Abstract
Consequences of prenatal stress on mother–young relationships are well-documented in altricial mammals but less so in precocial mammals. In this study, we investigated the effects of unpredictable aversive events on maternal behavior and mutual mother–young recognition in pregnant ewes while accounting for modulatory effects of ewe reactivity. From a population of 120 Romane-breed ewes, we selected 20 high-responsive (HR) and 20 low-responsive (LR) ewes according to pre-mating reactivity assessed in isolation tests. Over the final third of pregnancy, 10 HR ewes and 10 LR ewes were exposed daily to various aversive events such as social isolation, mixing and transport (stressed ewes), while the other 20 ewes were not exposed to aversive events (control ewes). Although the treatment induced chronic stress, physiologically confirmed by an increase in salivary cortisol following transport and sham shearing, maternal behavior of stressed ewes observed during the first 30 min postpartum and in the selectivity test 1 h 30 min later did not differ from controls. However, in a maternal motivation test performed 48 h postpartum, stressed ewes vocalized less than controls when separated from their lambs, and walked less readily past an unknown object to reach their lambs. Lambs of stressed ewes spent more time near their dam in a preference test performed 15 h after birth compared to control-ewe lambs. HR ewes spent more time grooming their lambs than LR ewes. We posit that domestication could have selected animals displaying robust expression of maternal behavior related to social reactivity and producing offspring that are better adapted to challenging situations.
Introduction
Prenatal stress is well documented in rodents and primates and shows major effects with heightened anxiety behaviors (Lupien et al., Citation2009; Weinstock, Citation2008). Studies have also shown that maternal care, specifically grooming, is reduced following gestational stress (Baker et al., Citation2008; Patin et al., Citation2002), which can accentuate some effects of prenatal stress on offspring behavior. Cross-fostering of rat pups born from stressed dams to non-stressed dams attenuates prenatal stress-induced behavioral alterations, and non-prenatally-stressed pups raised by stressed dams showed altered hormone (corticosterone, progesterone and estradiol) levels (Del Cerro et al., Citation2010). In addition, newborn rats are altricial and immature, with limited sensory, motor and thermoregulation abilities (Elwood & McCauley, Citation1983) and their neuroendocrine and neural development occurs mainly after birth (Matthews, Citation2002).
These consequences of prenatal stress are thus well documented in rodents but less so in more precocial mammals. Compared to rodents, new-born ungulates are born with a relatively mature brain, and their dams show social and maternal behaviors that have reached a higher level of complexity with the development of an attachment process (Gonzalez-Mariscal & Poindron, Citation2002). Overall, gestational stress in ungulates (e.g. pig and sheep) produces abnormal maternal care and reduced motivation of the dam for their young (Roussel et al., Citation2006; Rutherford et al., Citation2014). However in sheep, detailed accounts of gestational stress are still scarce, and studies have been focused on situations involving predictable repetitive stress such as social isolation, transport or aversive handling with contrasting results: for example, gestating ewes aversively treated by humans groom their young more (Hild et al., Citation2011).
There is a large inter-individual variability in gestational stress-induced alteration of maternal behaviors (e.g. in pig; Thodberg et al., Citation2002). A dam’s temperament may influence how it reacts to stressful situations and expresses maternal behavior (Dwyer et al., Citation2004). In pig, disturbed maternal behavior has been related to a high reaction pattern of sows during stress (Thodberg et al., Citation2002). In sheep, calmer dams (selected for their low reactivity to humans and to novelty) showed better maternal behavior with more time spent grooming their lambs (Murphy et al., Citation1994), a difference that was not shown by Bickell et al. (Citation2011) in their comparison between two ewe lines with different temperaments (calm or nervous).
The aim of this study was to assess whether long-term daily exposure to various unpredictable stressful events during gestation induces a state of chronic stress that alters mother–young interactions in the early post-partum period. We also took into account the enhancing effect of the ewe’s level of social reactivity. Thus, ewes selected for their divergent behavioral and physiological reactions to social isolation prior to being mated were exposed or not to stressful events during the final third of pregnancy. Maternal behavior and selectivity at parturition, maternal motivation tested at two days post-partum and the preference of the lambs for their mother were compared. We hypothesized that stressful experiences during pregnancy would impact adversely on mother–young interactions, and that these effects would be more pronounced for highly socially responsive mothers.
Methods
The experiment was performed in accordance with EC Council Directive of 24 November 1986 (86/609/EEC) and approved by a local ethics committee (CEMEA Auvergne no. CE38-12, France).
Animals, selection and housing
Two age- and weight-matched groups of multiparous Romane ewes (Romanov × Berrichon-du-Cher) were selected at 18 months of age from a single flock of 120 sheep on the basis of behavioral and cortisol responses measured in two individual challenge tests. The first test assessed the ewes’ behavioral responses to isolation and reunion with congeners (adapted from Ligout et al., Citation2011). The ewes were individually exposed to an arena test consisting of three consecutive 60-s phases: (1) in isolation, (2) in visual and acoustic presence of flock-mates and (3) after flock-mates were removed. The test was carried out in an enclosure divided into seven 1-m-wide zones surrounded by 2-m-high solid walls. Three flock-mates not used as subjects in this experiment were placed behind a grid barrier at one end of the testing pen where an opaque panel could be pulled down from a distance between the flock-mates and the tested ewe to prevent visual contact in the first and last phases of the test. Ewes showing the most vocalization and movement in phases 1 and 3 were defined as high-social. Ewes showing the least vocalization and movement were defined as low-social (). The second test measured the cortisol response to isolation from congeners (Deiss et al., Citation2009). The tests consisted in isolating the ewes for 1 min in a 2 m2 pen and collecting a 4 ml jugular vein blood sample with EDTA as an anticoagulant 15 min later to measure plasma cortisol concentration (). The blood samples were immediately chilled on ice, centrifuged at 3000 × g for 10 min at 4 °C and plasma stored at −20 °C until analysis. Cortisol concentrations were determined by RIA as described in Orgeur et al. (Citation1998). The detection limit was 0.02 ng/ml. Intra- and interassay CV were 11% and 22% for low (4 ng/ml) and 7% and 14% for high (32 ng/ml) controls.
Table 1. Behavioral measures and plasma cortisol concentrations in ewes selected for their high (HR, N = 20) or their low (LR, N = 20) responsiveness to isolation/reunion tests before mating and stress exposure.
Of the 120 ewes tested, we selected the 20 ewes showing the highest levels of social reactivity and plasma cortisol responses (high-responsive ewes (HR); ) and the 20 showing the lowest level of social reactivity and plasma cortisol response (low-responsive ewes (LR); ). The level of social reactivity and the plasma cortisol responses of the ewes were not correlated. The 20 HR ewes were thus selected from the 30 ewes showing the highest levels of social reactivity and from the 60 ewes showing the highest plasma cortisol responses. The 20 LR ewes were selected from the 30 ewes showing the lowest levels of social reactivity and from the 60 ewes showing the lowest plasma cortisol responses. These ewes were then mated at 21 months of age by natural service using 13 Romane rams after synchronization of estrus using progesterone. Paternity was balanced between the HR and LR ewes. They were then randomly allocated into eight pens (2.5 × 6 m) at five HR or five LR animals per pen on straw bedding under artificial lighting (07:30 h to 19:30 h). Ewes had free access to water and were fed twice daily (08:00 h and 16:00 h) with hay, straw ad libitum and a standard concentrate feed composed of barley and minerals (rations were 0.2 kg at mating, 1.2 kg one month before parturition and 0.9 kg after parturition). Ewes stayed with their lambs until weaning at two months old and then were returned to the experimental husbandry flock.
Stress exposure during pregnancy
Over the final third of pregnancy (seven weeks, at 94–143 d of gestation), gestationally stressed ewes (GS ewes: 10 HR and 10 LR) were exposed daily to repeated and uncontrollable challenging events, such as social isolation, mixing, dog presence, transport, sham shearing, delay in feeding times (stressors are described in more detail in ), while the 20 other control ewes were not exposed to any additional challenges (control ewes). As well as being uncontrollable, the aversive events were unpredictable, as they occurred at different times of the day or night with no forewarning. This seven-week exposure to unpredictable and uncontrollable aversive events has been shown to induce chronic mild stress and pessimistic judgment in sheep (Destrez et al., Citation2013a,Citationb), while the final third of gestation is a vulnerable period for brain development in sheep (McIntosh et al., Citation1979). To ensure that the control ewes had the same time of exposure to humans, three different familiar shepherds were rotated to sit still on a chair in the home pen for 10 min three times a week. To help the ewes to discriminate people via the color of their clothing (Rushen et al., Citation1999), experimenters wore white clothes for stressful events imposed on GS ewes and green clothes for normal farm handling. Control and GS groups were housed in two identical adjacent enclosed rooms (i.e. four pens per room with two pens of HR ewes and two pens of LR ewes).
Table 2. Aversive events used during the seven-week exposure to chronic gestational stress.
To assess the efficiency of exposure to repeated and uncontrollable stressors, physiological measures (cortisol concentrations in saliva and blood cell count parameters) and behavioral observations (time budget and human approach) were made twice over four consecutive days (one observation or measure per day) before (at 90 days of gestation) and at the end (at 139 days of gestation) of the treatment period.
Saliva sampling is less stressful than blood sampling and was chosen to limit stress in control ewes. To evaluate the basal cortisol concentration, samples were collected from each ewe at 14:00 h on days 90 and 139 of gestation without any interactions with the ewes before sample collection (adapted from Hild et al., Citation2011). A small enclosure was built within the pen with portable metal bars where the five ewes were in close physical contact with each other. This was meant to curb their movements so that restraining by the human during the saliva collection was moderate. To assess GS ewe cortisol concentrations in response to stressful events, samples were collected before two specific stressors (20 min of transport and 2 min of sham shearing) and again 20 min after exposure to detect activation of the hypothalamic–pituitary–adrenal axis. Saliva was extracted from cotton buds by 10-min centrifugation at 3000 g for 10 min at 4 °C on the day of sampling and immediately stored at −20 °C until analysis (Hild et al., Citation2011).
Blood samples of 4 ml were collected from the jugular vein (at 08:00 h) without anticoagulant and run through an electronic cell counter (Scil Vet abc, Scil Animal Care Company, Viernheim, Germany) to measure a series of blood cell parameters: number of white blood cells (103/mm3) and percent lymphocytes, granulocytes and monocytes; number of red blood cells (106/mm3), platelets (103/mm3), hemoglobin concentration (g/dL) and percent hematocrit (%).
Time budget was assessed from a 24-h observation period of the home pen starting at the 08:00 h concentrate feed. The 24-h-long video was analyzed by scan sampling every 10 min using the following ethogram: lying down, standing or moving, feeding (taking food into the mouth, chewing, searching for food or drinking) and in vigilance (erected head and ears).
Reaction to approach by a human was studied in each pen during a 10-min test. An unfamiliar human in green clothes quietly entered the home pen and sat on a chair. The home pen was divided into three equal zones: a 2-m-wide zone around the human, a 2-m-wide intermediate zone and a 2-m-wide zone around the entrance. Ewe position in one of these three zones was recorded every 30 s.
Mother–young interactions
Once a ewe was in labor, progress was monitored and assistance was provided when labor appeared excessively long, e.g. non-productive expulsion of the amniotic sac or presentation of a lamb head or limbs at the vulva. Once the first lamb was born, all ewes were caught and any subsequent lamb was manually delivered to standardize inter-birth duration and the beginning of the observations of maternal behavior. Variation in inter-birth duration would otherwise have been an additional factor of variability between individuals in the observations of maternal behavior. We consider this approach is unlikely to have affected maternal behavior as human interference has been shown to play only a minor role in effects of mother–young separation (Stevens et al., Citation1982) at birth in housed conditions, and we observed a similar behavioral reactivity among ewes to a human in the human test (see Results). The lambs were then ear-tagged and returned to their mother for behavioral observations to start. In the case of triplets, the third-born lamb was immediately removed and artificially hand-reared. Subsequent lamb health husbandry procedures (selenium injection, weighing and iodine application for umbilical cord care) were carried out after the behavioral tests at 2 h post-lambing. After birth, one GS group LR ewe and one control group LR ewe were not studied due to health issues, and one control group LR ewe was not taken into account in the analysis due to technical recording issues. Four GS ewes (one HR and three LR ewes) and 10 control ewes (six HR and four LR ewes) had a single lamb. All observations and tests were video-recorded and analyzed by an experimenter blind to treatment of the ewe.
Spontaneous mother–young interactions at parturition
The behavior of mothers and their offspring was video-recorded for 10 min at three periods: when the second lamb was born (T = 0), 30 min after (T = 30) and 1 h after (T = 1 h). This was performed from several meters outside the rearing pen to minimize human interference.
The following variables were recorded for each ewe: duration of sniffing and licking (grooming) their offspring (the single lamb or both twins), number of low-pitched bleats (maternal low vocalizations) and number of udder refusals. The following variables were recorded for each lamb: latencies to stand up and adopt a parallel inverse position at the udder, duration of suckling (time spent at the udder), number of vocalizations emitted by both lambs if twins (as it was difficult to distinguish which offspring vocalized, this number was divided by two and each lamb from the same litter was given the same number of bleats). The definitions of behaviors recorded are listed in . After the last observation period, the ewe and her lambs were placed in an individual 1 m2 pen to perform a selectivity test.
Table 3. Ewe and lamb behaviors.
Selectivity test
The ability of a ewe to provide selective care to her own offspring was tested at 1 h:30 min post-partum using a selectivity test adapted from Poindron et al. (Citation2010). The ewe’s own lamb(s) was taken away to another building so that the mother could not hear, see or smell it. An alien lamb of similar age and coat color was then placed in the pen, and the ewe’s behavioral responses were observed during three minutes. The alien lamb was then taken away, and the ewe was video-recorded with her own lamb for an additional 3-min test session. In cases of litters of more than one lamb, we only tested the ewes with one of their twins randomly chosen (the first or second lamb born). In each 3-min test session, we measured four variables indicative of acceptance of the lamb, i.e. number of low-pitched bleats emitted by the ewe, number of acceptances at the udder, time spent licking and duration of acceptance at the udder, and three variables indicative of lamb rejection, i.e. number of high-pitched or “protest” bleats (high-pitched vocalizations), number of rejections at the udder and number of head buttings. The definitions of behaviors recorded are listed in . The first minute of each 3-min phase was unusable due to human interaction, so only the second and third minutes were analyzed. In addition, to gain an overview of the data, the proportions of selective mothers in each group were determined according to criteria modified from Otten et al. (Citation1999). Ewes were categorized as selective if they rejected the alien lamb and accepted their own. A ewe was considered to reject a lamb if any of the following events occurred: (i) head-butting a lamb once or more, (ii) backing away from suckling attempts twice or more and (iii) emitting more high- than low-pitched vocalizations.
Maternal motivation test
Response to separation from their lambs and motivation to reach them was tested at two days post-partum in a 4 m × 1 m corridor (adapted from Perrin et al., Citation2007). Mothers were placed in one end of the starting pen (1 m2), while their lamb(s) were removed to another room. The ewes could not hear their lambs since they were in another building. Number of maternal low- and high-pitched vocalizations, circling and climbing on the fence was recorded for 5 min. The lamb(s) were then put in a holding pen (1 m2) 2 m away from the ewe, and a novel object (a 70-cm-high traffic cone) was placed halfway between the ewe and her lamb(s). Recording started once the door to the ewe’s starting pen was opened. Number of maternal low- and high-pitched bleats, number of times the ewe nosed the lamb’s pen (visits), latency to join the lambs and time spent 1 m away from the lambs were recorded.
Dam preference test in lambs
The ability of lambs to show a preference for their mother was assessed using a two-choice test performed at 15 h postpartum (adapted from Nowak et al., Citation1987). Testing preference of the lamb for the mother provides clues about an appropriate onset of maternal behavior as regulation of this onset heavily depends on lamb behavior (Nowak et al., Citation2011). The test consisted of a triangular enclosure (6 m sides) delimited by 2-m-high wooden panels. Two individual metal-barriered pens (1 m2) located at each end of the base of the enclosure contained the dam of the tested lamb or a familiar ewe (an ewe living in the same pen as the tested lamb). The tested lamb was put in the angle opposite the two ewes in a starting pen (1 m2). Both lambs were tested in the case of twins. The testing area was divided into three zones: two 1-m-wide contact zones in front of the two ewes’ pens and a neutral zone. Position of the dam and the familiar ewe was reversed at each test. Latency to reach a contact zone and total times spent in the contact zones near each ewe were recorded for 5 min. In order to assess a preference for the mother, a preference index (PI) was defined for each subject as follows: PI = (time spent near the mother−time spent near the alien ewe)/(total time near both ewes). The PI range was −1 to 1. To avoid neophobic reactions during the test, ewes were habituated to the experimental set-up for 10 min on two consecutive days prior to lambing.
Statistical analysis
Data were analyzed with the SAS software version ×9.2. (SAS Institute Inc., Cary, NC). For behavioral observations (time budget and human approach) and physiological measures (blood cell count parameters and cortisol concentrations) performed before parturition and behaviors recorded during the first hour after parturition, the effects of treatment (GS vs. control), level of social reactivity of the ewes (LR vs. HR), periods of observation and their interactions were tested using the MIXED procedure (generalization of the standard linear model with REML and ML estimation methods implemented with a Newton–Raphson algorithm (SAS Institute Inc., 1999) with the ewes as a random effect. For the selectivity and maternal motivation tests, the GLM procedure was used to analyze the effects of treatment (GS vs. control), level of social reactivity of the ewes (LR vs. HR) and their interaction on data collected in each part of the tests. For the two-choice test, the GLM procedure was used to analyze the effects of treatment (GS vs. control), level of social reactivity of the ewes (LR vs. HR), the ewes (dam or familiar ewe) and their interactions.
Litter size was initially included in the models but later removed due to the absence of any significant effect. Post hoc comparisons after ANOVA were run using Tukey tests. We checked the condition of normality and homogeneity of the variance, and number of circlings and time spent away from the lambs in the motivation test were log-transformed before being included in the model. Due to non-normally-distributed data, non-parametric statistics (Wilcoxon test – t statistics) were used (i) in the selectivity test for the number of backing away movements from suckling attempts, number of buttings and duration of suckling (only for the first part of the test); (ii) in the two-choice test for the time spent by lambs near the ewes and latency to reach the dam contact zone; and (iii) in the ewe motivation test for number of climbs on the fence, number of high-pitched vocalizations and latency to join the lambs in the second part of the test. The results are expressed as means ± standard error of the mean. Results with an associated probability less than or equal to 0.05 were considered significant. “N” indicates the number of animals in each group tested and “n” the number of events.
Results
Effects of exposure to stressful events during pregnancy
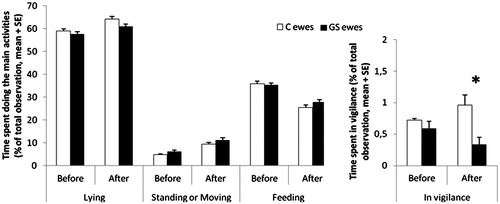
The time budget recorded over a 24-h period showed that the proportions of ewes lying, standing or moving and feeding in both GS and control groups were not significantly different (). However, the percentage of total observations where ewes were observed in vigilance mode after treatment was lower for GS ewes than control ewes (; F(1,35)=5.27, p = 0.028). In the human test, all ewes spent a majority of the 10 min in the zone opposite the human before (percentage of time: 94.2 ± 3.16% for control ewes and 89.1 ± 3.62% for GS ewes) and after exposure (84.6 ± 5% for control ewes and 88.3 ± 5.6% for GS ewes; treatment × period effect: F(1,34)=1.22, p = 0.28).
Figure 1. Percentage of total observations spent in central activities during the time budget (24 h) before and after the seven-week treatment during pregnancy. Control ewes (C, N = 20) and gestationally stressed ewes (GS, N = 20). Statistical MIXED procedure *p < 0.05.
Lymphocyte population was significantly higher and granulocyte population significantly lower after the stress protocol in GS than in control ewes (treatment × period effect: F(1,35)=12.5, p < 0.001; F(1,35)=8.36, p < 0.01; ). Other blood cell components were not significantly different (). The decrease in plasma cortisol concentrations with pregnancy (basal cortisol concentration before treatment minus basal cortisol concentration after treatment) was significantly lower in control ewes than in GS ewes (control ewes: 0.54 ± 0.08 vs. GS ewes: 0.82 ± 0.09 ng/mol, F(1,35)=8.97, p < 0.01). The stressful events led to higher salivary cortisol concentrations in GS ewes (0.24 ± 0.05 vs. 1.23 ± 0.2 ng/mol after transport, F(1,18)=27.5, p < 0.001 and 1.16 ± 0.06 vs. 1.7 ± 0.2 ng/mol after sham shearing, F(1,18)=6.75, p = 0.019). These treatment effects were not different between HR and LR ewes.
Table 4. Blood analysis of ewes (GS: gestationally stressed ewes N = 20; C: control ewes, N = 20) after daily exposure to repeated and uncontrollable aversive events over seven weeks during the final third of pregnancy.
Effects of stress on parturition and in the early postpartum period
Duration of pregnancy did not significantly differ between GS and control ewes (145.3 ± 0.3 d vs. 145.1 ± 0.3 d, respectively; (F(1,33)=0.25, p = 0.6, N = 37). There was an interaction effect between treatment and initial selection criteria: GS group LR ewes had a longer pregnancy duration (146 ± 0.5 d vs. 144.6 ± 0.5 d, F(1,33)=5.73, p = 0.02) than HR ewes. One twin of two LR ewes died in each group (GS and control ewes) in the two days following birth.
Maternal behavior at parturition
We did not observe any effect of stress during pregnancy on maternal behavior in the three observation periods, but there was a significant effect of social reactivity without interaction with observation periods (). HR ewes spent more time grooming their offspring (F(1,66)=6.37, p = 0.01; ), and LR ewes showed significantly more udder refusals (F(1,66)=4.61, p = 0.03; ), particularly in the GS group (treatment × selection effect, F(1,66)=4.9, p = 0.03; ).
Figure 2. Effect of selection for social reactivity on grooming and udder refusals by ewes during the first 30 min after parturition. LR: socially low-responsive, N = 17; HR: socially high-responsive, N = 20. Statistical MIXED procedure *p < 0.05. Data are mean ± SEM.
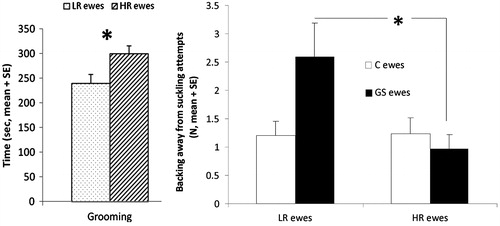
Table 5. Effects of chronic stress during gestation and periods of observation during the first hour post-partum on mother–young interactions.
Selectivity test
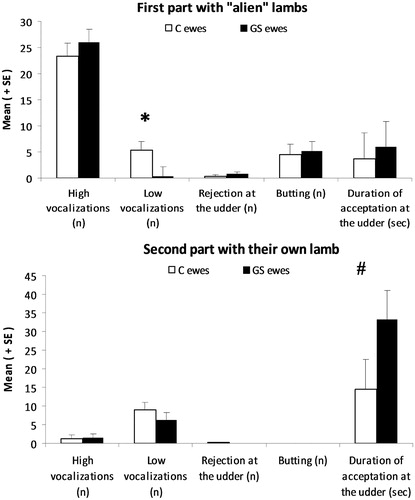
The proportion of selective ewes did not significantly differ between groups (16/18 control ewes vs. 19/19 GS ewes, 20/20 HR ewes vs. 15/17 LR ewes). However, on exposure to the alien lamb, GS ewes emitted significantly fewer maternal low-pitched bleats than control ewes (F(1,33)=4.08, p = 0.05; ), and HR ewes emitted significantly more high-pitched vocalizations than LR ewes (28.3 ± 2.3 vs. 21.1 ± 2.5, F(1,33)=4.49, p = 0.04). We did not observe any effect of treatment or differences between HR and LR ewes on the other behaviors ().
Figure 3. Effect of exposure to chronic stress during gestation on acceptance and rejection behaviors displayed towards alien or familiar lambs during a 3-min selectivity test performed at 1 h 30 min post-partum. GS, gestationally stressed and C, control, ewes, GS, N = 19 and C, N = 18. Statistical GLM procedure *p < 0.05; #p < 0.1; n: number of events. Data are mean ± SEM.
Maternal motivation test
GS ewes and control ewes showed no difference in responses to separation from their lambs (). At reunion with the lamb, control ewes emitted significantly more maternal low-pitched bleats and visited their lambs significantly more than GS ewes (). Ewe selection according to social reactivity and cortisol response also had an effect on high-pitched bleats, as HR ewes emitted vocalizing significantly more at both separation and reunion with the lamb ().
Table 6. Effects of exposure to chronic stress during gestation (GS and control ewes) and selection for social reactivity (LR and HR ewes) on the maternal motivation test.
Dam preference test in lambs
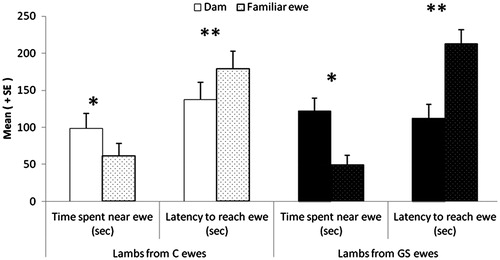
GS group lambs spent more time suckling at 1 h after birth (treatment × period; p < 0.05; ). Latency to stand up (20.4 ± 1.81 vs. 20.0 ± 1.75 min, F(1,33)=0.02, p = 0.9); latency to reach the udder (11 ± 1.72 vs. 7.9 ± 1.67 min, F(1,33)=1.71, p = 0.2); and number of vocalizations did not differ between treatments ().
During the preference test, lambs were significantly quicker to reach their dam than the familiar ewe (Z = −2.89, p < 0.01; ) and spent significantly more time near their dam than near the familiar ewe (Z = 3.08, p < 0.01; ). Stress induced during pregnancy had no effect on lamb behaviors. However, PI was significantly higher in GS group lambs than control group lambs (0.41 vs. 0, F(1,55)=4.05, p = 0.049).
Figure 4. Effect of exposure to chronic stress during gestation on lamb preferences between the mother and a familiar ewe observed in a two-choice test performed at 15 h post-partum. GS, gestationally stressed and C, control, ewes; GS, N = 19 and C, N = 18; lambs from GS ewes, N = 33; lambs from control ewes, N = 26. Statistical GLM procedure *p < 0.05; **p < 0.01. Data are mean ± SEM.
Discussion
This study found that experiencing stress over the final third of pregnancy in ewes had minor effects on onset of maternal behavior at parturition and early attachment to their offspring. The gestational stress-induced effects were minor, even though the events chosen have been reported as stressful in sheep (Destrez et al., Citation2013a) as physiologically confirmed in this study by an increase in salivary cortisol following transport and sham shearing. In addition, these gestational stress-induced effects were not modulated by the level of social reactivity of the ewes. Nevertheless, HR ewes of both the GS and control groups displayed enhanced maternal responses at parturition and 48 h later at separation and reunion with their lamb in comparison to LR ewes of both the GS and control groups.
Unlike in previous studies where gestational stress had detrimental effects on mother–young interaction (e.g. Patin et al., Citation2002; Ringgenberg et al., Citation2012), the ewes showed no effects on maternal behavior just after lambing. This lack of effect on the onset of maternal behavior was not due to the efficacy of the stressors used. After the seven-week treatment, ewes exposed to aversive challenges during gestation were less vigilant than their control counterparts. Dantzer & Mormede (Citation1983) hypothesized that experience of unavoidable aversive events leads to apathy. GS ewes showed a stronger pregnancy-induced decrease in cortisol concentrations than controls. A decrease in basal cortisol concentration, linked to a disrupted stress physiology (McEwen, Citation1998), has also been reported in other studies on chronic stress in non-pregnant animals (Cyr & Romero, Citation2007; Dickens et al., Citation2009). This physiological stress-related response associated with behavioral apathy seems to appear in chronically stressed animals when they are unable to develop a coping strategy, as shown in tethered bulls (Ladewig & Smidt, Citation1989). Furthermore, GS ewes showed lower blood granulocyte populations and higher lymphocyte populations. Destrez et al. (Citation2013a) reported significantly fewer granulocytes in chronically stressed ewe-lambs, while Bellingrath et al. (Citation2010) observed a drop in total white blood cells (leukocytes) in depressed human patients. However, Zager et al. (Citation2007) observed that chronic stress led to a decrease in total lymphocyte counts in rats. Our results therefore indicate that the gestational exposure to stressors may induce a chronic stress state in pregnant ewes, characterized inter alia by changes in physiological stress-related responses and a low granulocyte count. Nevertheless, the minor behavioral impacts observed are possibly related to a reduction in anxiety or in fear-related behaviors evident in pregnant ewes (Viérin & Bouissou, Citation2001), and the increase in lymphocyte counts could indicate an adaptive response to gestational stress in ewes. Ducsay (Citation1998) found a biological maternal adaptation to chronic hypoxia in ewes during gestation that, by reducing responsiveness of the myometrium, prevented preterm delivery.
The observed mild effects of gestational stress on the maternal behavior of ewes may be related to the amount, intensity or duration of the exposure to stressors (Braastad, Citation1998). In the study of Hild et al. (Citation2011), aversive handling during gestation had no effect on multiparous ewes as the increased grooming duration was restricted to primiparous aversively treated ewes. This suggests that in multiparous ewes the effects of gestational stress on maternal behavior are buffered by multiple lambing experiences. The onset of maternal behavior, particularly in primiparous ewes that are more likely to show fearful behavior toward the lamb and may fail to show maternal care, is dependent on a coordinated sequence of neuroendocrine activation and sensory stimuli, and disruptions to this process in stressful situations can result in behavioral disturbances. Minimizing disruption and stress around lambing time thus allows the normal onset of maternal care (Dwyer, Citation2008). An alternative explanation could be that gestational stress treatment only has observable effects in stressful situations that activate the HPA axis. Indeed, in the selectivity test and maternal motivation tests combining novel environment and separation from their lamb, gestation-stressed ewes (GS ewes) were highly stressed and emitted less maternal bleats than control ewes. They were also slower to approach their lambs behind a barrier at reunion in the maternal motivation test. This suggests that maternal behavior was more disturbed in GS mothers as the HPA axis was activated. A lower maternal responsiveness in the motivation test in GS mothers might be caused by lower post-stress-period cortisol concentrations. It has been proposed that the level of cortisol concentration in late pregnancy is involved in the expression of maternal behavior, as cortisol concentration correlates positively to degree of maternal responsiveness (in primates; Nguyen et al., Citation2008). Nevertheless, a negative relationship between cortisol and maternal care has also been suggested in sheep (Dwyer et al., Citation2004). In contrast, at parturition, the ewes were in non-challenging environmental conditions with space and familiar social partners that did not disturb the maternal behavior in GS ewes and are shown to have beneficial effects on the development of filial bonding in sheep (Val-Laillet & Nowak, Citation2006). Dysregulation of the oxytocin system could also be involved in the low expression of maternal behavior. Psychosocial stress early in life, as well as later adverse life experience, associated with low release of oxytocin, has been shown to affect the ability of mothers to provide sensitive and responsive care (Zelkowitz et al., Citation2014).
Similar minor effects of gestational stress treatment were observed by Klaus et al. (Citation2013) in guinea pigs where pup grooming and anogenital licking behavior were unaffected in dams stressed by repeated exposure to high-frequency strobe lighting in an unfamiliar dark room during gestation. In both sheep and guinea pigs, young at birth are highly precocial compared to altricial rat pups. This difference in maturity may explain the observed difference in effect of gestational stress on maternal behavior where the offspring of precocial species participate more actively in interactions with their mother compared to rats. Nowak et al. (Citation2011) claim maternal behavior arises from interplays between the mother and cues received from the offspring and that maternal motivation is regulated by young behavior. In this study, preference for the mother in the two-choice test was more pronounced in lambs born from GS mothers than lambs born to control mothers. This could reflect a higher emotional reactivity in lambs born from GS mothers. Indeed, it has been shown that previous stressing ewes during gestation increases emotional reactivity in offspring (Coulon et al., Citation2011), probably due to maternal glucocorticoids crossing the placenta and modifying the development of the fetal HPA axis (Rutherford et al., Citation2012). Thus, young that have been exposed prenatally to glucocorticoids may more readily react to maternal calls or demand more attention and protection that require additional care (Nguyen et al., Citation2008). Note that the GS group lambs spent more time suckling at 1 h after birth than controls. Another possibility is an adaptive effect of prenatal stress. Early exposure to stress does not create vulnerability but instead enhances arousal regulation and resilience (Lyons et al., Citation2010) as suggested in recent studies in sheep (Hild et al., Citation2011; Pickup & Dwyer, Citation2011). Domestication may have selected animals that, faced with successfully adverse situations, display a more robust expression of maternal behavior and produce offspring better adapted to challenging situations. There is ample evidence in farm animals that some breeds and populations that have evolved over centuries in adverse and stressful environments have a range of unique adaptive traits (e.g. disease and heat resistance and ability to cope with poor quality feed) enabling them to survive better and be more efficient in these environments (Mirkena et al., Citation2010).
Some effects of gestational stress were specifically observed in LR ewes, which had a longer pregnancy duration and displayed more udder refusals when their neonate attempted to suckle. However, we did not observe any effects on treatment × social reactivity interactions in ewes except that LR ewes showed significantly more udder refusals, particularly in the GS group. These observations may be linked to the lack of differential effects of the treatment on the stress responses of the ewes according to their social reactivity. One explanation is that the ewes were selected for their reactivity to social isolation whereas a majority of the aversive events used during the stress treatment were on groups. Selecting ewes according to one trait or dimension of temperament (i.e. sociality) may not be sufficient to investigate the effect of gestational stress on maternal behavior, and it may be more productive to test reactivity to novelty, suddenness or human reactions (Boissy & Erhard, Citation2014; Plush et al., Citation2011). However, HR ewes did display better maternal behavior at lambing, with more time spent grooming their offspring and less udder refusals at suckling attempts. They also vocalized more when separated from their lambs in selectivity and motivation tests. Previous studies have reported interactions between emotional reactivity and maternal behavior (Murphy et al., Citation1994). Plush et al. (Citation2011) showed a positive correlation between agitation score in social isolation, litter survival and improved maternal score in ewes. In primates, Maestripieri (Citation2011) suggested that differences in maternal behavior are linked to maternal level of sociality and glucocorticoids, where social reactivity is positively correlated to the maternal anxiety that activates behaviors promoting proximity and protection of the offspring (Maestripieri, Citation2011).
In conclusion, in sheep, in contrast to less precocial animals, repeated exposure to stressful events during gestation had minor effects on onset of maternal care at parturition (Rutherford et al., Citation2012; Weinstock, Citation2008). This difference may be due to the variety of the stressors used, the experience of ewes with lambing and the fact that lambs participate more actively in interactions with their mother. Effects of gestational stress cannot thus be generalized to all mammals and are highly dependent on the characteristics of the species studied.
Declaration of interest
Each of the authors declares that he has no conflict of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the work submitted that could inappropriately influence, or be perceived to influence his work.
This work was funded by the French National Research Agency fund (ANR 11 PDOC Psysheep, No. 01601).
Acknowledgements
We thank the staff of the INRA-UE1354 (at Theix) and INRA-UE0332 (at Bourges) experimental farms for taking special care of the animals, Agathe Labalette for help throughout the study and Fabien Cornilleau and the UMRH-ACS team for their participation in the experiments at lambing.
References
- Baker S, Chebli M, Rees S, LeMarec N, Godbout R, Bielajew C. (2008). Effects of gestational stress: 1. Evaluation of maternal and juvenile offspring behavior. Brain Res 1213:98–110
- Baldock NM, Sibly RM. (1990). Effects of handling and transportation on the heart rate and behaviour of sheep. Appl Anim Behav Sci 28:15–39
- Bellingrath S, Rohleder N, Kudielka BM. (2010). Healthy working school teachers with high effort–reward-imbalance and overcommitment show increased pro-inflammatory immune activity and a dampened innate immune defence. Brain Behav Immun 24:1332–9
- Bickell S, Poindron P, Nowak R, Ferguson D, Blackberry M, Blache D. (2011). Maternal behaviour and peripartum levels of oestradiol and progesterone show little difference in Merino ewes selected for calm or nervous temperament under indoor housing conditions. Animal 5:608–14
- Boissy A, Erhard HW. (2014). How studying interactions between animal emotions, cognition and personality can contribute to improve farm animal welfare. In: Grandin T, Deesing MJ, editors. Genetics and the behavior of domestic animals. San Diego: Academic Press. p 81–113
- Braastad BO. (1998). Effects of prenatal stress on behaviour of offspring of laboratory and farmed mammals. Appl Anim Behav Sci 61:159–80
- Coulon M, Hild S, Schroeer A, Janczak AM, Zanella AJ. (2011). Gentle vs. aversive handling of pregnant ewes: II. Physiology and behavior of the lambs. Physiol Behav 103:575–84
- Cyr NE, Romero LM. Chronic stress in free-living European starlings reduces corticosterone concentrations and reproductive success. Gen Comp Endocrinol 2007;151:82–9
- Dantzer R, Mormede P. (1983). Stress in farm animals: a need for reevaluation. J Anim Sci 57:6–18
- Deiss V, Temple D, Ligout S, Racine R, Bouix J, Terlouw C, Boissy A. (2009). Can emotional reactivity predict stress responses at slaughter in sheep? Appl Anim Behav Sci 119:193–202
- Del Cerro MC, Perez-Laso C, Ortega E, Martin JL, Gomez F, Perez-Izquierdo MA, Segovia S. (2010). Maternal care counteracts behavioral effects of prenatal environmental stress in female rats. Behav Brain Res 208:593–602
- Destrez A, Deiss V, Leterrier C, Boivin X, Boissy A. (2013a). Longterm exposure to unpredictable and uncontrollable aversive events alters fearfulness in sheep. Animal 7:476–84
- Destrez A, Deiss V, Lévy F, Calandreau L, Lee C, Chaillou-Sagon E, Boissy A. (2013b). Chronic stress induces pessimistic-like judgment and learning deficits in sheep. Appl Anim Behav Sci 148:28–36
- Dickens MJ, Delehanty DJ, Romero LM. (2009). Stress and translocation. Alterations in the stress physiology of translocated birds. Proc R Soc B 276:2051–6
- Doyle RE, Lee C, Deiss V, Fisher AD, Hinch GN, Boissy A. (2011). Measuring judgement bias and emotional reactivity in sheep following long-term exposure to unpredictable and aversive events. Physiol Behav 102:503–10
- Ducsay CA. (1998). Fetal and maternal adaptations to chronic hypoxia: prevention of premature labor in response to chronic stress. Comp Biochem Physiol A Mol Integr Physiol 119:675–81
- Dwyer CM, Gilbert CL, Lawrence AB. (2004). Prepartum plasma estradiol and postpartum cortisol, but not oxytocin, are associated with interindividual and breed differences in the expression of maternal behaviour in sheep. Horm Behav 46:529–43
- Dwyer CM. (2008). Genetic and physiological determinants of maternal behavior and lamb survival: implications for low-input sheep management. J Anim Sci 86:E246–58
- Elwood RW, McCauley PJ. (1983). Communication in rodents: infants to adults. In: Elwood RW, editor. Parental behaviour of rodents. New York: Wiley. p 127–49
- Gonzalez-Mariscal G, Poindron P. (2002). Parental behavior in mammals: immediate internal and sensory factors of control. In: Pfaff DW, Arnold AP, Etgen AM, Fahfbach SE, Rubin RT, editors. Hormones, brain and behavior. San Diego: Academic Press. p 215–98
- Hild S, Coulon M, Schroeer A, Andersen IL, Zanella AJ. (2011). Gentle vs. aversive handling of pregnant ewes: I. Maternal cortisol and behavior. Physiol Behav 104:384–91
- Kendrick KM. (2008). Sheep senses, social cognition and capacity for consciousness. In: Dwyer CM, editor. The welfare of sheep. New York: Springer Science+Business Media BV. p. 135–57
- Klaus T, Schöpper H, Huber S. (2013). Effects of chronic stress during pregnancy on maternal performance in the Guinean pig (Cavia aperea f. porcellus). Behav Proc 94:83–8
- Ladewig J, Smidt D. (1989). Behavior, episodic secretion of cortisol, and adrenocortical reactivity in bulls subjected to tethering. Horm Behav 23:344–60
- Ligout S, Foulquie D, Sèbe F, Bouix J, Boissy A. (2011). Assessment of sociability in farm animals: the use of arena test in lambs. Appl Anim Behav Sci 135:57–62
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. (2009). Effects of stress throughout the lifespan on the brain, behaviour, cognition. Nature Review 10:434–45
- Lyons DM, Parker KJ, Schatzberg AF. (2010). Animal models of early life stress: implications for understanding resilience. Dev Psychobiol 52:402–10
- Maestripieri D. (2011). Emotions, stress and maternal motivation in primates. Am J Primatol 73:516–29
- Matthews SG. (2002). Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab 13:373–80
- McEwen BS. (1998). Seminars in medicine of the Beth Israel Deaconess Medical Center: protective and damaging effects of stress mediators. N Engl J Med 338:171–9
- McIntosh GH, Baghurst KI, Potter BJ, Hetzel BS. (1979). Foetal brain development in the sheep. Neuropathol Appl Neurobiol 5:103–14
- Miranda-de la Lamaa GC, Villarroel M, Maríaa GA. (2012). Behavioural and physiological profiles following exposure to novel environment and social mixing in lambs. Small Rumin Res 103:158–63
- Mirkena T, Duguma G, Haile A, Tibbo M, Okeyo AM, Wurzinger M, Sölkner J. (2010). Genetics of adaptation in domestic farm animals: a review. Livest Sci 132:1–12
- Murphy PM, Purvis IW, Lindsay DR, Neindre PL, Orgeur P, Poindron P. (1994). Measures of temperament are highly repeatable in merino sheep and some are related to maternal behaviour. Proc Aust Soc Anim Prod 20:247–50
- Nguyen N, Gesquiere LR, Wango EO, Alberts SC, Altmann J. (2008). Late pregnancy glucocorticoid levels predict responsiveness in wild baboon mothers (papio cynocephalus). Anim Behav 75:1747–56
- Nowak R, Keller M, Levy F. (2011). Mother–young relationships in sheep: a model for a multidisciplinary approach of the study of attachment in mammals. J Neuroendocrinol 23:1042–53
- Nowak R, Poindron P, Le neindre P, Putu IG. (1987). Ability of 12-hour-old merino and crossbred lambs to recognise their mothers. Appl Anim Behav Sci 17:263–71
- Orgeur P, Mavric N, Yvore P, Bernard S, Nowak R, Schaal B. (1998). Artificial weaning in sheep: Consequences on behavioural, hormonal and immuno-pathological indicators of welfare. Appl Anim Behav Sci 58:87–103
- Otten W, Puppe B, Kanitz E, Schon PC, Stabenow B. (1999). Effects of dominance and familiarity on behaviour and plasma stress hormones in growing pigs during social confrontation. J Vet Med A Physiol Path Clin Med 46:277–92
- Patin V, Lordi B, Vincent A, Thoumas JL, Vaudry H, Caston J. (2002). Effects of prenatal stress on maternal behavior in the rat. Brain Res Dev Brain Res 139:1–8
- Perrin G, Meurisse M, Lévy F. (2007). Inactivation of the medial preoptic area or the bed nucleus of the stria terminalis differentially disrupts maternal behavior in sheep. Horm Behav 52:461–73
- Pickup HE, Dwyer CM. (2011). Breed differences in the expression of maternal care at parturition persist throughout the lactation period in sheep. Appl Anim Behav Sci 132:33–41
- Plush KJ, Hebart ML, Brien FD, Hynd PI. (2011). The genetics of temperament in merino sheep and relationships with lamb survival. Appl Anim Behav Sci;134:130–5
- Poindron P, Otal J, Ferreira G, Keller M, Guesdon V, Nowak R, Levy F. (2010). Amniotic fluid is important for the maintenance of maternal responsiveness and the establishment of maternal selectivity in sheep. Animal 4:2057–64
- Ringgenberg N, Bergeron R, Meunier-Salaün M-C, Devillers N. (2012). Impact of social stress during gestation and environmental enrichment during lactation on the maternal behavior of sows. Appl Anim Behav Sci 136:126–35
- Roussel S, Hemsworth PH, Leruste H, White C, Duvaux-Ponter C, Nowak R, Boissy A. (2006). Repeated transport and isolation during pregnancy in ewes: effects on the reactivity to humans and to their offspring after lambing. Appl Anim Behav Sci 97:172–89
- Rushen J, Taylor AA, de Passillé AM. (1999). Domestic animals’ fear of humans and its effect on their welfare. Appl Anim Behav Sci 65:285–303
- Rutherford KMD, Donald RD, Arnott G, Rooke JA, Dixon L, Mehers JJM, Turnbull J, Lawrence AB. (2012). Farm animal welfare: assessing risks attributable to the prenatal environment. Anim Welf 21:419–29
- Rutherford KMD, Piastowska-Ciesielska A, Donalda RD, Robsona SK, Ison SH, Jarvis S, Brunton PJ, et al. (2014). Prenatal stress produces anxiety prone female offspring and impaired maternal behaviour in the domestic pig. Physiol Behav 129:255–64
- Schwartzkopf-Genswein KS, Booth-McLean ME, Shah MA, Entz T, Bach SJ, Mears GJ, Schaefer AL, et al. (2007). Effects of prehaul management and transport duration on beef calf performance and welfare. Appl Anim Behav Sci 108:12–30
- Stevens D, Alexander G, Lynch JJ. (1982). Lamb mortality due to inadequate care of twins by Merino ewes. Appl Anim Ethology 8:243–52
- Terlouw EMC, Boissy A, Blinet P. (1998). Behavioural responses of cattle to the odours of blood and urine from conspecifics and to the odour of faeces from carnivores. Appl Anim Behav Sci 57:9–21
- Thodberg K, Jensen KH, Herskin MS. (2002). Nursing behaviour, postpartum activity and reactivity in sows. Effects of farrowing environment, previous experience and temperament. Appl Anim Behav Sci 77:53–76
- Val-Laillet D, Nowak R. (2006). Socio-spatial criteria are important for the establishment of maternal preference in lambs. Appl Anim Behav Sci 96:269–80
- Viérin M, Bouissou M-F. (2001). Pregnancy is associated with low fear reactions in ewes. Physiol Behav 72:579–87
- Weinstock M. (2008). The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev 32:1073–86
- Zager A, Andersen ML, Ruiz FS, Antunes IB, Tufik S. (2007). Effects of acute and chronic sleep loss on immune modulation of rats. Am J Physiol Regul Integr Comp Physiol 293:504–9
- Zelkowitz P, Goldd I, Feeley N, Hayton B, Carter CS, Tulandi T, Abenhaim HA, Levin P. (2014). Psychosocial stress moderates the relationships between oxytocin, perinatal depression, and maternal behavior. Horm Behav 66:351–60
