Abstract
This study assessed the effects of premenstrual syndrome (PMS) and menstrual phases on the hypothalamic–pituitary–adrenal (HPA) axis, sympathetic nervous system axis and psychological responses to the Trier Social Stress Test (TSST). Thirty-six PMS women (mean age 21.69 ± 2.16 years) and 36 control women (mean age 22.03 ± 2.48 years) participated in the TSST task, either in the follicular phase or in the late luteal phase (each group N = 18). Saliva samples, heart rate and subjective stress levels were collected for seven time points throughout the test (10, 20, 30, 40, 55, 70 and 100 min). The results indicated that in comparison with control women, PMS women displayed blunted cortisol stress responses to the TSST irrespective of the menstrual phases, as indexed by the cortisol levels across time, area under the curve with respect to ground (AUCg) and peak change scores of cortisol. The results also demonstrated that the measurements indexed by cortisol levels across time, AUCg and peak change scores of heart rate were smaller in women tested during the late luteal phase than during the follicular phase. Correlation results indicated that AUCg was negatively correlated with PMS scores. These results suggest that measures of cortisol, rather than heart rate or subjective responses to stress, may be most closely associated with PMS. Furthermore, hypo-reactivity of the HPA axis may be pathologically relevant to PMS because it predicts heightened PMS severity.
Introduction
Premenstrual syndrome (PMS) is a mix of mood, physical and behavior symptoms that cyclically occur during the luteal phase and vanish after the onset of menses (Halbreich, Citation2003). PMS afflicts 10–20% women in their reproductive years (Qiao et al., Citation2012). Despite the most recent 80 years of research into this disorder, the precise pathophysiological mechanisms remain unknown.
PMS is associated with increased daily stress and is deteriorated by stressful life events. Consequently, several studies have focused on examination of the stress responses in PMS (Deuster et al., Citation1999; Perkonigg et al., Citation2004; Sadler et al., Citation2010). Exposure to an acute stressor can alter numerous indices of biological and psychological function, such as the hypothalamic–pituitary–adrenal (HPA) axis, sympathetic nervous system (SNS) and subjective negative experience (Allen et al., Citation2014), while chronic stress can induce chronic stimulation of these stress systems, leading to persistent alterations in neurobiological systems (Heim et al., Citation2000), and changes in subsequent reactivity to an acute stressor (Chatkoff et al., Citation2010; Roth et al., Citation2012). Thus, examining the effects of acute stress in the context of disorders associated with chronic stress, such as PMS, is a promising way to investigate the underlying etiology of PMS and to elucidate possible treatment and prevention of PMS.
Despite several experimental studies attempting to examine the stress responses in PMS women, results remain equivocal, with some studies reporting increased ovine corticotropin-releasing hormone stimulated plasma cortisol levels (Rabin et al., Citation1990) and elevated mental stress stimulated norepinephrine and total peripheral resistance in PMS women than in control women (Girdler et al., Citation1998), while others report more blunted adrenocorticotropic hormone (ACTH) and cortisol responses to serotonergic agents in PMS women than in control women (Bancroft et al., Citation1991; Su et al., Citation1997). Furthermore, studies using laboratory physiological or psychological stressors showed the activity of either one or both of the HPA and SNS axis were blunted in PMS women as compared with control women (Girdler et al., Citation1993, Citation1998, Citation2001; Klatzkin et al., Citation2010, Citation2014; Roca et al., Citation2003). Although the majority of evidence suggests hypo-reactive stress responses in PMS women, the ability to effectively draw conclusions from these discrepant findings is limited by methodological differences across the studies.
For example, the prior studies employed different stress tasks. Some studies used the pharmacological stimulation (Bancroft et al., Citation1991; Rabin et al., Citation1990; Su et al., Citation1997), whereas others used multiple stressors (Girdler et al., Citation1993; Klatzkin et al., Citation2010, Citation2014) or used only psychological (Girdler et al., Citation1998, Citation2001) or physiological stressors (Roca et al., Citation2003). Different stress responses may be specific to the type of stressor that is used (Lustyk et al., Citation2010). Accordingly, a standardized acute stressor that can produce reliably psychophysiological stress responses in humans is needed. The Trier Social Stress Test (TSST) is a standard stress protocol that employs a combination of elements (public speaking, mental arithmetic, anticipation and social evaluation) to produce reliable stress responses in a majority of participants (Kirschbaum et al., Citation1993). Furthermore, a cross-cultural comparison verified it applicability to Chinese populations (Yang et al., Citation2011).
In addition, the prior studies employed a wide variety of physiological stress responses. Some studies only measured SNS axis activity (Girdler et al., Citation1993; Klatzkin et al., Citation2014), whereas others measured only the HPA axis (Girdler et al., Citation2001; Roca et al., Citation2003) or combined HPA and SNS axes activity (Girdler et al., Citation1998; Klatzkin et al., Citation2010). Some studies analyzed HPA axis activity using one (baseline; Klatzkin et al., Citation2010) or two time points (during baseline and after stress; Girdler et al., Citation1998, Citation2001). Use of only one or two time points can introduce high variability in cortisol measurement due to cortisol pulsatility and cortisol diurnal pattern (Young et al., Citation2004). Furthermore, although previous studies observed comparable HPA axis and heart rate stress responses to the TSST in the morning and afternoon (Kudielka et al., Citation2004), due to diurnal patterns, most studies have restricted the time window for conducting the TSST to afternoon hours (Allen et al., Citation2014). More importantly, the three above-mentioned studies that used analog TSST tasks only analyzed blood cortisol levels but not saliva cortisol (Girdler et al., Citation1998, Citation2001; Klatzkin et al., Citation2010). Cortisol levels in blood and saliva showed significantly different steroid patterns; in saliva, only the free hormone fraction will be determined, whereas blood reflects both free cortisol and that bound to plasma proteins (total cortisol) (Foley & Kirschbaum, Citation2010). Moreover, collection of saliva is noninvasive and therefore relatively easy to perform in comparison with blood, and because of this, Foley & Kirschbaum (Citation2010) argued that it is the best characterized HPA marker for the response to the TSST.
Finally, different studies assessed different menstrual phases. Some studies only used the luteal phase (Girdler et al., Citation2001; Klatzkin et al., Citation2010, Citation2014), whereas others used both the follicular phase and the luteal phase (Girdler et al., Citation1993, Citation1998; Roca et al., Citation2003). Participants in those studies used two menstrual phases exposed to the stress task for repeated measures, which may introduce the potential confound of habituation (Kudielka et al., Citation2006).
This study examined the variation in HPA axis, SNS axis and psychological activity (manifested by salivary cortisol, heart rate and subjective stress, respectively) at multiple time points in PMS and control women during the follicular phase and the late luteal phase, using the well-validated TSST task. Because PMS is a chronic stress-related disorder, combined with the findings showed that chronic stress mainly influences the HPA axis activity (Checkley, Citation1992; Juruena et al., Citation2011), heart rate and subjective stress responses may be less specific to stressful stimuli than is HPA axis activity (Gordis et al., Citation2006). We hypothesized that the hypo-reactivity stress responses in PMS women as compared with control women are mainly reflected in the HPA axis rather than the SNS axis or psychological activity. We also anticipated that the hypo-reactivity stress responses in PMS women are mainly reflected in the late luteal phase because it is the symptomatic phase. To explore the role of the HPA axis in PMS severity, we predicted that the hypo-reactivity of the HPA axis could predict heightened PMS severity.
Methods
Participants
Potential participants were recruited via posters or online ads in universities seeking women with PMS. Of the 251 women who were instructed to assess themselves daily in terms of the American College of Obstetricians and Gynecologists (ACOG) recommendations (Cirillo et al., Citation2012) for PMS diagnosis for two consecutive cycles, 227 women completed the survey (the completion rate was 90.44%).
The ACOG recommendations were translated into Chinese to examine the incidence of PMS in China (Qiao et al., Citation2012). Therefore, this study used the translated ACOG recommendations as the diagnostic criteria for PMS. These recommendations outlined a total of 10 emotional and physical symptoms: depression, angry outbursts, irritability, anxiety, confusion, social withdrawal, breast tenderness, abdominal bloating, headache and swelling of extremities. To be confirmed as having a diagnosis of PMS, participants had to show that (a) at least one symptom occurred during the five days before menses in each of the three previous menstrual cycles and also that (b) it occurred reproducibly during the next two cycles; (c) these symptoms must be relieved or improved during the 4–13 d of the menstrual cycle and (d) be associated with identifiable dysfunction in work, study or life; additionally, (e) these symptoms must occur in the absence of any pharmacologic therapy, hormone ingestion or alcohol abuse (Cirillo et al., Citation2012).
A diary form of questionnaires that contained the 10 symptoms and an additional 4 PMS diagnostic criterion (above-mentioned criteria (a, c, d and e) was designed in our study to operationalize the ACOG recommendations. Participants were instructed to indicate whether these symptoms and criterion occurred or not for two consecutive cycles (except both the criteria (a) and (c), which were completed once on the first complete day), and also to indicate the days of menses, thus the criteria (b) were prospectively validated. Participants were asked to complete the printed versions of the questionnaires for two consecutive cycles every night before going to bed. Those who met the above-mentioned five criteria were confirmed as PMS women, whereas others were confirmed as non-PMS women. Ultimately, 77 (33.92%) women met the criteria for PMS, the other 150 (66.08%) women were confirmed as non-PMS, and the incidence of PMS was consistent with other reports (Klatzkin et al., Citation2014).
Of the 77 PMS women, 38 women volunteered to participate in the TSST and were compared with 38 healthy volunteers who were selected from the pool of 150 non-PMS women (control). All PMS and control women were unmarried, were physically healthy and did not have a cardiovascular disorder or endocrine disorder as confirmed by a complete hospital physical examination; were not taking hormones and medications; had regular menstrual cycles; did not have alcohol and substance abuse; did not currently have Axis I psychiatric disorders that were ascertained using the Chinese version of Mini International Neuropsychiatry Interview, based on DSM-IV and ICD-10 (Si et al., Citation2009); and self-reported of no treatment for depression prior to the screening.
The menstrual cycle phases of each participant were calculated with regard to the self-reported date of her last menses, the average length of a single menstrual cycle and the predicted time of the next menses, which could be found in demographic data that were collected before the experiment (). Women were either tested in the follicular phase (1–4 d after offset of menstruation; 19 PMS and 19 control women) or in the late luteal phase (1–4 d before menses; 19 PMS and 19 control women) because repeated exposure to the TSST generally leads to rapid habituation effects of the HPA axis responses (Kudielka et al., Citation2006). The test days for each participant were adjusted according to her cycle length and the duration of menstrual flow. The late luteal phase was validated according to the exact time of the onset of the next menses. In addition, salivary estradiol and progesterone assessments performed during the baseline period of TSST were used to verify self-reported menstrual cycle phases ().
Table 1. The demographic variables, PMS scores and sex hormones levels of PMS and control women tested in the follicular phase and the late luteal phase (mean ± standard deviation).
Three women were excluded because the hormone values and the test days did not correspond to the late luteal phase, and one woman was excluded due to insufficient saliva samples. Consequently, 36 women with PMS (18 tested in the follicular phase and 18 tested in the late luteal phase) and 36 control women (18 tested in the follicular phase and 18 tested in the late luteal phase) composed of the sample for this report. All participants provided written informed consent to participate in this study. This study protocol was approved by the Institutional Review Board of the State Key Laboratory of Cognitive Neurosciences and Learning of Beijing Normal University. This study was performed in accordance with the Declaration of Helsinki.
Confirmation of severity of PMS
The PMS scale, which consists of 12 items related to emotional and physical symptoms, can provide the degree of severity of PMS (Bancroft, Citation1993). All items are scored on a four-point Likert scale, with 0 “no symptoms”, 1 “mild symptoms”, 2 “symptoms affecting life, study and work, but tolerable” and 3 “symptoms seriously affecting life, study and work, needing treatment”. Total scores of 6–10, 11–20 and >20 points indicate mild, moderate and severe PMS, respectively. The validated Chinese PMS scale has good reliability and validity (Shi & Yu, Citation1999; Yu, Citation2008). The PMS scores of PMS and control women tested in the follicular phase and the late luteal phase are listed in .
Procedures
All TSST laboratory testing began at 2:00 p.m. to control for diurnal variations of cortisol secretion. Participants were asked to refrain from any food or drink with the exception of water for at least 2 h before testing. The TSST were conducted based on standardized procedures as previously described (Kirschbaum et al., Citation1993). Briefly, participants rested for 10 min in room A (baseline phase). Subsequently, they were escorted by a research assistant to an adjacent room (room B), where two other research assistants (one man and one women) acting as TSST judges were seated, and participants were introduced to the task. Following the instructions, participants returned to room A for a 10-min preparation (preparation phase). Next, they were guided back to room B for a 10-min performance in which participants delivered a 5-min public speaking and 5-min arithmetic (serial subtraction of the number 13 from 1022) in front of the two judges while being videotaped and recorded (TSST phase). After the performance, participants were taken back to room A for a 70-min recovery (recovery phase). A total of seven segments of heart rate, salivary cortisol samples and subjective stress (Likert scale, ranging from 1: “extremely stressed” to 5: “not at all stressed”) measures were obtained throughout the test: baseline (10 min), preparation (20 min), TSST (30 min), first recovery (40 min), second recovery (55 min), third recovery (70 min) and fourth recovery (100 min).
Cardiovascular measures
Heart rate was calculated from the raw blood volume pulse signal recorded with a sample rate of 128 Hz by a mobile and wireless NeXus-10 recording device with BioTrace+ software (Mind Media B.V., Herten, the Netherlands). The heart rate signal was sampled continuously, and the mean heart rate during successive 10-min periods was calculated into seven segments as described above.
Biochemical assays
All saliva samples were collected with the Salivette sampling device (DRG Inc., Marburg, Germany) and were stored at −20 °C until assayed. Cortisol, estradiol and progesterone analyses were determined by a competitive enzyme-linked immunosorbent assay. The intra- and inter-assay coefficients of variation were all below 12%.
Statistical analyses
All statistics were conducted using SPSS, version 19.0 (Chicago, IL), and the significance level was set at p < 0.05. Where appropriate, results were corrected by the Greenhouse–Geisser procedure. Partial eta squared () is presented as a measure of effect sizes.
The demographic variables, PMS scores and sex hormones levels of PMS and control women tested in the follicular phase and the late luteal phase were compared using two-way ANOVAs, with group (PMS and controls) and menstrual cycle (the follicular phase and the late luteal phase) as between-subjects factors (for details, see ). None of these demographic variables result in any significant main or interaction effects (all F(1,68) < 3.11, all p > 0.08), therefore, none were included in the following analysis.
Because raw cortisol values are typically skewed, the data were log transformed to yield a normal distribution before statistical analysis. For illustrative reasons, and below present non-transformed data. The impact of the TSST upon heart rate, cortisol and subjective stress level across time were assessed using three-way mixed ANOVAs (time by group by menstrual cycle), with group and menstrual cycle as between-subjects factors, and the repeated measure of time (10, 20, 30, 40, 55, 70 and 100 min) as within-subjects factor (for details, see ).
Figure 1. Changes in salivary cortisol (A), heart rate (B) and subjective stress (C) after the TSST for PMS and control women in the follicular phase and the late luteal phase. Data represent mean ± SEM (baseline from 0 to 10 min, preparation from 10 to 20 min, TSST from 20 to 30 min, first recovery from 30 to 40 min, second recovery from 40 to 55 min, third recovery from 55 to 70 min and fourth recovery from 70 to 100 min).
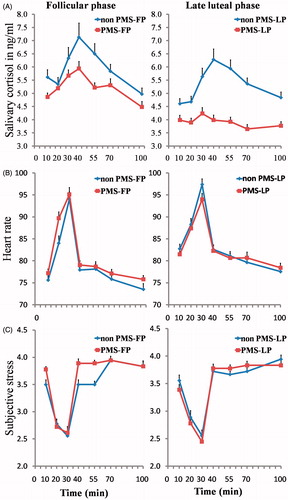
Table 2. Salivary cortisol, heart rate and subjective stress levels throughout the TSST of PMS and control women tested in the follicular phase and the late luteal phase (mean ± standard deviation).
To parallel measures employed in previous studies (Klatzkin et al., Citation2010, Citation2014; Roca et al., Citation2003), we also conducted two-way ANOVAs (group by menstrual cycle) for two measures of stress-induced responses as in Ruttle et al. (Citation2014): (1) stress reactivity (peak sample minus baseline), i.e. the maximum positive or negative change from the baseline; and (2) total cortisol output (area under the curve with respect to ground [AUCg]). The AUCg was calculated using the formula described by Pruessner et al. (Citation2003).
Results
Sex hormones assay
The two-way ANOVA results for progesterone showed a main effect of menstrual cycle, F(1,68) = 5.96, p = 0.02, = 0.08, with women tested in the late luteal phase having higher progesterone levels compared with women tested in the follicular phase. No other main effect or interaction effect was found (all F < 0.37, all p > 0.54).
Analysis of estradiol levels demonstrated no significant main effects or interaction effect (all F < 2.17, all p > 0.15).
PMS scores
As expected, a main effect of group was found, F(1,68) = 17.90, p < 0.001, = 0.21, with significantly higher PMS scores in PMS women than in control women. No other main effects or interaction effects were found (all F < 0.16, all p > 0.69).
Salivary cortisol measures
and illustrate the cortisol responses to the TSST. The three-way mixed ANOVA was conducted to test the salivary cortisol responses to the TSST, a main effect of time was found, F(6,408) = 5.46, p < 0.001, = 0.07. Post-hoc analysis showed that cortisol levels reflecting the typical course of the TSST, specifically, cortisol levels assessed at TSST (30 min) were significantly higher than at baseline (10 min), preparation (20 min) and fourth recovery (100 min); cortisol levels assessed at first recovery (40 min) were significantly higher than at baseline (10 min), preparation (20 min), third recovery (70 min) and fourth recovery (100 min); cortisol levels assessed at second recovery (55 min) were significantly higher than at baseline (10 min) and fourth recovery (100 min); cortisol levels assessed at third recovery (70 min) were significantly higher than at fourth recovery (100 min), all p < 0.05. With respect to group, the main effect of group was significant, F(1,68) = 5.15, p = 0.03,
= 0.07, with significantly lower cortisol levels in PMS women compared with control women. There was also a main effect of menstrual cycle, F(1,68) = 7.20, p = 0.01,
= 0.10, with women tested in the late luteal phase having lower cortisol levels compared with women tested in the follicular phase. No interaction effects were found (all F < 1.48, all p > 0.23).
Analysis of AUCg confirmed these findings; PMS women exhibited significantly lower AUCg than control women [F(1,68) = 6.07, p = 0.02, = 0.08], and women tested in the late luteal phase had lower AUCg than women tested in the follicular phase [F(1,68) = 7.12, p = 0.01,
= 0.10]. Analysis of peak change scores revealed a main effect of group, F(1,68) = 4.53, p = 0.04,
= 0.06, with PMS women exhibiting significantly smaller increases in cortisol than control women.
Cardiovascular measures
Exposure to the TSST significantly activated the heart rate activity (see and ). A three-way mixed ANOVA revealed a significant main effect of time, F(6,408) = 145.15, p < 0.001, = 0.68. Post-hoc analysis showed that heart rate at TSST (30 min) was significantly higher than at other phases, and heart rate at fourth recovery (100 min) was significantly lower than at other phases, all p < 0.001. A trend for significance was observed for a menstrual cycle × time, F(6,408) = 2.32, p = 0.06,
= 0.03. Follow-up simple effect analysis showed that women tested in the late luteal phase had higher heart rates compared with women tested in the follicular phase at baseline (10 min), F(1,68) = 6.33, p = 0.01. No other main effects or interaction effects were found (all F < 1.97, all p > 0.17), whereas analysis of peak change scores revealed a main effect of menstrual cycle, F(1,68) = 6.18, p = 0.02,
= 0.08, with women tested in the late luteal phase having smaller increases in heart rate than women tested in the follicular phase.
Subjective measures
The TSST significantly increased scores upon the subjective stress scores (see and ). A three-way mixed ANOVA revealed a significant main effect of time, F(6,408) = 54.08, p < 0.001, = 0.44. Post-hoc analysis showed that stress scores assessed at TSST (30 min) were significantly lower than other phases, and stress scores assessed at baseline (10 min), preparation (20 min) and TSST (30 min) were significantly lower than at first recovery (40 min), second recovery (55 min), third recovery (70 min) and fourth recovery (100 min), all p < 0.05. No other main effects or interaction effects were found (all F < 0.81, all p > 0.39). Analysis of peak change scores also demonstrated no significant effects of group or menstrual cycle (all F < 2.18, all p > 0.14).
Correlational analyses
Pearson correlations were calculated between the outcome measures in the sample as a whole. As expected, AUCg and peak change scores of cortisol were correlated (r = 0.30, p = 0.01). Correlation results also showed a positive relation between AUCg and peak change scores of heart rate (r = 0.27, p = 0.02). Moreover, AUCg were negatively correlated with PMS scores (r = −0.24, p < 0.05).
Discussion
This study aimed to examine stress responses in PMS and control women during the follicular phase and the late luteal phase. The results indicated that exposure to the TSST activated physiological and subjective stress responses in women, as demonstrated by increased cortisol, heart rate and subjective stress level. These changes reflected the typical course of the TSST. As expected, our results are consistent with the hypothesis that PMS women displayed blunted cortisol responses to the TSST compared with control women, as indexed by the cortisol levels across time, AUCg and peak change scores of cortisol. Despite the differences in cortisol responses to the TSST between PMS and control women, no differences in heart rate and subjective stress level were reported. With respect to menstrual cycle, women tested in the late luteal phase showed smaller levels of measurements indexed by cortisol levels across time, AUCg and peak change scores of heart rate than women tested in the follicular phase. Moreover, the negative correlation between AUCg and PMS scores suggests that the hypo-reactivity of the HPA axis may be pathologically relevant. These results suggest that lower cortisol secretion (rather than heart rate responses or subjective stress perception) may be closely associated with PMS.
In line with previous studies, the TSST induced increased salivary cortisol, heart rate and subjective stress level in women (Kirschbaum et al., Citation1999). Furthermore, these stress responses to the TSST reflected the expected typical course. With respect to cortisol, the peak occurred approximately 10–20 min after the TSST onset, which is in accordance with Foley & Kirschbaum (Citation2010). Heart rate and subjective stress level peaked throughout the TSST exposure and returned quickly to the baseline level, as in Duchesne & Pruessner (Citation2013). Correlation result showed that AUCg is positively correlated with peak change scores of heart rate. This finding adds to the previous studies concerning the interaction between the HPA axis and SNS (Andrews et al., Citation2012; Oei et al., Citation2010). Theoretically, different stress systems (HPA axis, SNS axis and subjective stress) represent the same construct indicator, leading to “response coherence” or interrelationship between these different systems (Lazarus, Citation1991; Mauss et al., Citation2005). However, the interactions between different stress systems have been rarely investigated. As in the review of Campbell & Ehlert (Citation2012), the authors reviewed 359 studies that employed the TSST, only 22 (6.1%) studies focused on all three stress systems simultaneously. Our results highlight the importance of inclusion of different indices of different stress systems. The lack of relationship between subjective stress level and the physiological stress responses may be because the subjective stress measures were determined prior to and after the TSST in our study. In light of the study of Hellhammer & Schubert (Citation2012), the physiological responses to the TSST relates to subjective measures of stress during but not before or after the test. It would be interesting in future studies to look at the psychological stress measures during the stressor itself. It is also worth noting that we did not use a well-established instrument for assessment of stress (e.g. the Perceived Stress Scale; Cohen et al., Citation1983), and thus our test may have been insufficient to assess subjective stress.
The chief finding of our study was that PMS women showed blunted cortisol responses to the TSST compared with control women, as indexed by the cortisol levels across time, AUCg and peak change scores of cortisol. To our knowledge, this is the first study demonstrating the hypo-reactivity HPA axis in PMS women’s responses to the TSST reflected by salivary cortisol at multiple time points. Cortisol plays a critical role in regulating individuals’ ability to respond to the demands of a stressful situation (Fries et al., Citation2009). It has been speculated that an insufficient cortisol response to stressors leads to increased vulnerability for somatic and mental health problems. PMS is a chronic stress-related disorder; chronic stress exposure elicits long-term activation of the stress systems, which may result in a wearing out of the stress systems, leading to hypo-responsiveness of the systems to subsequent stressors (DeBellis & Thomas, Citation2003; Lindley et al., Citation2004; Young & Breslau, Citation2004). Although reduced cortisol response to stressors has been documented in a number of studies (Girdler et al., Citation2001; Roca et al., Citation2003), the mechanisms underlying this pattern of “blunting” are not well understood and are probably complex. It will be important for future research to better characterize this pattern and evaluate its clinical relevance. In contrast with the HPA axis, the cardiovascular and subjective responses to stress are adaptive in the short term and thus may be less specific to stressful stimuli (Gordis et al., Citation2006). This may be the reason for the lack of differences in heart rate and subjective stress responses to the TSST in PMS and control women. While blunted heart rate has been previously documented in premenstrual dysphoric disorder (PMDD; Klatzkin et al., Citation2010) or menstrually related mood disorder (MRMD; Klatzkin et al., Citation2014), the two aforementioned studies only observed the luteal phase, and the severity of both PMDD and MRMD are higher than PMS, according to the PMS scores in our study, which reflected that the overall premenstrual symptoms of PMS women were relatively mild. Thus, the heart rate effect may have been magnified by the severity of premenstrual symptoms in the symptomatic period in previous studies. Furthermore, the differences in cortisol responses to the TSST between PMS and control women irrespective of the menstrual phases may also be related to the relatively mild premenstrual symptoms of PMS women.
Another main finding of our study was that women tested in the late luteal phase showed lower cortisol levels over time, AUCg and peak change scores of heart rate than women tested in the follicular phase. These results seem contrary to previous studies using the TSST or similar mental stress tasks, which found the luteal phase to be associated with increases in salivary cortisol (Kirschbaum et al., Citation1999; Walder et al., Citation2012) or heart rate (Lustyk et al., Citation2010; Manhem et al., Citation1991) responses to stress. However, with respect to salivary cortisol results, the menstrual phases compared in previous studies were the early follicular phase (marked by the lowest relative estradiol and progesterone levels) and the mid-luteal phase (marked by relatively high estradiol and peak progesterone levels) (Kirschbaum et al., Citation1999; Walder et al., Citation2012); thus, both the estradiol and progesterone levels were different between the two phases, while the menstrual phases compared in our study were the mid-follicular phase (marked by relatively high estradiol and relatively low progesterone levels) and the late luteal phase (marked by relatively high estradiol and progesterone levels) – only the progesterone levels were different between the two phases, which is in line with the existing research (Farage et al., Citation2008; Wactawski-Wende et al., Citation2009). Based on previous studies, it is not possible to differentiate the influence of progesterone alone from progesterone plus estradiol on stress reactivity. The late luteal phase is a symptomatic period that may provide a “context of vulnerability” for PMS women, according to previous findings that exposure to an acute stressor leads to the release of chemicals that can help cope with the stressor (Joels & Baram, Citation2009; Schwabe et al., Citation2012) and that cortisol regulates metabolism and immunity (Heim et al., Citation2000; Meinlschmidt & Heim, Citation2005). The smaller amount of cortisol in the late luteal phase in our study may indeed reflect the inability to respond to the demands of stress, leading to increasing vulnerability for somatic and mental health problems in the late luteal phase. There was no effect of menstrual phases on peak change scores of cortisol, consistent with findings reported by Bouma et al. (Citation2009) and Childs et al. (Citation2010). This suggests that when exposed to stress, the absolute amount (reflect by cortisol levels across time and AUCg) rather than the change amount index (reflect by peak change scores of cortisol) are more likely to manifest the menstrual cycle effect, but this assertion needs further confirmation.
In contrast, the autonomic results indicate that the menstrual cycle effects were manifest as change in heart rate (peak change scores of heart rate). However, care should be taken in interpreting the heart rate results. In line with Gordon & Girdler (Citation2014), our results showed that the baseline levels of heart rate were higher in women tested during the late luteal phase than women tested during the follicular phase. Thus, the smaller increase in heart rate in women tested during the late luteal phase than during the follicular phase may be explained by the high baseline levels. Taken together, we speculate that there is greater heart rate reactivity in the late luteal phase compared with the follicular phase.
We found no effect of group or menstrual cycle on subjective stress level, which is consistent with previous studies (Kirschbaum et al., Citation1999) but contrary to Childs et al. (Citation2010) and Klatzkin et al. (Citation2010). Further studies concerning various negative moods, such as anger, hostility and anxiety, rather than only the subjective stress report, may be more suitable for revealing subtle emotional changes. Finally, although preliminary, the negative correlation between AUCg and PMS scores could implicate that hypo-reactivity of the HPA axis as a physiological mechanism mediates the severity of PMS.
There are limitations to this study. First, the relatively young and narrow age range of our participants may limit the generalizability of the results to other distinct reproductive phases such as puberty and the menopause transition phase, and thus replication is required in more diverse samples. Second, we did not assess measures other than salivary cortisol and heart rate to reflect the HPA axis and SNS axis activity; thus, we cannot conclude that the different physiological stress responses in groups and menstrual cycle in our study could be generalized to other HPA axis measures, such as ACTH and serum cortisol or markers of the SNS axis, such as catecholamine and hemodynamic activity. Third, we did not use techniques to measure the differences between PMS and control women in functional impairment during different menstrual cycle phases, which limits our ability to clarify the deterioration of functional symptoms (i.e. dysfunction in work and study or life) in PMS women in the late luteal phase. Finally, although the TSST represents a standardized stressor that is most frequently used in stress research, future research would do well to develop comparably potent alternative stress protocols to explore whether the effects observed in our study might be “TSST specific” (Kudielka & Wüst, Citation2009).
In summary, our study reveals that PMS women displayed blunted HPA axis responses to stress compared to control women, as reflected by salivary cortisol, and that this blunted HPA axis stress reactivity, as reflected by ACUg, may be pathologically relevant to PMS because it predicts heightened PMS severity. The findings have implications for dysfunctions of HPA axis that may result in maladaptive responses and failure to cope with stress. Based on the experimental manipulation conducted by Pilgrim et al. (Citation2014), which demonstrated that attentional training enhances salivary cortisol responses to the TSST, further studies should specifically investigate whether behavioral interventions that affect the HPA axis can be used to treat and prevent PMS.
Declaration of interest
The authors have declared that no competing interests exist.
This work was funded by the National Basic Research Program of China (No. 2011CB505101) and the key lab open project of Beijing University of Chinese Medicine (2011-SYSKFKT03), as well as the Shangshan funding. We would like to express our gratitude for the support of these projects.
References
- Allen AP, Kennedy PJ, Cryan JF, Dinan TG, Clarke G. (2014). Biological and psychological markers of stress in humans: focus on the Trier Social Stress Test. Neurosci Biobehav R 38:94–124
- Andrews J, D’Aguiar C, Pruessner JC. (2012). The combined dexamethasone/TSST paradigm – a new method for psychoneuroendocrinology. PLoS One 7:e38994
- Bancroft J. (1993). The premenstrual syndrome: a reappraisal of the concept and the evidence. Psychol Med 24:3–7
- Bancroft J, Cook A, Davidson D, Bennie J, Goodwin G. (1991). Blunting of neuroendocrine responses to infusion of L-tryptophan in women with perimenstrual mood change. Psychol Med 21:305–12
- Bouma E, Riese H, Ormel J, Verhulst FC, Oldehinkel AJ. (2009). Adolescents’ cortisol responses to awakening and social stress; effects of gender, menstrual phase and oral contraceptives. The TRAILS study. Psychoneuroendocrinology 34(6):884–93
- Campbell J, Ehlert U. (2012). Acute psychosocial stress: does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology 37(8):1111–34
- Chatkoff DK, Maier KJ, Klein C. (2010). Nonlinear associations between chronic stress and cardiovascular reactivity and recovery. Int J Psychophysiol 77:150–6
- Checkley S. (1992). Neuroendocrine mechanisms and the precipitation of depression by life events. Br J Psychiatry 160:7–17
- Childs E, Dlugos A, De Wit H. (2010). Cardiovascular, hormonal, and emotional responses to the TSST in relation to sex and menstrual cycle phase. Psychophysiology 47(3):550–9
- Cirillo PC, Passos RBF, Bevilaqua MCDN, López JRRA, Nardi AE. (2012). Bipolar disorder and Premenstrual Syndrome or Premenstrual Dysphoric Disorder comorbidity: a systematic review. Rev Brasileira Psiquiatria 34(4):467–79
- Cohen S, Kamarck T, Mermelstein R. (1983). A global measure of perceived stress. J Health Soc Behav 24(2):385–96
- DeBellis MD, Thomas LA. (2003). Biologic findings of post-traumatic stress disorder and child maltreatment. Curr Psychiat Rep 5:108–17
- Deuster PA, Adera T, South-Paul J. (1999). Biological, social, and behavioral factors associated with premenstrual syndrome. Arch Fam Med 8(2):122–8
- Duchesne A, Pruessner JC. (2013). Association between subjective and cortisol stress response depends on the menstrual cycle phase. Psychoneuroendocrinology 38(12):3155–9
- Farage MA, Osborn TW, MacLean AB. (2008). Cognitive, sensory, and emotional changes associated with the menstrual cycle: a review. Arch Gynecol Obstet 278(4):299–307
- Foley P, Kirschbaum C. (2010). Human hypothalamus–pituitary–adrenal axis responses to acute psychosocial stress in laboratory settings. Neurosci Biobehav Rev 35:91–6
- Fries E, Dettenborn L, Kirschbaum C. (2009). The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol 72:67–73
- Girdler SS, Pedersen CA, Stern RA, Light KC. (1993). Menstrual cycle and premenstrual syndrome: modifiers of cardiovascular reactivity in women. Health Psychol 12(3):180–92
- Girdler SS, Pedersen CA, Straneva PA, Leserman J, Stanwyck CL, Benjamin S, Light KC. (1998). Dysregulation of cardiovascular and neuroendocrine responses to stress in premenstrual dysphoric disorder. Psychiat Res 81(2):163–78
- Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. (2001). Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiat 49(9):788–97
- Gordis EB, Granger DA, Susman EJ, Trickett PK. (2006). Asymmetry between salivary cortisol and alpha-amylase reactivity to stress: relation to aggressive behavior in adolescents. Psychoneuroendocrinology 31:976–87
- Gordon JL, Girdler SS. (2014). Mechanisms underlying hemodynamic and neuroendocrine stress reactivity at different phases of the menstrual cycle. Psychophysiology 51:309–18
- Halbreich U. (2003). The etiology, biology, and evolving pathology of premenstrual syndromes. Psychoneuroendocrinology 28:55–99
- Heim C, Ehlert U, Hellhammer DH. (2000). The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology 25(1):1–35
- Hellhammer J, Schubert M. (2012). The physiological response to Trier Social Stress Test relates to subjective measures of stress during but not before or after the test. Psychoneuroendocrinology 37(1):119–124
- Joels M, Baram TZ. (2009). The neuro-symphony of stress. Nat Rev Neurosci 10:459–466
- Juruena M, Baes CVW, de Carvalho Tofoli SM, Martins CMS. (2011). Early life stress, HPA axis, and depression. Psychol Neurosci 4:229–34
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. (1999). Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med 61:154–62
- Kirschbaum C, Pirke KM, Hellhammer DH. (1993). The ‘Trier Social Stress Test’ – a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28:76–81
- Klatzkin RR, Bunevicius A, Forneris CA, Girdler S. (2014). Menstrual mood disorders are associated with blunted sympathetic reactivity to stress. J Psychosom Res 76(1):46–55
- Klatzkin RR, Lindgren ME, Forneris CA, Girdler SS. (2010). Histories of major depression and premenstrual dysphoric disorder: evidence for phenotypic differences. Biol Psycho 84:235–47
- Kudielka BM, Schommer NC, Hellhammer DH, Kirschbaum C. (2004). Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology 29:983–92
- Kudielka BM, von Känel R, Preckel D, Zgraggen L, Mischler K, Fischer JE. (2006). Exhaustion is associated with reduced habituation of free cortisol responses to repeated acute psychosocial stress. Biol Psychol 72:147–53
- Kudielka BM, Wüst S. (2009). Human models in acute and chronic stress: assessing determinants of individual hypothalamus–pituitary–adrenal axis activity and reactivity. Stress 13:1–14
- Lazarus R. (1991). Emotion and adaptation. London: Oxford University Press
- Lindley SE, Carlson EB, Benoit M. (2004). Basal and dexamethasone suppressed salivary cortisol concentrations in a community sample of patients with posttraumatic stress disorder. Biol Psychiat 55:940–5
- Lustyk MKB, Olson KC, Gerrish WG, Holder A, Widman L. (2010). Psychophysiological and neuroendocrine responses to laboratory stressors in women: implications of menstrual cycle phase and stressor type. Biol Psychol 83(2):84–92
- Manhem K, Jern C, Pilhall M, Shanks G, Jern S. (1991). Haemodynamic responses to psychosocial stress during the menstrual cycle. Clin Sci 81(Pt 1):17–22
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. (2005). The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion 5:175–90
- Meinlschmidt G, Heim C. (2005). Decreased cortisol awakening response after early loss experience. Psychoneuroendocrinology 30:568–76
- Oei NY, Tollenaar MS, Elzinga BM, Spinhoven P. (2010). Propranolol reduces emotional distraction in working memory: a partial mediating role of propranolol-induced cortisol increases? Neurobiol Learn Mem 93:388–95
- Perkonigg A, Yonkers KA, Pfister H, Lieb R, Wittchen H-U. (2004). Risk factors for premenstrual dysphoric disorder in a community sample of young women: the role of traumatic events and posttraumatic stress disorder. J Clin Psychiat 65(10):1314–22
- Pilgrim K, Ellenbogen MA, Paquin K. (2014). The impact of attentional training on the salivary cortisol and alpha amylase response to psychosocial stress: importance of attentional control. Psychoneuroendocrinology 44:88–99
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28:916–31
- Qiao M, Zhang H, Liu H, Luo S, Wang T, Zhang J, Ji L. (2012). Prevalence of premenstrual syndrome and premenstrual dysphoric disorder in a population-based sample in China. Eur J Obstet Gyn R B 162:83–6
- Rabin DS, Schmidt PJ, Campbell G, Gold PW, Jensvold M, Rubinow DR, Chrousos GP. (1990). Hypothalamic–pituitary–adrenal function in patients with the premenstrual syndrome. J Clin Endocr Metab 71:1158–62
- Roca CA, Schmidt PJ, Altemus M, Deuster P, Danaceau MA, Putnam K, Rubinow DR. (2003). Differential menstrual cycle regulation of hypothalamic-pituitary-adrenal axis in women with premenstrual syndrome and controls. J Clin Endocr Metab 88:3057–63
- Roth MK, Bingham B, Shah A, Joshi A, Frazer A, Strong R, Morilak DA. (2012). Effects of chronic plus acute prolonged stress on measures of coping style, anxiety, and evoked HPA-axis reactivity. Neuropharmacology 63:1118–26
- Ruttle PL, Klein MH, Slattery MJ, Kalin NH, Armstrong JM, Essex MJ. (2014). Adolescent adrenocortical activity and adiposity: differences by sex and exposure to early maternal depression. Psychoneuroendocrinology 47:68–77
- Sadler C, Smith H, Hammond J, Bayly R, Borland S, Panay N, Inskip H. (2010). Lifestyle factors, hormonal contraception, and premenstrual symptoms: the United Kingdom Southampton women’s survey. J Womens Health 19(3):391–6
- Schwabe L, Joëls M, Roozendaal B, Wolf OT, Oitzl MS. (2012). Stress effects on memory: an update and integration. Neurosci Biobehav Rev 36:1740–9
- Shi W, Yu Y. (1999). A study on the change of serum Ca2+, Mg2+ level of the patients with premenstrual syndrome. Trace Elem Health Res 16:31–3
- Si TM, Shu L, Dang WM, Su YA, Chen JX, Dong WT, Kong QM, Zhang WH. (2009). Evaluation of the reliability and validity of Chinese version of the Mini-International Neuropsychiatric Interview in patients with mental disorders. Chin Ment Health J 23:493–7
- Su T-P, Schmidt PJ, Danaceau M, Murphy DL, Rubinow DR. (1997). Effect of menstrual cycle phase on neuroendocrine and behavioral responses to the serotonin agonist m-chlorophenylpiperazine in women with premenstrual syndrome and controls. J Clin Endocr Metab 82:1220–8
- Wactawski-Wende J, Schisterman EF, Hovey KM, Howards PP, Browne RW, Hediger M, Trevisan M. (2009). BioCycle study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatr Perinat Ep 23(2):171–84
- Walder DJ, Statucka M, Daly MP, Axen K, Haber M. (2012). Biological sex and menstrual cycle phase modulation of cortisol levels and psychiatric symptoms in a non-clinical sample of young adults. Psychiat Res 197(3):314–21
- Yang J, Hou Y, Yang Y, Zhang Q. (2011). Impact of Trier Social Stress Test (TSST) on Salivary Cortisol Secretion. Acta Psychol Sin 43(4):403–9
- Young EA, Abelson J, Lightman SL. (2004). Cortisol pulsatility and its role in stress regulation and health. Front Neuroendocrinol 25:69–76
- Young EA, Breslau N. (2004). Cortisol and catecholamines in posttraumatic stress disorder. Arch Gen Psychiatry 61:394–401
- Yu C. (2008). Relationship between premenstrual syndrome and type D personality in female college students. Master’s thesis, Central South University; 2008: 1–38
