experimental protocol. All WAS/sham sessions were performed between 09:00 h and 12:00 h. The 1-h WAS protocol represents a validated, mild psychological stressor that activates the hypothalamic–pituitary–adrenal (HPA) axis with a concurrent increase in adrenocorticotropic hormone and cortisol levels; a stress response to WAS can be non-invasively assessed by counting the number of fecal pellets excreted during WAS/sham procedures (Bradesi et al., Citation2005; Million et al., Citation1999).
Visceral sensitivity
The visceromotor response (VMR) to colorectal distension was used as an objective measure of visceral sensitivity (Deiteren et al., Citation2014; Vermeulen et al., Citation2013). Three-to-five days prior to testing, rats were equipped with Teflon-coated wire electrodes for electromyography (Advent Research Materials Ltd, Oxford, UK) that were sutured into the external oblique abdominal muscle and tunnelled subcutaneously to be exteriorized at the base of the neck for future access. No antibiotics were administered post-surgery. On the day of VMR assessment, rats were placed in a custom-made fabric glove, to which they were accustomed during the preceding 3–5 days. The electrodes were connected to a data acquisition system. A lubricated latex balloon (length 5 cm) was gently introduced into the distal colon up to 0.5 cm past the anal verge. The balloon catheter was secured to the base of the tail and connected to a barostat system (Distender Series II™ Barostat, G&J Electronics, Ontario, Canada) for balloon distension. Colorectal balloon distensions were performed in a graded and pressure-controlled fashion (10–80 mmHg, 20 s distension, 4 min interval). The abdominal electromyographic signal was recorded (NL100AK headstage), amplified (NL104), filtered (NL125/126; NeuroLog, Digitimer Ltd, Welwyn Garden City, UK; band pass 50–5000 Hz) and digitized (CED 1401, Cambridge Electronic Design) to a PC for offline analysis using Spike2 version 5.16 for Windows (Cambridge Electronic Design, Cambridge, UK). After correction for movement and breathing, the analog signal was rectified and integrated. To quantify the magnitude of the VMR at each distension pressure, the area under the curve (AUC) pre-distension (20 s) was subtracted from the AUC during distension (20 s).
Assessment of estrous cycle phase
Vaginal smears were used to assess the estrous cycle phase following VMR assessment (Hubscher et al., Citation2005). The vaginal cavity was flushed with 0.9% saline (100 µl) and the estrous cycle phase (metestrus, diestrus and (pro)estrus) was determined by evaluating the wet smear under 100× magnification as previously described by Hubscher et al. (Citation2005).
Corticosterone concentrations
Blood (300 µl) was drawn from the tail vein of consciously restrained rats between 09:00 and 12:00 h and serum was collected by centrifugation for 10 min at 13 000g. Serum corticosterone concentrations were assayed using an enzyme-linked immunosorbent assay kit according to the manufacturer’s instructions (Enzo Life Sciences, Farmingdale, NY). The quality control data for the corticosterone assay were as follows: sensitivity 67 pg/ml, interassay variability 6.4% and intra-assay variability 2.4%.
In vivo signs of inflammation
To evaluate the extent of inflammation and to follow-up the healing process in time, colonoscopy was performed at several time points during the (post-)inflammatory course using a neonate gastroscope (Olympus Europa GmbH). Rats were anesthetized with pentobarbital (45 mg kg−1) and placed in a supine position. After lubrication and under direct endoscopic vision, the endoscope was gently introduced for 10 cm into the colon. During withdrawal of the endoscope, mucosal damage was assessed using our previously published scoring system (score 0–19), comprised of the sum of the subscores for ulceration (0–6), stenosis (0–1), edema (0–1), bleeding (0–1) and the extent of damage (0–10 cm) (Deiteren et al., Citation2014; Vermeulen et al., Citation2011).
Post-mortem signs of inflammation
Rats were killed by exsanguination by cutting open the ventricles under deep pentobarbital anesthesia (100 mg kg−1), and the distal colon was excised to assess macroscopic mucosal damage using a standardized scoring system, based on the presence and extent of ulcerations and ranging from 0 (normal) to 10 (severe damage over >5 cm) () (Vermeulen et al., Citation2011; Wallace & Keenan, Citation1990). Next, two 1-cm full-thickness segments were harvested from areas with representative inflammation on macroscopic inspection for microscopic evaluation and a myeloperoxidase activity (MPO) assay. Previously, we demonstrated that using this standardized approach, there is a high correlation between the four signs of inflammation (from colonoscopy, macroscopy, microscopy and MPO activity) both during acute TNBS-colitis and during the healing process (Vermeulen et al., Citation2011). One segment was fixed in 4% formaldehyde for 24 h and embedded in paraffin for hematoxylin–eosin staining (5 µm transverse sections). Histological specimens were scored microscopically for the presence of an inflammatory infiltrate (0–3), the number of infiltrated layers (0–3), mucosal architectural distortion (0–3) and edema (0–1) (total score 0–10) (Vermeulen et al., Citation2011). The other segment was used in the MPO activity assay, according to our previously published method (Vermeulen et al., Citation2011). In brief, full-thickness colonic segments were blotted dry and placed in a potassium phosphate buffer pH 6.0 containing 0.5% hexadecyltrimethylammonium bromide (5 g tissue per 100 ml buffer). The samples were placed on ice, homogenized for 30 s and subjected to two sonication and freeze-thawing cycles. The suspension was centrifuged at 15 000 g for 15 min at 4 °C. Aliquots (100 µl) of the supernatant were added to 2.9 ml of o-dianisidine solution (16.7 mg of o-dianisidine in 1 ml 0.9% saline, 98 ml of 50 mmol potassium phosphate buffer, pH 6.0 and 1 ml of a 0.05% H2O2 solution as a substrate for MPO enzyme). The change in absorbance was read at 460 nm over 60 s using a Spectronic Genesys 5 spectrophotometer (Milton Roy, Ivyland, PA). One unit of MPO activity was defined as the quantity able to convert 1 µmol H2O2 to H2O per min at 25 °C and was expressed as units per gram of tissue (U g−1).
Table 1. Macroscopic damage score.
Experimental design
Four experimental protocols were performed, each consisting of a short-term and a long-term arm (). In the first protocol (), we assessed the effects of TNBS-colitis on visceral sensitivity. In the short-term experiment (n = 8/group), colorectal balloon distensions were performed 3 days after the induction of TNBS-colitis whereas the long-term protocol (n = 4–8/group) was designed as a follow-up experiment in which rats underwent sensitivity testing on Days 3, 28 and 56 after induction. The second condition () was designed to evaluate the effects of acute and repetitive WAS. In the short-term protocol (n = 8/group), rats were exposed to 1 h of WAS and visceral sensitivity was evaluated 24 h later; whereas in the long-term protocol (n = 8/group), rats underwent 1 h stress exposures during 10 consecutive days and colorectal distensions were performed the day before the first session and VMRs were compared with those on the day after the final stress session. We opted for 10 consecutive WAS sessions as this approach was previously shown to induce visceral hypersensitivity in male Wistar rats (Bradesi et al., Citation2005; Larauche et al., Citation2008). In the third series of experiments (), the potential additive effect of inflammation and stress was evaluated. In the short-term protocol (n = 7–8/group), rats were exposed to 1 h WAS 2 days after TNBS-induction and VMRs to colorectal balloon distension were quantified 1 day later. To assess long-term effects (n = 6–8/group), TNBS-induced rats were exposed either to acute (1×) or repetitive (7×) WAS during acute colitis and the post-inflammatory effects of combining colitis and stress were assessed after the resolution of colitis (Day 28); saline-instilled controls were subjected to sham stress on the corresponding days. We opted to subject rats to one or seven WAS sessions in this experiment to facilitate comparison with the study of Liebregts et al. (Citation2007) who previously showed that this protocol induced visceral hypersensitivity in female Lewis rats.
Figure 1. Scheme of the experimental protocol. (A) Short- and long-term effects of TNBS-induced colitis on visceral sensitivity as assessed by VMRs. Rats received an intracolonic enema of TNBS or saline on Day 0 and VMRs were assessed on Day 3 only (short-term) or on Days 3, 28 and 56 post-induction (long-term). (B) Effect of WAS on visceral sensitivity. In the short-term protocol, 1 h WAS was followed by a VMR assessment the next day, whereas in the long-term protocol VMRs were registered before and after 10 consecutive WAS sessions. (C) Protocol exploring a possible additive effect of inflammation and stress. In the short-term experiment, induction of TNBS-colitis was followed by WAS 2 days later and the effect on visceral sensitivity was assessed on Day 3. In the long-term protocol, TNBS-induced rats were exposed to (i) acute (1×) or (ii) repetitive (7×) WAS during acute colitis and VMRs were assessed on Day 28 post-TNBS enema. In all three experimental protocols (A, B and C), colonoscopic evaluation of the colon was performed following each VMR assessment. (D) Protocol exploring the effects of the short- and long-term experiments on the activation of the HPA axis. Serum corticosterone (cort) concentrations were measured before the experiment and at the end of the experiment. In the short-term protocol, the end of the experiment was on Day 3, in the long-term protocol this was on Day 11 (repetitive WAS) or Day 28 (TNBS or TNBS plus repetitive WAS).
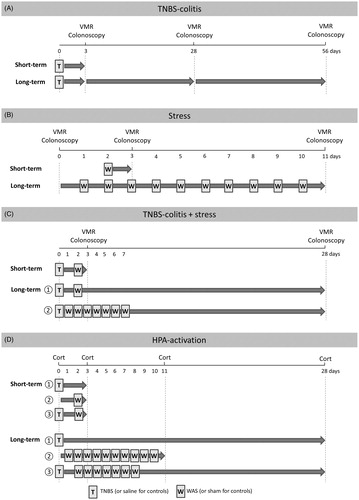
The fourth protocol () was designed to assess the effects of TNBS-colitis, WAS and their combination on the activation of the HPA axis. Each short-term and long-term experimental design paralleled the first three protocols (n = 6–7/group). However, rats did not undergo VMR assessment; instead, serum corticosterone concentrations were determined before the start and at the end of each experimental protocol.
In the first three protocols, colonoscopy and vaginal flushing to determine the estrous cycle stage were performed after every VMR assessment and rats were killed at the end of the experiment to evaluate the presence of remaining inflammation. Additionally, in the TNBS/saline experiments colonoscopy was performed 3 days after the induction to confirm colitis was indeed present in all rats.
Solutions and drugs
TNBS was purchased from Sigma (St. Louis, MO). Pentobarbital was obtained from Ceva, Libourne, France. Hexadecyltrimethylammonium bromide and o-dianisidine dihydrochloride were purchased from Sigma-Aldrich Inc. (St. Louis, MO), and hydrogen peroxide, formaldehyde and hematoxylin were from Merck, Darmstadt, Germany. Eosin was from Acros Organics (Fair Lawn, NJ) and paraffin from McCormick (St. Louis, MO).
Data presentation and statistical analysis
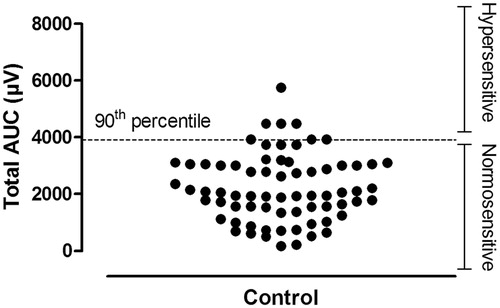
Visceral sensitivity is expressed as the AUC of the electromyographic signal and this AUC was used (i) to determine the magnitude of the VMR at each distension pressure (10, 20, 30, 40, 60 and 80 mmHg, µV 20 s−1), (ii) to calculate the total AUC as follows: AUC10 mmHg + AUC20 mmHg + AUC30 mmHg + AUC40 mmHg + AUC60 mmHg + AUC80 mmHg (µV) and (iii) to establish whether a rat displayed visceral hypersensitivity, based on a pre-defined cutoff. This cutoff was constructed on the 90th percentile of the total AUC for all control rats included in this manuscript (these rats received a saline enema and/or were exposed to sham stress) and was used as a boundary to define the normal range of sensitivity to colorectal distension (normosensitive). This method has been previously used by others to define VMR-based visceral hypersensitivity to colorectal balloon distensions (van den Wijngaard et al., Citation2013). Including 70 controls, the 90th percentile for the total AUC was 3911 µV (). Thus, all rats with a total AUC exceeding 3911 µV were considered hypersensitive.
Figure 2. VMR to colorectal distension: definition of normosensitivity. A range for normosensitivity was defined by the 90th percentile of the total AUC (µV) for all 70 controls in this study. The individual total AUCs are presented in the plot. The 90th percentile was calculated to be 3911 µV. All rats with a total AUC exceeding 3911 µV were considered hypersensitive. Therefore, by definition, 7 out of 70 controls were considered hypersensitive.
All data are presented as mean ± standard error of mean (SEM), for the number (n) of rats per group. Due to considerable variation in the corticosterone concentrations among rats, concentrations at the end of the experiment were expressed as a change relative to the baseline value. Most variables were analyzed using unpaired Student’s t-test, one-way or two-way analysis of variance (ANOVA) followed by Student–Newman–Keuls (SNK) post hoc test when appropriate. Analysis of VMR data was performed by generalized estimating equations followed by least significant difference (LSD) post hoc test when appropriate. Fisher’s exact test was used to compare estrous cycle phases between groups and the effect of the different cycle phases on inflammation/stress-induced alterations in visceral sensitivity was assessed in a two-way ANOVA design. Correlation was assessed by computing Pearson’s or Spearman’s ρ as correlation coefficients. Statistical significance was set at p < 0.05 for all tests.
Results
Protocol 1: effect of TNBS-colitis
Short-term
TNBS instillation resulted in marked colonic inflammation on Day 3. Large ulcers extending longitudinally over several centimeters of the distal colon were noted in all rats on colonoscopy [score 9.9 ± 0.6 for TNBS versus 0.0 ± 0.0 for controls; n = 8/group; t(7) = 16.1; p < 0.001]. Macroscopic [7.4 ± 0.6 versus 0.0 ± 0.0 for controls; n = 8/group; t(7) = 11.8; p < 0.001] and microscopic [6.8 ± 0.6 versus 0.0 ± 0.0 for controls; n = 8/group; t(7) = 12.1; p < 0.001] damage scores were increased accordingly. Similarly, MPO activity was significantly higher in TNBS-colitis rats compared to controls [28.8 ± 2.0 versus 0.7 ± 0.3 U g−1, respectively; n = 8/group; t(7) = 13.7; p < 0.001].
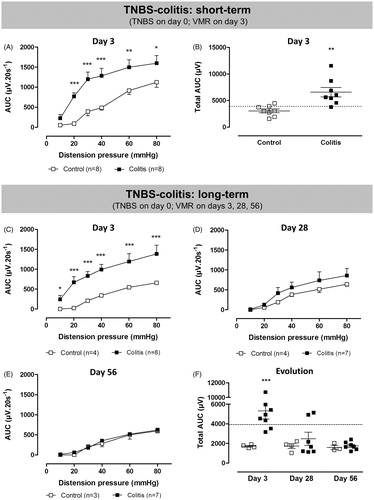
VMRs to colorectal distension on Day 3 post-induction increased in a pressure-dependent fashion (χ2 = 475; df = 5; p <0.001 for the factor distension pressure, ). At a distension pressure of 20 mmHg or higher, abdominal contractions were more vigorous in TNBS-colitis rats, indicating the presence of visceral hypersensitivity (χ2 = 74; df = 5; p < 0.001 for the interaction colitis*pressure, post hoc LSD). Similarly, the total AUC was significantly higher in the acute colitis group [t(14) = 3.7; p < 0.01; ]. Using the 90th percentile cutoff, seven out of eight TNBS-colitis rats were considered hypersensitive. In TNBS-treated rats, the total AUC did not correlate with colonoscopic, macroscopic or microscopic damage scores, nor with the MPO activity (data not shown). At the time of VMR assessment, the proportion of rats in the different estrous cycle phases was similar in control and TNBS-colitis rats [metestrus/diestrus/(pro)estrus was 6/1/1 for controls and 5/2/1 for TNBS; p = 1, Fisher’s exact test] and the cycle phase did not affect the total AUC (two-way ANOVA, no significant effect of the factor cycle phase and no significant interaction between TNBS and cycle phase).
Figure 3. Short- and long-term effects of TNBS-induced colitis (filled squares) or saline enema (open squares) on VMRs to colorectal distension. In the short-term protocol, VMRs were evaluated 3 days after induction of TNBS-colitis (A and B). In the long-term experiment, visceral sensitivity was assessed longitudinally in the same rats on Days 3, 28 and 56 post-induction of colitis (C–F). Data are presented as the AUC for the individual distension pressures (A, C–E; analyzed by generalized estimating equations) or as the total AUC (B and F, unpaired Student’s t-test). The dashed line in B and F represents the 90th percentile for controls as an upper limit for normosensitivity. Results are expressed as mean ± SEM; *p < 0.05, **p < 0.01, ***p < 0.001 compared to controls.
Long-term
In the TNBS-colitis group, overt inflammation was present 3 days after induction as colonoscopic damage scores were significantly increased compared to controls [9.3 ± 0.3 for TNBS versus 0.0 ± 0.0 for controls; n = 4–8/group; t(7) = 20; p < 0.001]. At Days 28 and 56, the colonoscopic picture was indistinguishable from controls (0.0 ± 0.0 for TNBS versus 0.0 ± 0.0 for controls at both time points; n = 4–8/group) evidencing endoscopic resolution of colitis.
Significantly increased VMRs were present during the acute inflammatory phase over the full range of distension pressures applied [χ2 = 81; df = 5; p < 0.001 for the interaction colitis*pressure, post hoc LSD for ; t(10) = 3; p < 0.05 for ]. However as colonic inflammation resolved, visceral hypersensitivity waned. At Day 28, VMRs remained slightly but non-significantly elevated [χ2 = 2; df = 1; p = 0.1 for the factor colitis in ; t(9) = 1; ns for ], mostly due to the presence of visceral hypersensitivity in two out of seven rats. By Day 56 post-induction, VMR had declined into the range of normosensitivity in all rats [χ2 = 0.5; df = 1; ns for the factor colitis in ; t(8) = 1; ns for ]. Control rats showed comparable VMRs to colorectal distension at all three time points investigated. Of note, due to technical failure of the EMG electrode, VMRs could not be assessed in one colitis rat on Days 28 and 56 as well as in one control rat on Day 56. In TNBS-treated rats, there was no significant correlation between the total AUC and any of the signs of inflammation on Days 3, 28 or 56 (data not shown). At the time of VMR assessment, the proportion of rats in the different estrous cycle phases was similar in control and TNBS-colitis rats on Days 3, 28 and 56 [metestrus/diestrus/(pro)estrus on Day 3 was 1/2/1 for controls and 3/3/2 for TNBS, p = 0.45; on Day 28 1/2/1 for controls and 2/5/1 for TNBS, p = 0.62; on Day 56 2/0/2 for controls and 1/5/2 for TNBS, p = 0.10; Fisher’s exact test] and the cycle phase did not affect the total AUC (two-way ANOVA, no significant effect of the factor cycle phase and no significant interaction between TNBS and cycle phase at any time point).
Protocol 2: effect of stress
Acute stress
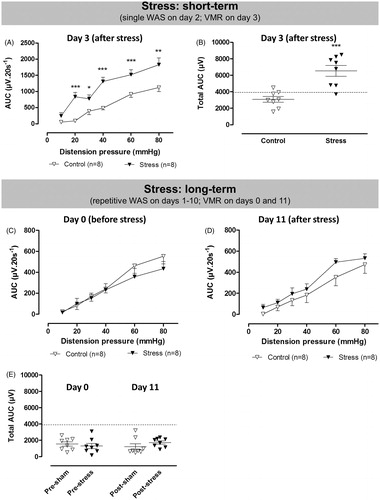
Single WAS exposure did not induce any significant changes in mucosal appearance as assessed by colonoscopy [0.3 ± 0.3 for WAS versus 0.0 ± 0.0 for controls; n = 8/group; t(7) = 1.0; ns], macroscopy [0.3 ± 0.3 versus 0.0 ± 0.0 for controls; n = 8/group; t(7) = 1.0; ns] or microscopy [0.0 ± 0.0 versus 0.0 ± 0.0 for controls; n = 8/group], nor did it affect MPO activity [0.9 ± 0.2 versus 0.7 ± 0.3 U g−1 for controls; n = 6–8/group; t(12) = 0.7; ns]. Nevertheless, a small ulcer was present in the distal colon of one rat subjected to acute stress. Fecal pellet output was significantly augmented during WAS [8.3 ± 1.4 pellets for WAS versus 1.4 ± 0.6 for controls; n = 8/group; t(9) = 4.4; p < 0.01], indicating that a 1-h exposure effectively induced stress. Visceral sensitivity was assessed 24 h after WAS and showed significantly increased VMRs in WAS-exposed rats at a distension pressure of ≥20 mmHg [χ2 = 40; df = 5; p < 0.001 for the interaction WAS*pressure, post hoc LSD in ; t(14) = 5; p < 0.001 for ]. In WAS-exposed rats, there was no significant correlation between the total AUC and the number of feces excreted during WAS [ρ = 0.66; p = 0.078). The proportion of rats in the different estrous cycle phases was similar in the control and stressed groups at the time of VMR assessment [metestrus/diestrus/(pro)estrus was 1/6/1 for control and 5/2/1 for WAS, p = 0.13, Fisher’s exact test] and the cycle phase did not affect the total AUC [two-way ANOVA, no significant effect of the factor cycle phase and no significant interaction between WAS and cycle phase].
Repetitive stress
Before the start of the repetitive stress protocol, VMRs to colorectal distension were comparable between the stress and control groups [χ2 = 0.4; df = 1; ns for the factor WAS on ; t(15) = 1; ns for ]. Subsequent colonoscopy confirmed that no colonic damage was induced by the balloon distensions (0.0 ± 0.0 for WAS versus 0.0 ± 0.0 for controls; n = 8).
Figure 4. The effects of acute and repetitive WAS (filled triangles) or sham stress (open triangles) on VMRs to colorectal distension. In the acute setting, VMRs were evaluated 24 h after single WAS (A and B). In the repetitive stress experiment, visceral sensitivity was assessed longitudinally in the same rats the day before the start of the protocol and 24 h after the last stress exposure (C–E). Data are presented as the AUC for the individual distension pressures (A, C and D; analyzed by generalized estimating equations) or as the total AUC (E, unpaired Student’s t-test). The dashed line in B–E represents the 90th percentile for controls as an upper limit for normosensitivity. Results are expressed as mean ± SEM; *p < 0.05, **p < 0.01, ***p < 0.001 compared to controls.
Repetitive stress exposure did not induce significant inflammation since colonoscopic (0.3 ± 0.3 for WAS versus 0.1 ± 0.1 for controls; n = 8/group; t(14) = 0.9; ns], macroscopic [0.4 ± 0.3 versus 0.1 ± 0.1 for controls; n = 8/group; t(14) = 0.9; ns] and microscopic [0.4 ± 0.3 versus 0.1 ± 0.1 for controls; n = 8/group; t(14) = 0.9; ns] scores were comparable in both group although MPO activity was slightly, but non-significantly increased [1.1 ± 0.4 versus 0.3 ± 0.2 U g−1 for controls; n = 8/group; t(14) = 1.1; ns]. A small ulcer was present in the distal colon of one stress rat whereas hyperemia was noted in one control rat. Fecal pellet output was increased during the first WAS exposure (8.4 ± 1.0 pellets for WAS versus 1.6 ± 0.4 for controls; n = 8/group) and remained equally elevated throughout all 10 stress sessions. During the last WAS protocol, stressed rats expelled 8.8 ± 1.1 fecal pellets compared to 1.1 ± 0.4 in the control group. Two-way ANOVA confirmed that the difference in fecal pellet output between the two groups [F(1,20) = 64.0; p < 0.001] was not influenced by time [F(1,20) = 0.01; ns; no interaction, F(1,20) = 0.9; ns].
VMR assessment 24 h after the last WAS session showed no significant difference in visceral pain perception between control and stressed rats [χ2 = 1; df = 1; ns for the factor WAS in ; t(15) = 1; ns for ]. In WAS-exposed rats, there was no correlation between the total AUC on Day 11 and the number of fecal pellets excreted during the first or last WAS session (data not shown). The proportion of rats in the different estrous cycle phases was similar in control and stressed groups [metestrus/diestrus/(pro)estrus was 2/4/2 for control and 2/3/3 for WAS, p = 1, Fisher’s exact test]. There was a significant interaction between the cycle phase and WAS [F(2,10) = 16; p = 0.019], although no significant effects were present between groups on post hoc comparison. Of note, the rats demonstrating the highest total AUC in this experiment were in the proestrus phase.
Protocol 3: effect of inflammation and stress
Short-term
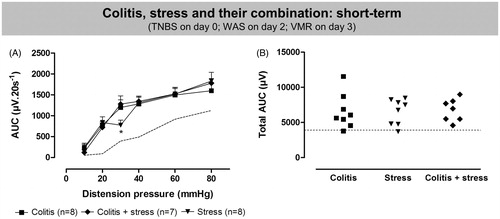
In rats that were exposed to both TNBS and 1 h WAS, colonoscopy showed signs of severe inflammation 3 days after TNBS-induction [score 10.9 ± 0.9 versus 0.0 ± 0.0 for controls; n = 7–8/group; t(6) = 11.9; p < 0.001]. Similarly, macroscopic [8.0 ± 0.7 versus 0.0 ± 0.0 for controls; t(6) = 12.2; p < 0.001] and microscopic [7.3 ± 0.4 versus 0.0 ± 0.0 for controls; t(6) = 17.3; p < 0.001] damage scores confirmed the presence of colitis, and MPO activity was significantly increased [35.0 ± 7.6 versus 0.7 ± 0.3 U g−1 for controls; t(6) = 4.4; p < 0.01]. VMRs during acute TNBS-colitis plus stress were markedly elevated compared to controls and significant visceral hypersensitivity was present in all seven rats [χ2 = 56; df = 5; p < 0.001 for the interaction colitis plus WAS*pressure, post hoc LSD in ; t(13) = 5; p < 0.001 for ]. compares the short-term effects of acute TNBS, acute stress and their combination on visceral sensitivity. Only at the 30 mmHg distension, were increased responses more apparent after TNBS or TNBS plus stress [χ2 = 92; df = 10; p < 0.001 for the interaction group*pressure, post hoc LSD in ; F(2,20) = 0.02; ns for ]. At the time of VMR assessment, the proportion of rats in the different estrous cycle phases was similar in control and TNBS plus stress rats [metestrus/diestrus/(pro)estrus was 2/5/1 for control and 4/2/1 for TNBS plus WAS, p = 0.51, Fisher’s exact test] and the cycle phase did not affect the total AUC (two-way ANOVA, no significant effect of the factor cycle phase and no significant interaction).
Figure 5. Short- and long-term effects of TNBS-induced colitis combined with exposure to WAS (filled diamonds) compared to saline enema and sham stress (open diamonds). In the short-term protocol, VMRs were evaluated 3 days after induction of TNBS-colitis and WAS was performed on Day 2 (A and B). In the long-term experiment, visceral sensitivity was assessed 28 days post-induction of colitis and rats were exposed either to single WAS on Day 2 (C) or repetitive WAS on Days 1–7 (D). Data are presented as the AUC for the individual distension pressures (A, C and D; analyzed by generalized estimating equations) or as the total AUC (B and E, unpaired Student’s t-test). The dashed line in B and E represents the 90th percentile for controls as an upper limit for normosensitivity. Results are expressed as mean ± SEM; ***p < 0.001 compared to controls.
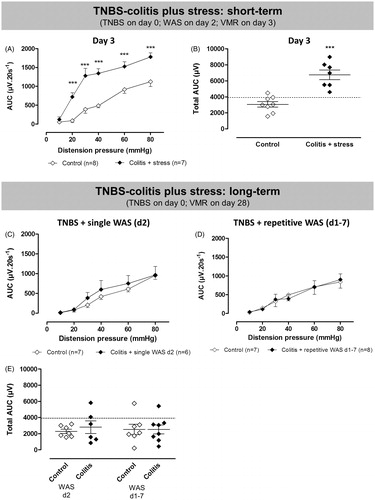
Figure 6. Comparison of the short-term effect of acute TNBS-induced colitis (filled squares), acute WAS (filled triangles) and their combination (filled diamonds) on visceral pain responses. Data are presented as the AUC for the individual distension pressures (A; analyzed by generalized estimating equations) or as the total AUC (B; one-way ANOVA). The dashed line represents the normal range of sensitivity. Results are expressed as mean ± SEM; *p < 0.05, significantly different compared to acute colitis and acute colitis plus stress.
Long-term
In both long-term protocols evaluating the additive effects of colitis and WAS (), intrarectal TNBS instillation resulted in severe inflammation at Day 3 (). By Day 28, there were no significant differences in colonoscopic, macroscopic and histological scores between the control and colitis plus stress groups, as well as no statistical differences in MPO activity.
Table 2. Inflammatory markers on Day 28 after TNBS and WAS.
Twenty-eight days after TNBS-induction, VMRs to colorectal distension were similar when comparing colitis plus stress groups to their respective controls [χ2 = 2; df = 1; ns for the factor TNBS + 1× WAS in ; χ2 = 0.01; df = 1; ns for the factor TNBS + 7× WAS in ]. Using the 90th percentile () cutoff two of six rats were considered hypersensitive after colitis plus single WAS [t(11) = 1; ns] and one of eight after colitis plus repetitive WAS [t(13) = 0.02; ns]. At the time of VMR assessment, more rats were in the diestrus phase after TNBS plus single WAS compared to its respective control group [metestrus/diestrus/(pro)estrus was 1/4/2 for control and 0/5/1 for TNBS plus single WAS, p = 0.03, Fisher’s exact test] but this did not affect the total AUC. The proportion of rats in the different estrous cycle phases was similar in control and TNBS plus repetitive stress groups [metestrus/diestrus/(pro)estrus was 3/4/0 for control and 4/4/0 for TNBS plus repetitive WAS, p = 1, Fisher’s exact test] and the cycle phase did not affect the total AUC (two-way ANOVA, no significant effect of the factor cycle phase, no significant interaction between cycle phase and TNBS plus repetitive WAS).
Protocol 4: activation of the HPA axis
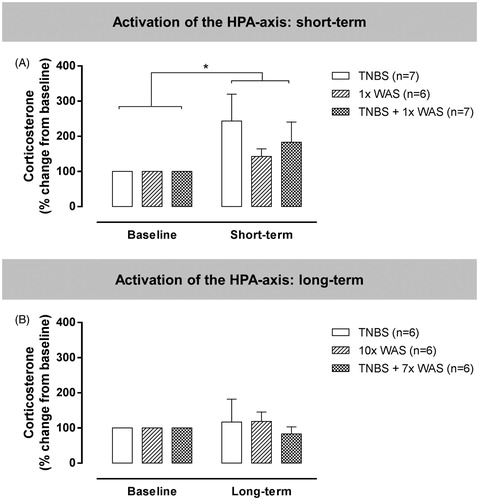
Acute TNBS-colitis, acute WAS and their combination all resulted in a similar increase in serum corticosterone concentration compared to baseline levels [; F(1,17) = 7; p < 0.05]. However, in the long-term protocols, corticosterone concentrations were no longer increased at the time of VMR assessment [; F(1,15) = 0.1; ns]. In other words, no alteration in HPA axis activation was seen following repetitive WAS or after the resolution of TNBS-colitis or TNBS-colitis combined with repetitive WAS.
Figure 7. Serum corticosterone concentrations in a separate set of rats to assess activation of the HPA axis. The short-term (A) and long-term (B) protocols were designed to parallel the visceral sensitivity experiments. Corticosterone concentrations were measured before the start and at the end of each experiment, consistent with the timing of registration of VMRs in the previous experiments. Data are presented as mean ± SEM. Repeated two-way ANOVA. *p < 0.05, significantly different compared to baseline.
Discussion
In this study, we assessed the effects of colitis and stress and a possible interaction between both on colonic sensitivity in female Wistar rats. TNBS-colitis and acute WAS activated the HPA axis and resulted in visceral hypersensitivity in separate sets of rats. However, these increased VMRs and corticosterone levels resolved spontaneously in the long-term. We found no evidence of an interaction between TNBS-colitis and WAS in the modulation of visceral sensitivity.
The well-validated TNBS-colitis model, reminiscent of an acute flare of Crohn’s disease, is associated with visceral hypersensitivity during acute inflammation (Adam et al., Citation2006; De Schepper et al., Citation2008b; Gschossmann et al., Citation2002; Vermeulen et al., Citation2013). It is also the most commonly used approach when studying post-inflammatory visceral hypersensitivity which can remain after the TNBS-colitis has resolved (Qin et al., Citation2011). However, whereas some groups have documented post-inflammatory hypersensitivity 4 weeks after TNBS instillation in 90–95% of rats (Greenwood-Van Meerveld et al., Citation2005; Gschossmann et al., Citation2004), others have reported persistent hypersensitivity to colorectal distension in only ∼25% of rats (Zhou et al., Citation2008a,Citationb,Citationc), and some were not able to demonstrate any remaining hypersensitivity (Feng et al., Citation2012; Gschossmann et al., Citation1999a; Tarrerias et al., Citation2002). Also in our study, robust visceral hypersensitivity was present during acute TNBS-colitis, but during convalescence rats recovered towards the range of normosensitivity, indicating that colitis does not always result in long-term sensitization to noxious stimuli.
The explanations underlying the wide discrepancy in post-inflammatory hypersensitivity are presently unclear. Methodological differences such as TNBS dosage, administered volume and ethanol percentage could contribute (Qin et al., Citation2012). Adam et al. (Citation2006) previously reported that the severity of colitis is associated with enhanced post-inflammatory nociception. Although we could not confirm the correlation between the degree of inflammation and visceral sensitivity, our dose of TNBS induced a severe colitis with extensive ulcerations; hence, it is unlikely that the degree of colitis was insufficient. Gender and strain also contribute to discrepancies in post-inflammatory hypersensitivity; whereas, Lewis rats are prone to develop persistent post-inflammatory hypersensitivity, this is not the case for Fisher rats and this seems to relate to a longer duration of TNBS-colitis in Lewis compared to Fisher rats (Adam et al., Citation2013). Also Sprague–Dawley rats seem highly susceptible to developing post-inflammatory hypersensitivity (Diop et al., Citation2002; Greenwood-Van Meerveld et al., Citation2005; Qin et al., Citation2012). However, long-lasting post-inflammatory visceral hypersensitivity has also been reported in Wistar rats (Distrutti et al., Citation2006) and their high stress responsiveness makes them an interesting strain to simultaneously assess the effect of both factors on visceral sensitivity (Bradesi et al., Citation2005).
To support the role of the degree of inflammation, strain and gender, we could demonstrate in a pilot experiment that inducing a mild colitis in male Sprague–Dawley rats and monitoring the inflammation individually over time was capable of inducing post-inflammatory visceral hypersensitivity in 80% of rats (Deiteren et al., Citation2012).
Whereas colitis is a peripherally driven process, stress affects sensory processing at multiple levels of the brain–gut axis. A single WAS exposure resulted in robust hypersensitivity, similar to acute TNBS-colitis, but repetitive stress did not affect visceral nociception. In a separate set of rats, corticosterone levels were increased only after acute but not repetitive stress, as was also recently reported by another group (Lauffer et al., Citation2014). Although fecal pellet output remained high during the entire repetitive WAS protocol, our data seem to indicate that female Wistar rats quickly habituate to repeated stress. In contrast, the 10× WAS protocol was previously shown to effectively induce visceral hypersensitivity and persistently increased corticosterone levels in male Wistar and Sprague–Dawley rats (Bradesi et al., Citation2009; Hong et al., Citation2009; Larauche et al., Citation2008). In contrast, our experiments were performed in female rats. Overall, female rats display enhanced responses to colorectal distension compared to males (Gschossmann et al., Citation1999b) and exhibit greater corticosterone release in response to stress (Chaloner & Greenwood-Van Meerveld, Citation2013). Sex differences are also known to differentially modulate sensory signaling in response to stress and this sexual dimorphism could underlie the increased prevalence of IBS in females (Chaloner & Greenwood-Van Meerveld, Citation2013). Therefore, by studying female rats we would be more likely to find stress-induced alterations. As our protocols were not designed to investigate the effect of the estrous cycle phase on hypersensitivity in these different set-ups and thus were probably underpowered in this regard, we need to interpret these results with caution. Recently Larauche et al. (Citation2010, Citation2012a,Citationb) convincingly demonstrated that the effect of repetitive WAS on sensitivity is not only related to the animals’ sex, but also highly dependent on the methods used to record VMRs, housing conditions and the handling by the investigator, which could all account for part of the discrepancy between our results and previous reports from other groups.
Based on clinical observations of an interaction between inflammation and stress on the disease course of IBS and IBD, it was hypothesized that whereas inflammation serves as a trigger, concomitant stress might be responsible for long-term symptom persistence (Bernstein et al., Citation1996; Gwee et al., Citation1999). However, whether this is also true for visceral hypersensitivity is unclear as in these clinical studies visceral pain perception was never compared between stressed and non-stressed patients. We expected that, similar to the human findings of increased inflammation- and stress-induced visceral sensitivity, acute TNBS-colitis and WAS would enhance visceral sensitivity in female Wistar rats and hypothesized that a combination of both factors might result in long-lasting post-inflammatory hypersensitivity. In the short-term protocol, acute TNBS-colitis combined with single WAS resulted in significant visceral hypersensitivity and HPA- axis activation but VMRs and corticosterone levels were increased to a comparable degree after TNBS or WAS alone. In the long-term protocol, evaluating the combination of colitis with either acute or repetitive stress, significant post-inflammatory hypersensitivity remained absent and thus corticosterone levels had returned to baseline. As VMRs and corticosterone levels were assessed in separate sets of rats, it remains speculative whether persistent HPA- axis activation is a prerequisite to develop long-lasting post-inflammatory hypersensitivity. Three other groups previously evaluated the effect of combined inflammation and stress on post-inflammatory hypersensitivity (Supplementary Table S1). In support, Larsson et al. (Citation2009) documented no effect of 10 consecutive WAS sessions before the induction of DSS-colitis in female mice, leading the authors to question the link between inflammation/stress and visceral hypersensitivity. Besides species differences, our studies differ in experimental protocol: they used mice that were exposed to repetitive WAS before the induction of DSS-colitis, whereas we exposed rats to repeated WAS after a TNBS enema. Taken together, both studies suggest a lack of long-lasting alterations in sensory signaling after colitis combined with stress, extending across species and inflammatory models.
It is not exactly clear why our results (and those of others) do not always match the clinical findings. Most likely, other elements not considered in these animal models also contribute to an individual’s susceptibility to develop IBS/hypersensitivity such as smoking and psychosocial factors including depression and hypochondriasis, social learning and early life stressors (Spiller & Garsed, Citation2009). Early life stressors are the key component of the maternal stress protocol, in which pups are separated from the dam for several hours a day during the early postnatal period and the resulting visceral hypersensitivity is even transferred across generations (van den Wijngaard et al., Citation2013). To our knowledge, none of the currently available models for visceral hypersensitivity accurately reflects all the different factors modulating visceral sensitivity.
Alternatively, although the VMR to colorectal distension is a nociceptive brainstem reflex that integrates both central and peripheral signaling and parallels the human experimental paradigms, it might not be the most sensitive method to study alterations in sensory transmission as recently demonstrated by Chen et al. (Citation2013). They compared VMRs and direct afferent nerve firing in response to colorectal distension during DSS-colitis in male Sprague–Dawley rats: the firing of high threshold afferents was increased after DSS-exposure, indicating peripheral afferent sensitization, but VMRs remained unaltered. The concurrent exposure to inflammation and stress showed synergistic effects in their study with sensitization of peripheral low and high threshold afferents which was paralleled by an increase in VMRs. Finally, Liebregts et al. (Citation2007) used the same long-term protocol as we did and subjected female Lewis rats to repetitive WAS during the 7 days following TNBS-induction. Concomitant exposure to TNBS plus stress had an additive effect on VMRs 28 days post-induction and resulted in a longer duration of low-grade inflammation. Of note, a prolonged inflammatory response is a known risk factor for PI-IBS (Spiller & Garsed, Citation2009). However, a major difference from our study is that Liebregts et al. (Citation2007) also reported significant inflammatory changes in the colon after repeated WAS alone, which coincides with the notion of Lewis rats being highly susceptible to enterocolitis (Adam et al., Citation2013). Overall, these results, in conjunction with our own data, seem to suggest that the effect of stress on colitis and post-inflammatory nociception is—at least partially—governed by genetic differences. These could possibly involve differences in HPA-axis activation and mechanisms regulating the extent and duration of the initial inflammation, which will need to be addressed in future studies.
Conclusion
Clinical data suggest that both stress and inflammation are important factors in the pathophysiology of visceral hypersensitivity. Although both acute TNBS-colitis and acute WAS potently modulated visceral sensory function and activated the HPA axis in female Wistar rats, long-term effects were absent. In addition, we found no evidence of an interaction between colitis and stress on the development and/or maintenance of inflammation-induced visceral hypersensitivity and on activation of the HPA axis. At the least, these findings highlight the complexity of the mechanisms contributing to visceral pain perception and the difficulty of translating disorders with an intricate pathophysiology such as IBS and IBD into animal models.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
AD is an aspirant of the Fund for Scientific Research (FWO), Flanders. This work was supported by the FWO, Flanders (Grant Nos G.0249.09 and G.0341.13) and the University of Antwerp (NOI-BOF).
Supplementary material available online.
Supplementary Table S1
Supplementary Material
Download PDF (40.5 KB)References
- Adam B, Liebregts T, Gschossmann JM, Krippner C, Scholl F, Ruwe M, Holtmann G. (2006). Severity of mucosal inflammation as a predictor for alterations of visceral sensory function in a rat model. Pain 123:179–86
- Adam B, Tsopelas C, Liebregts T, Bartholomeusz FD, Holtmann G. (2013). Host immune response determines visceral hyperalgesia in a rat model of post-inflammatory irritable bowel syndrome. J Gastroenterol 48:1119–27
- Barbara G, Cremon C, De Giorgio R, Dothel G, Zecchi L, Bellacosa L, Carini G, et al. (2011). Mechanisms underlying visceral hypersensitivity in irritable bowel syndrome. Curr Gastroenterol Rep 13:308–15
- Bennett EJ, Tennant CC, Piesse C, Badcock CA, Kellow JE. (1998). Level of chronic life stress predicts clinical outcome in irritable bowel syndrome. Gut 43:256–61
- Bernstein CN, Niazi N, Robert M, Mertz H, Kodner A, Munakata J, Naliboff B, Mayer EA. (1996). Rectal afferent function in patients with inflammatory and functional intestinal disorders. Pain 66:151–61
- Bradesi S, Martinez V, Lao L, Larsson H, Mayer EA. (2009). Involvement of vasopressin 3 receptors in chronic psychological stress-induced visceral hyperalgesia in rats. Am J Physiol Gastrointest Liver Physiol 296:G302–9
- Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, et al. (2005). Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol 289:G42–53
- Chaloner A, Greenwood-Van Meerveld B. (2013). Early life adversity as a risk factor for visceral pain in later life: importance of sex differences. Front Neurosci 7:13. doi:10.3389/fnins.2013.00013
- Chen J, Winston JH, Sarna SK. (2013). Neurological and cellular regulation of visceral hypersensitivity induced by chronic stress and colonic inflammation in rats. Neuroscience 248C:469–78
- Chitkara DK, van Tilburg MA, Blois-Martin N, Whitehead WE. (2008). Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol 103:765–74; quiz:775
- De Schepper HU, De Man JG, Moreels TG, Pelckmans PA, De Winter BY. (2008a). Review article: gastrointestinal sensory and motor disturbances in inflammatory bowel disease – clinical relevance and pathophysiological mechanisms. Aliment Pharmacol Ther 27:621–37
- De Schepper HU, De Winter BY, Van Nassauw L, Timmermans JP, Herman AG, Pelckmans PA, De Man JG. (2008b). TRPV1 receptors on unmyelinated C-fibres mediate colitis-induced sensitization of pelvic afferent nerve fibres in rats. J Physiol 586:5247–58
- Deiteren A, De Man JG, Ruyssers NE, Moreels TG, Pelckmans PA, De Winter BY. (2014). Histamine H4 and H1 receptors contribute to postinflammatory visceral hypersensitivity. Gut. [Epub ahead of print]. doi:10.1136/gutjnl-2013-305870
- Deiteren A, Vermeulen W, De Man JG, Moreels TG, Pelckmans PA, De Winter BY. (2012). Different methods to induce visceral hypersensitivity in rats: short- and long-term effects of inflammation and stress. Gastroenterology 142:S894
- Diop L, Raymond F, Fargeau H, Petoux F, Chovet M, Doherty AM. (2002). Pregabalin (CI-1008) inhibits the trinitrobenzene sulfonic acid-induced chronic colonic allodynia in the rat. J Pharmacol Exp Ther 302:1013–22
- Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Russo G, Caliendo G, et al. (2006). 5-Amino-2-hydroxybenzoic acid 4-(5-thioxo-5H-[1,2]dithiol-3yl)-phenyl ester (ATB-429), a hydrogen sulfide-releasing derivative of mesalamine, exerts antinociceptive effects in a model of postinflammatory hypersensitivity. J Pharmacol Exp Ther 319:447–58
- Feng B, La JH, Tanaka T, Schwartz ES, McMurray TP, Gebhart GF. (2012). Altered colorectal afferent function associated with TNBS-induced visceral hypersensitivity in mice. Am J Physiol Gastrointest Liver Physiol 303:G817–24
- Greenwood-Van Meerveld B, Johnson AC, Foreman RD, Linderoth B. (2005). Spinal cord stimulation attenuates visceromotor reflexes in a rat model of post-inflammatory colonic hypersensitivity. Auton Neurosci 122:69–76
- Gschossmann JM, Adam B, Liebregts T, Buenger L, Ruwe M, Gerken G, Mayer EA, Holtmann G. (2002). Effect of transient chemically induced colitis on the visceromotor response to mechanical colorectal distension. Eur J Gastroenterol Hepatol 14:1067–72
- Gschossmann JM, Holtmann G, Adam B, Liebregts T, Zeeh JM, Ruwe M, Schmid KW, et al. (1999a). Strain differences in the visceromotor response to colorectal distension during trinitrobenzenesulfonic acid-induced colitis. Gastroenterology 116:A607
- Gschossmann JM, Holtmann G, Miller JC, Cheng HS, Martinez V, Tache Y, Gerken G, Mayer EA. (1999b). Sex-related differences in visceral pain response to colorectal distension in rats. Gastroenterology 116:A607
- Gschossmann JM, Liebregts T, Adam B, Buenger L, Ruwe M, Gerken G, Holtmann G. (2004). Long-term effects of transient chemically induced colitis on the visceromotor response to mechanical colorectal distension. Dig Dis Sci 49:96–101
- Gwee KA, Leong YL, Graham C, McKendrick MW, Collins SM, Walters SJ, Underwood JE, Read NW. (1999). The role of psychological and biological factors in postinfective gut dysfunction. Gut 44:400–6
- Hong S, Fan J, Kemmerer ES, Evans S, Li Y, Wiley JW. (2009). Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat. Gut 58:202–10
- Hubscher CH, Brooks DL, Johnson JR. (2005). A quantitative method for assessing stages of the rat estrous cycle. Biotech Histochem 80:79–87
- Larauche M, Bradesi S, Million M, McLean P, Tache Y, Mayer EA, McRoberts JA. (2008). Corticotropin-releasing factor type 1 receptors mediate the visceral hyperalgesia induced by repeated psychological stress in rats. Am J Physiol Gastrointest Liver Physiol 294:G1033–40
- Larauche M, Gourcerol G, Million M, Adelson DW, Tache Y. (2010). Repeated psychological stress-induced alterations of visceral sensitivity and colonic motor functions in mice: influence of surgery and postoperative single housing on visceromotor responses. Stress 13:343–54
- Larauche M, Mulak A, Tache Y. (2012a). Stress and visceral pain: from animal models to clinical therapies. Exp Neurol 233:49–67
- Larauche M, Mulak A, Yuan PQ, Kanauchi O, Tache Y. (2012b). Stress-induced visceral analgesia assessed non-invasively in rats is enhanced by prebiotic diet. World J Gastroenterol 18:225–36
- Larsson MH, Miketa A, Martinez V. (2009). Lack of interaction between psychological stress and DSS-induced colitis affecting colonic sensitivity during colorectal distension in mice. Stress 12:434–44
- Lauffer A, Vanuytsel T, Fornari F, Farré R. (2014). Differential effects of acute, chronic and combined stress paradigms on small intestinal and colonic permeability in rats. Gastroenterology 146:S490
- Liebregts T, Adam B, Bertel A, Lackner C, Neumann J, Talley NJ, Gerken G, Holtmann G. (2007). Psychological stress and the severity of post-inflammatory visceral hyperalgesia. Eur J Pain 11:216–22
- Maunder RG, Levenstein S. (2008). The role of stress in the development and clinical course of inflammatory bowel disease: epidemiological evidence. Curr Mol Med 8:247–52
- Million M, Tache Y, Anton P. (1999). Susceptibility of Lewis and Fischer rats to stress-induced worsening of TNB-colitis: protective role of brain CRF. Am J Physiol 276:G1027–36
- Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. (1989). Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 96:795–803
- Posserud I, Agerforz P, Ekman R, Bjornsson ES, Abrahamsson H, Simren M. (2004). Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut 53:1102–8
- Qin HY, Wu JC, Tong XD, Sung JJ, Xu HX, Bian ZX. (2011). Systematic review of animal models of post-infectious/post-inflammatory irritable bowel syndrome. J Gastroenterol 46:164–76
- Qin HY, Xiao HT, Wu JC, Berman BM, Sung JJ, Bian ZX. (2012). Key factors in developing the trinitrobenzene sulfonic acid-induced post-inflammatory irritable bowel syndrome model in rats. World J Gastroenterol 18:2481–92
- Simren M, Axelsson J, Gillberg R, Abrahamsson H, Svedlund J, Bjornsson ES. (2002). Quality of life in inflammatory bowel disease in remission: the impact of IBS-like symptoms and associated psychological factors. Am J Gastroenterol 97:389–96
- Spiller R, Garsed K. (2009). Postinfectious irritable bowel syndrome. Gastroenterology 136:1979–88
- Tarrerias AL, Millecamps M, Alloui A, Beaughard C, Kemeny JL, Bourdu S, Bommelaer G, et al. (2002). Short-chain fatty acid enemas fail to decrease colonic hypersensitivity and inflammation in TNBS-induced colonic inflammation in rats. Pain 100:91–7
- te Velde AA, Verstege MI, Hommes DW. (2006). Critical appraisal of the current practice in murine TNBS-induced colitis. Inflamm Bowel Dis 12:995–9
- van den Wijngaard RM, Stanisor OI, van Diest SA, Welting O, Wouters MM, Cailotto C, de Jonge WJ, Boeckxstaens GE. (2013). Susceptibility to stress induced visceral hypersensitivity in maternally separated rats is transferred across generations. Neurogastroenterol Motil 25:e780–90
- Vermeulen W, De Man JG, De Schepper HU, Bult H, Moreels TG, Pelckmans PA, De Winter BY. (2013). Role of TRPV1 and TRPA1 in visceral hypersensitivity to colorectal distension during experimental colitis in rats. Eur J Pharmacol 698:404–12
- Vermeulen W, De Man JG, Nullens S, Pelckmans PA, De Winter BY, Moreels TG. (2011). The use of endoscopy to follow the inflammatory time course of TNBS-colitis in rats. Acta Gastroenterol Belg 74:304–11
- Vermeulen W, De Man JG, Pelckmans PA, De Winter BY. (2014). Neuroanatomy of lower gastrointestinal pain disorders. World J Gastroenterol 20:1005–20
- Wallace JL, Keenan CM. (1990). An orally active inhibitor of leukotriene synthesis accelerates healing in a rat model of colitis. Am J Physiol 258:G527–34
- Zhou Q, Price DD, Caudle RM, Verne GN. (2008a). Visceral and somatic hypersensitivity in a subset of rats following TNBS-induced colitis. Pain 134:9–15
- Zhou Q, Price DD, Caudle RM, Verne GN. (2008b). Visceral and somatic hypersensitivity in TNBS-induced colitis in rats. Dig Dis Sci 53:429–35
- Zhou Q, Price DD, Verne GN. (2008c). Reversal of visceral and somatic hypersensitivity in a subset of hypersensitive rats by intracolonic lidocaine. Pain 139:218–24
