Abstract
Skeletal muscle protein loss, known as atrophy, occurs during inactivity, disease, and aging. Atrophy may be the result of increased catabolic factors, e.g. glucocorticoids, or reduced influence of anabolic factors, e.g. insulin. The purpose of this study was to investigate atrophy, signaling mechanisms, and apoptosis in a rat model of restraint stress in 40 adult male Wistar rats. Due to the anxiolytic effects of Sutherlandia frutescens, we also determined if any of the molecular events in gastrocnemius muscle would be affected by daily treatment with S. frutescens. Rats were randomly assigned to four experimental groups: control placebo (CP); control Sutherlandia (CS) treatment; Restraint Placebo (RP) and Restraint Sutherlandia (RS) treatment. Restraint resulted in a significant increase in myostatin which was significantly reduced with Sutherlandia treatment. In addition, MyoD expression was significantly attenuated in RP and this effect was also counteracted by Sutherlandia treatment. Restraint also resulted in a significant attenuation of the PI3-Kinase/Akt signaling pathway and increased apoptosis which was reversed with Sutherlandia treatment. This study demonstrates for the first time that psychological stress elevates markers of muscle atrophy and apoptosis, whilst a herbal remedy, Sutherlandia, inhibits apoptosis, and signaling pathways associated with muscle atrophy.
Introduction
Glucocorticoids have numerous homeostatic and stress response functions. A delicate balance exists between the protective effects of secreted glucocorticoid in response to stressful experiences, and the negative consequences that glucocorticoid may have on many target tissues. For example, in skeletal muscle, glucocorticoid deficiency leads to debilitating fatigue, myalgia, and general muscle weakness (Orth and Kovacs Citation1998), whereas excess glucocorticoid causes decreased rates of protein synthesis (Bullock et al. Citation1972), excess protein catabolism (Kayali et al. Citation1987; Attaix et al. Citation1998; Combaret et al. Citation2004), and muscle atrophy (Hickson et al. Citation1995; Pellegrino et al. Citation2004). The loss of muscle protein due to excessive glucocorticoid levels is a feature of immobilization, inactivity, and various diseases (Zamecnik et al. Citation2008). The functional and morphological consequences common to atrophy are well known and include decreased muscle fiber cross-sectional area (Uchikawa et al. Citation2008) and increased insulin resistance (Kandarian and Jackman Citation2006). However, atrophy is not only associated with disease. Aging is associated with both obesity and sarcopenia (Zamboni et al. Citation2008) and employees in various job situations report work-associated psychological stress (Lundberg Citation1999; McCann et al. Citation2009) which may contribute to central fat deposition (Epel et al. Citation2000) and muscle atrophy.
Multiple processes related to atrophic events may contribute to the reduction of muscle fiber size and potential cellular role players include myostatin and p38 MAPK (Tracey Citation2002). Myostatin, a TGF-β family member, is involved in several forms of muscle wasting in adulthood (Durieux et al. Citation2008). The activity of p38 in skeletal muscle is elevated during procatabolic states, which include limb immobilization (Childs et al. Citation2003), acute quadriplegic myopathy, neurogenic atrophy (Di Giovanni et al. Citation2004) and milder conditions, such as physiological aging (Williamson et al. Citation2003). Apoptosis may play a role in muscle atrophy, however in skeletal muscle this is a complicated scenario because single muscle cells contain thousands of myonuclei and apoptosis assists only in the elimination of segments and not of the entire fiber (Allen et al. Citation1999; Borisov and Carlson Citation2000). A decrease in the mean number of myonuclei per fiber has been reported in atrophying muscle under several experimental conditions (Allen et al. Citation1997; Fitts et al. Citation2001). Furthermore, although the expression of p53 is well conserved in mature muscle (Jin et al. Citation2001), it has been hypothesized that p53 may also be related to the activation of apoptosis during muscle disuse-atrophy (Siu and Alway Citation2005a, Citation2005b).
Skeletal muscle mass is a function of the balance between anabolic and catabolic stimuli. These may occur simultaneously, even in the face of denervation-induced atrophy (Borisov et al. Citation2001). Myogenesis was evident within 2 weeks of denervation and was still evident 2–4 months later. However, MyoD, a myogenic regulatory factor that acts as a master transcriptional switch for muscle differentiation and development, has recently been shown to be reduced as a common catabolic consequence (Sun et al. Citation2008).
The phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway is now recognized as one of the most critical pathways involved in regulation of cell viability and maintenance of skeletal muscle mass (Brunet et al. Citation1999; Song et al. Citation2005). Akt is a serine/threonine kinase which is mostly activated in response to survival signals from growth factors via mechanisms involving PI3K. Once Akt is fully activated it dissociates from the plasma membrane and phosphorylates both cytoplasmic and nuclear target proteins which include FOXO proteins, a subgroup of the Forkhead transcription factors. Phosphorylation of FOXO proteins by Akt promotes FOXO sequestration by 14-3-3 proteins in the cytoplasm leading to inhibition of their transcriptional functions. In contrast, dephosphorylation of FOXO leads to nuclear entry and growth suppression or apoptosis (Brunet et al. Citation1999; Tran et al. Citation2003). No studies have investigated these pathways in skeletal muscle in the context of their potential roles in psychological stress-induced atrophy.
Medicinal plants are widely used throughout the African continent for healthcare, despite an apparent lack of scientific evidence for their quality, safety, and efficacy (Johnson et al. Citation2007). One such therapeutically useful plant is Sutherlandia frutescens, which contains several biologically active chemicals including l-canavinine, pinitol, gamma-aminobutyric acid (GABA), methyl and propyl parabens, saponins, as well as chemicals with unknown effects on human physiology including sigma-4-en-3-one and gamma sisterol (Gericke et al. Citation2001; Mills et al. Citation2005; Johnson et al. Citation2007). It has been previously commented that the bioactivity of S. frutescens might be attributed to synergistic effects of several compounds present in S. frutescens extracts, rather than a single compound, resulting in a relative complex mechanism of action (Tai et al. Citation2004).
Sutherlandia may elicit anti-atrophy effects via more than one mechanism. Firstly, pinitol is a known treatment for wasting in cancer and AIDS, resulting in increased availability of glucose for cell metabolism (Bates et al. Citation2000). Secondly, the inhibitory neurotransmitter GABA is well known for its anti-anxiety action (Johnston Citation2005), characterized by decreased glucocorticoid production. In vitro studies indicate that Sutherlandia also suppresses adrenal steroidogenesis via inhibition of the P450 enzyme (Prevoo et al. Citation2004). Therefore, its potential to inhibit atrophy may be to reduce exposure to excess glucocorticoids, or through a direct effect on muscle signaling, or both.
The aims of this study were therefore twofold: (1) to determine the effects of brief daily exposure to psychological stress on muscle atrophy and related intracellular signaling and (2) to determine the effects of daily treatment with an extract of S. frutescens ssp. microphylla on signaling events and apoptosis in skeletal muscle, in a rat model of chronic restraint stress.
Materials and methods
Ethical approval for this project was obtained from the research ethics committee of the University of Stellenbosch (Faculty of Natural Science) and conforms to the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health (NIH publication no. 85-23, revised 1985).
Experimental animals
Forty adult male Wistar rats (average body mass of 339 ± 40 (mean ± SEM) g at the start of the intervention protocol) were used in this study. All rats were housed in groups of four in standard, cages with a 12 h:12 h light:dark cycle (lights on 07.00 h). The animals were fed standard rat chow and tap water ad libitum. All rats were allowed 4 weeks to acclimatize after transport to the animal house, before the start of the stress intervention and supplementation protocols. Rats were separated into four mass-matched groups (10 rats/group). Two groups represented the controls. One of these received treatment with Sutherlandia extract (control Sutherlandia, CS), while the other received isotonic saline (control placebo, CP) for the same period. The third and fourth groups were subjected to chronic restraint stress in addition to receiving either Sutherlandia extract (restraint Sutherlandia, RS) or isotonic saline (restraint placebo, RP).
Procedures
All rats were weighed and handled once a day for 3 weeks before the start of the restraint protocol to accustom them to the investigators, preventing stress caused by handling from being a confounding factor in this study. Restraint was achieved by placing rats individually into small Perspex cages (8 × 6 × 18 cm) designed for this purpose, which did not allow free movement, but allowed normal respiration via ventilation holes. The duration of restraint was 2 h, once daily during the lights-on cycle, between 09.00 and 11.00 h, for 28 consecutive days.
Commercially available Sutherlandia is sold as 700 mg tablets, each containing 300 mg Sutherlandia leaf powder. Manufacturers of these tablets recommended a dose of one tablet twice daily. This recommended dose for the commercial product equals 9 mg per kg body mass for humans. This corresponds to a daily dose of about 3.4 mg per rat, which was rounded up to 4 mg per rat, to allow for weight gain during the study period. Specimens of Sutherlandia were harvested in the vicinity of Murraysburg, western Cape province, South Africa by W. Grobler. The plants were identified as S. frutescens subspecies microphylla by B.E. van Wyk of the Botany Department, Rand Afrikaans University [voucher specimen form W. Grobler: C. Albrecht s.n sub. B.E. van Wyk 4126 (JRAU)]. Since the traditional way of administration is in the form of a herbal infusion, a warm water extract was prepared; sterile boiling water was added to Sutherlandia leaves (8 mg/ml) and left to infuse overnight at room temperature. The infusion was not filtered, as this might have resulted in the removal of an active component, but poured through a sieve to remove any big leaf particles. Thereafter, the extract was diluted 1:2 with 1.7% saline to produce an extract of 4 mg/ml in isotonic saline. Placebo consisted of a solution of sterile 0.85% saline. All rats were subjected to intraperitoneal injections twice per day at 07.00 and 19.00 h, of either 0.5 ml of 0.85% saline (CP and RP) or 0.5 ml of Sutherlandia extract (CS and RS).
Sample collection
At the end of the protocol, all rats were killed between 11:00 and 13:00 h by decapitation, without anesthesia; rats were not restrained, nor injected on the day of sacrifice, to prevent the possible confounding effect of an acute stress response. The rats were taken from the housing cage one at a time, placed into a weighing basket and carried to another room, where they were weighed and then decapitated. Trunk blood was collected into serum separation tubes (Vacutainers; BD Vacutainer Systems, Plymouth, UK) used for assessment of circulating hormone and cytokine levels, presented elsewhere (Smith and Myburgh Citation2004). The right gastrocnemius muscles were removed, and divided into two pieces by a medial section through the mid-belly. One piece was snap-frozen in liquid nitrogen, and stored at − 80°C for subsequent analyses. The other piece was frozen embedded in mounting medium (Tissue-Tek OCT, Miles, Naperville, IL, USA) and stored at − 80°C for subsequent histological and morphometric analysis.
Histology and morphometry
From the medial part of the gastrocnemius muscle, cryosections of 10 μm were made at − 18°C, mounted onto slides with poly-l-lysine and then stained with haematoxylin and eosin. Digital images of the distal part of the medial gastrocnemius muscle (fast twitch, glycolytic fibers) were taken using a 40 × objective and fibers were analyzed semi-automatically for cross-sectional area using image analysis software (Simple PCI). A minimum of 75 and maximum of 200 fibers per sample were counted. Average number (range) of fibers per rat for each group was: CS 103(79–186), RS 139(111–190), RP 112(93–132) and CP 119(107–126). Mean fiber cross-sectional area measurements for each individual rat were used in the statistical analysis (ANOVA and Bonferroni post hoc tests).
Western-blot analysis
Skeletal muscle proteins were extracted with a lysis buffer, pH 7.4, containing (in mM): Tris 20, p-nitrophenylphosphate 20, EGTA 1, sodium fluoride (NaF) 50, sodium orthovanadate 0.1, phenylmethyl sulfonyl fluoride (PMSF) 1, dithiothreitol (DTT) 1, aprotinin 10 μg/ml, leupeptin 10 μg/ml. Mitogen-activated protein kinase phosphatase-1 (MKP-1) protein was extracted with a lysis buffer, pH 7.4 containing (in mM): Hepes 50, EDTA 10, EGTA 10, PMSF 1, Aprotinin (1 ug/ml), Leupeptin (1 ug/ml), and Triton (0.5%). The tissue lysates were diluted in Laemmli sample buffer, boiled for 5 min and 20 μg protein for kinases, or 50 μg protein (caspase-3, PARP) were separated by 10% PAGE-SDS-gel electrophoresis. The lysate protein content was determined using the Bradford technique (Bradford Citation1976). The separated proteins were transferred to a PVDF membrane (ImmobilonTM P, Millipore, Bedford, MA, USA), which were routinely stained with Ponceau Red for visualization of proteins and stripped and reprobed with anti-actin antibody to ensure equal loading. Non-specific binding sites on the membranes were blocked with 5% fat-free milk in Tris-buffered saline-0.1% Tween 20 (TBST) and then incubated with the primary antibodies that recognize phosphospecific and total ERK p42/44 (Thr202/Tyr204), p38-MAPK (Thr180/Tyr182), JNK p54/46 (Thr183/Tyr185), PTEN, PKB Ser473, PKB Thr308, CREB, FOX01, MyoD, myostatin, PI3-K p85 and p110, caspase-3 (p17 fragment pAb), and PARP (p85 fragment pAb). All antibodies were obtained from Cell Signaling Technology. Membranes were subsequently washed with large volumes of TBST (5 × 5 min) and the immobilized antibody conjugated with a diluted horseradish peroxidase-labelled secondary antibody (Amersham LIFE SCIENCE, Arlington Heights, IL, USA). After thorough washing with TBST, membranes were covered with ECLTM detection reagents and quickly exposed to an autoradiography film (Hyperfilm ECL, RPN 2103) to detect light emission through a non-radioactive method (ECLTM western blotting). Films were densitometrically analysed (UN-SCAN-IT, Silkscience, Orem, UT, USA) and phosphorylated protein values were corrected for minor differences in protein loading, if required. Experiments were performed to ensure that all signals were within the linear range of detection on the autoradiograph films under our assay and gel loading conditions (data not shown).
DNA agarose gel eletrophoresis
The method used is an adaptation of the method published by Tilly and co-workers (Citation1991). The fragments of gastrocnemius muscle were homogenized in 400 μl of 0.1 M NaCl, 10 mM EDTA, 0.3 M Tris-HCl, pH 8, 0.2 M sucrose, and 0.01% SDS in Eppendorf tubes and incubated for 1 h in a water bath at 65°C. Next, 70 μl of 8 M potassium acetate was added and the preparation was incubated on ice for 60 min and centrifuged at 3000 g for 10 min at 4°C. The upper phase was transferred to a new tube and DNA extraction was performed with 1 volume of phenol:chloroform:isoamylalcohol solution (25:24:1) and precipitated with two volumes of 100% ethanol overnight at − 70°C. Ten microgram of DNA was electrophoresed on a 2% agarose gel with ethidium bromide and the DNA bands were photographed by UV translumination.
Statistical analysis
All results are expressed as means ± SEM. One-way ANOVA was performed for each group of treatments, using Bonferroni's post hoc test. P values < 0.05 were regarded as significant. The number of experiments is indicated in the figure legends, where applicable. The degrees of freedom for all experiments were 35 (treatment between columns = 3 and residual within columns = 32).
Results
The effect of restraint and Sutherlandia treatment on body mass and fiber size as assessed in gastrocnemius muscle
All experimental groups had similar average body mass at the start of the restraint protocol, and maintained normal growth curves throughout the protocol, resulting in all groups still having similar average body mass on the day they were killed. However, in the RP group only, gastrocnemius muscle fiber cross-sectional area was significantly smaller (p < 0.01) when compared to all other groups ().
Table I. The effect of Sutherlandia treatment and daily brief restraint in rats on body mass and muscle fiber size.
The effect of restraint and Sutherlandia treatment on myostatin in gastrocnemius muscle
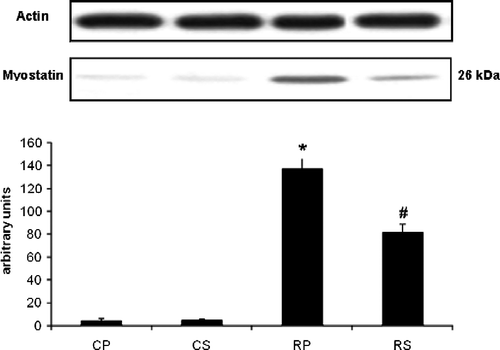
Basal levels of myostatin in both the control groups (CP and CS) were low. Restraint significantly increased myostatin levels (∼36-fold vs. CP, p < 0.001) which was attenuated by Sutherlandia treatment (∼15-fold vs. RP, p < 0.001; ).
Figure 1 The effect of restraint and Sutherlandia supplementation on myostatin in gastocnemius muscle. Samples were analysed by western blotting with antibodies recognizing myostatin. Results are expressed as means ± SEM for eight independent experiments, *p < 0.001 vs. CP; #p < 0.001 vs. RP, F = 86.86. CP, control placebo; CS, control Sutherlandia; RP, restraint placebo; RS, restraint Sutherlandia.
The effect of restraint and Sutherlandia treatment on MyoD in gastrocnemius muscle
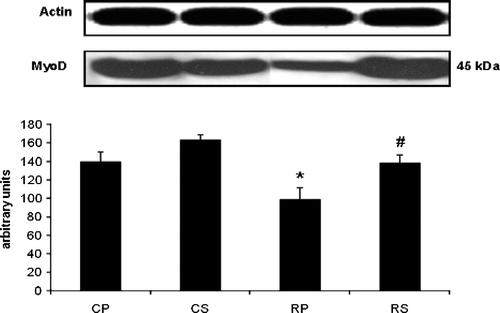
Restraint significantly attenuated MyoD (∼30% decrease vs. CP, p < 0.05); an effect which was abolished after Sutherlandia treatment (∼30% increase vs. RP, p < 0.05; ).
Figure 2 The effect of restraint and Sutherlandia supplementation on MyoD in gastocnemius muscle. Samples were analysed by western blotting with antibodies recognizing MyoD. Results are expressed as means ± SEM for eight independent experiments, *p < 0.05 vs. CP; #p < 0.05 vs. RP, F = 7.298. CP, control placebo; CS, control Sutherlandia; RP, restraint placebo; RS, restraint Sutherlandia.
The effect of restraint and Sutherlandia treatment on p38 MAPK phosphorylation in gastrocnemius muscle
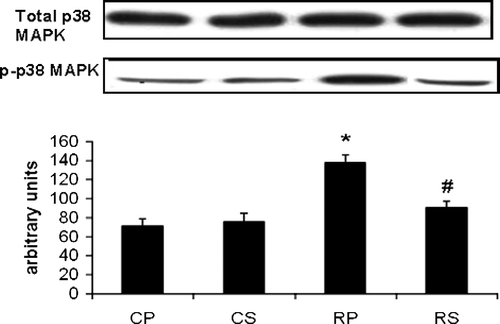
Restraint significantly increased p38 (∼94% increase vs. CP, p < 0.001) while Sutherlandia treatment significantly attenuated p38 phosphorylation in restrained rats to almost reach control (CS) levels (67% decrease vs. RP, p < 0.01; ).
Figure 3 The effect of restraint and Sutherlandia supplementation on p38-MAPK phosphorylation in gastocnemius muscle. Samples were analysed by western blotting with antibodies recognizing the dual phosphorylated MAPK. Results are expressed as means ± SEM for eight independent experiments, *p < 0.001 vs. CP; #p < 0.01 vs. RP, F = 15.5. CP, control placebo; CS, control Sutherlandia; RP, restraint placebo; RS, restraint Sutherlandia.
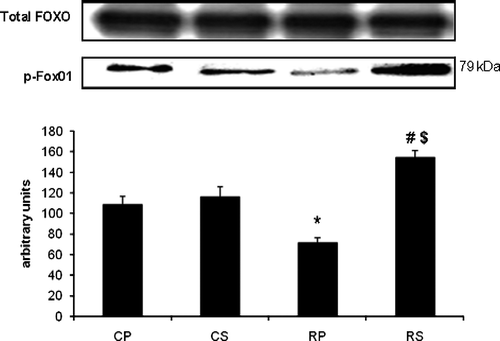
The effect of restraint and Sutherlandia treatment on the PI3-Kinase/Akt signaling pathway in gastrocnemius muscle
Restraint significantly decreased p-Akt (∼52% decrease vs. CP, p < 0.001) while Sutherlandia treatment significantly increased Akt phosphorylation (∼30% increase vs. RP, p < 0.01). FOX01, a substrate of Akt, showed the same phosphorylation pattern as observed with Akt. There was a significant decrease in FOX01 phosphorylation in the restraint group (∼35% decrease vs. CP, p < 0.01) which was counteracted by Sutherlandia treatment (∼76% increase vs. RP, p < 0.001). Interestingly, the increase in FOX01 in the RS group was also significantly higher compared with the CS group (∼35% increase vs. CS, p < 0.01; ).
Figure 4 The effect of restraint and Sutherlandia supplementation on Akt phosphorylation in gastocnemius muscle. Samples were analysed by western blotting with antibodies recognizing phospho- and total Akt. Results are expressed as means ± SEM for eight independent experiments, *p < 0.001 vs. CP; #p < 0.01 vs. RP, F = 16.36. CP, control placebo; CS, control Sutherlandia; RP, restraint placebo; RS, restraint Sutherlandia.
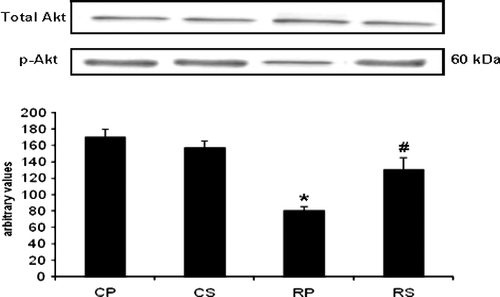
Figure 5 The effect of restraint and Sutherlandia supplementation on FKHR phosphorylation in gastocnemius muscle. Samples were analysed by western blotting with antibodies recognizing phospho- and total FKHR. Results are expressed as means ± SEM for eight independent experiments, *p < 0.001 vs. CP; #p < 0.01 vs. RP, $p < 0.01 vs. CS, F = 20.59. CP, control placebo; CS, control Sutherlandia; RP, restraint placebo; RS, restraint Sutherlandia.
The effect of restraint and Sutherlandia treatment on parameters of apoptosis in gastrocnemius muscle
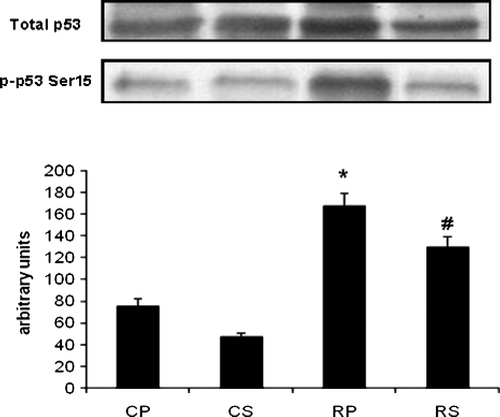
Restraint significantly increased caspase-3 cleavage in gastrocnemius muscle (∼40% increase vs. CP, p < 0.05) and Sutherlandia treatment significantly attenuated caspase-3 cleavage (∼90% decrease vs. RP, p < 0.001). The anti-apoptotic effect was also observed in the DNA ladder pattern where no indication of apoptosis was observed in both of the Sutherlandia groups. p53 is another potent regulator of apoptosis which was significantly upregulated in the restrained group (∼123% increase vs. CP, p < 0.001) and also significantly inhibited by Sutherlandia treatment (∼51% decrease vs. RP, p < 0.05; ).
Figure 6 (A) The effect of restraint and Sutherlandia supplementation on cleavage of caspase-3 in gastocnemius muscle. Samples were analysed by western blotting with antibodies recognizing cleaved caspase-3. Results are expressed as means ± SEM for eight independent experiments, *p < 0.05 vs. CP; #p < 0.001 vs. RP, F = 13.03. CP, control placebo; CS, control Sutherlandia; RP, restraint placebo; RS, restraint Sutherlandia. (B) The effect of restraint and Sutherlandia supplementation on DNA fragmentation in gastocnemius muscle. Lane 1: DNA sample from the RP group showing a ladder pattern, lane 2: DNA samples from the CS group, lane 3: DNA samples from the RS group and lane 4: DNA samples from the CP group. CP, control placebo; CS, control Sutherlandia; RP, restraint placebo; RS, restraint Sutherlandia.
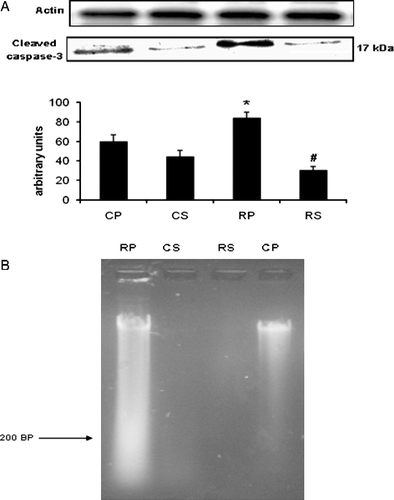
Figure 7 The effect of restraint and Sutherlandia supplementation on p53 phosphorylation in gastocnemius muscle. Samples were analysed by western blotting with antibodies recognizing phospho- and total p53. Results are expressed as means ± SEM for eight independent experiments, *p < 0.001 vs. CP; #p < 0.05 vs. RP, F = 41.36. CP, control placebo; CS, control Sutherlandia; RP, restraint placebo; RS, restraint Sutherlandia.
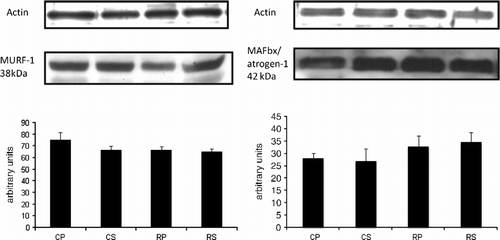
The effect of restraint and Sutherlandia treatment on two muscle specific E3 ligases in gastrocnemius muscle
The ubiquitin–proteasome pathway is one of the major regulatory systems of muscle mass (Taillandier et al. Citation2004). Proteins that are degraded by the ubiquitin–proteasome are first covalently bound to ubiquitin, a process which is partly regulated by ubiquitin (E3) ligases. MURF-1 and atrogin-1 are examples of E3 ligases that are involved in skeletal muscle atrophy (Bodine et al. Citation2001; Gomes et al. Citation2001). MURF-1 and atrogin-1 levels remained unchanged after restraint stress ().
Figure 8 The effect of restraint and Sutherlandia supplementation on muscle RING finger 1 (MURF-1) and muscle atrophy F-box (MAFbx)/atrogin-1 in gastocnemius muscle. Samples were analysed by western blotting with antibodies recognizing MURF-1 and MAFbx/atrogin-1. Results are expressed as means ± SEM for eight independent experiments. F = 1.113 for MURF-1 and F = 0.7410 for MAFbx. CP, control placebo; CS, control Sutherlandia; RP, restraint placebo; RS, restraint Sutherlandia.
Discussion
Members of our group have previously demonstrated that intermittent restraint stress induced in rats could significantly increase basal serum corticosterone levels. Unpublished data by our group indicate that corticosterone levels may increase up to 10-fold from baseline (to a level of ≈ 80–100 ng/ml) after an acute restraint stress exposure, and does not return to baseline levels within 24 h after cessation of stress exposure. It was also shown that Sutherlandia treatment decreased the corticosterone response to chronic intermittent restraint stress in these animals when compared to placebo-supplemented rats as controls (Smith and Myburgh Citation2004). The first aim of this study therefore was to determine whether this model of intermittent restraint stress could also induce a molecular response in the gastrocnemius muscle of the rats; and secondly to determine whether Sutherlandia treatment could counteract the stress response induced in the muscle.
We demonstrated that intermitted restraint stress, while having no effect on total body mass or growth, resulted in significant atrophy of the gastrocnemius muscle (). The finding that a significant decrease in muscle fiber cross-sectional area of the gastrocnemius muscle in the placebo restraint group was not associated with a significant loss of body mass, may be explained by possible concomitant increases in fat mass, although this was not assessed. A recent study in human subjects (Brooks et al. Citation2008) using a model of spaceflight indicated reductions in mid-thigh muscle size assessed by CT scans with increases in fat mass and intramuscular fat, but no change in body mass. Although their study was an extreme model of unloading, it also lasted 28 days which is sufficient time to allow for substantial body composition redistribution. Our data illustrate a significant increase in myostatin in rat gastrocnemius muscle in the RP group and that this increase was significantly attenuated with Sutherlandia treatment (). Durieux and co-workers (2007) have demonstrated that in vivo overexpression of myostatin induces severe atrophy in adult skeletal muscle. Furthermore, Ma and co-workers (Citation2003) also demonstrated that glucocorticoid administration to rats dose-dependently induces up to a 4-fold increase in intramuscular myostatin protein expression. We therefore postulate that the increased myostatin levels induced by our stress model are also associated with the induction of atrophy and that Sutherlandia treatment partly counteracted this atrophy response of myostatin.
MyoD is another transcription factor which needs to be tightly regulated to achieve normal muscle structure and function. The transcriptional activities of MyoD are negatively regulated by a family of inhibitors of DNA-binding (Id) proteins. It has recently been shown that glucocorticoids, via the glucocorticoid receptors, selectively induce a decrease in protein abundance of MyoD, but not of its negative regulator Id1. This decrease in MyoD resulted from accelerated degradation after glucocorticoid exposure (Sun et al. Citation2008). The decreased levels of MyoD in the RP group observed in our results might also be indicative of accelerated protein catabolism induced by high corticosterone levels in the stressed rats. This effect was totally reversed by Sutherlandia supplementation in the RS group.
p38 MAPK is a stress-activated protein kinase that has been identified as a likely mediator of catabolic signaling in skeletal muscle (Tracey Citation2002). This is consistent with our results where increased phosphorylation of p38 MAPK was observed in the RP group compared with the control group. Sutherlandia treatment attenuated p38 phosphorylation to control levels. Although it was demonstrated by Kewalramani and co-workers (Citation2008) that dexamethasone induces a rapid p38 MAPK response in cardiomyocytes, no evidence exists in the literature for glucocorticoid inducing an increase in p38 MAPK phosphorylation in skeletal muscle. Di Giovanni and co-workers (Citation2004) also reported elevated p38 phosphorylation in atrophic muscles of patients with acute quadriplegic myopathy. The involvement of p38 in muscle atrophy was further supported by the results of Li and co-workers (Citation2005) who demonstrated that the atrogin1/MAFbx gene is a downstream target of p38 MAPK signaling. The current results indicate that only brief daily exposure to a stressor is sufficient to elicit this response.
Glucocorticoids can cause muscle atrophy by inhibiting the growth factor related signaling pathways which include the PI3-kinase pathway (Gayan-Ramirez et al. Citation1999; Gilson et al. Citation2007). An intriguing aspect of PI3-K/Akt signaling is the wide range of cellular functions that is mediated by these kinases. It was also demonstrated by Amirouche and co-workers (2009) that myostatin overexpression induced atrophy in tibialis anterior muscle through negative regulation of the Akt/mTOR signaling pathway. Although the PI3-Kinase/Akt signaling pathway is linked with both synthesis and degradation of muscle proteins, recent data indicate that the Akt/mTORC1 pathway specifically controls protein synthesis, whereas the Akt/mTORC2 pathway controls protein degradation (Zhao et al. Citation2007; Mammucari et al. Citation2007). mTOR is part of two multiprotein complexes: mTORC1 contains raptor, is rapamycin sensitive and is required for signaling to S6K and 4E-BP1; mTORC2 contains rictor and is required for signaling to FOXO (Guertin et al. Citation2006). Our results demonstrated a significant decrease in Akt phosphorylation in the RP group compared with the control group and a significant increase in phosphorylation in the Sutherlandia-treated group.
Interestingly, although there was a significant decrease in FOX01 phosphorylation in the RP group, the increase in phosphorylation in the RS-treated group was significantly higher compared with both the control groups without restraint as well as the with the RP group. The inactivation of FOXO through phosphorylation in the RS group confirms the protective effect of S. frutescens against FOXO-induced muscle proteolysis. Furthermore, our results are in accordance with the work of Sandri and co-workers (Citation2004) who demonstrated that C2C12 myotubes treated with dexamethasone resulted in dephosphorylation of FOX01.
Although apoptosis is necessary for maintaining the integrity of highly proliferative tissue such as epithelial cells, the role of apoptosis in post-mitotic tissue such as skeletal muscle is less well defined. Apoptosis of myonuclei likely contributes to the loss of muscle mass, but the mechanisms underlying this process are largely unknown. The fact that apoptosis plays an important role in skeletal muscle atrophy can be deduced from the observation that it is increased in a number of pathological and physiological conditions such as muscular dystrophy, motor neuron disorders, spinal cord injury and skeletal muscle atrophy due to hind limb restraint. A hallmark of the apoptotic pathway is the cleavage of caspase-3. Restraint significantly increased caspase-3 cleavage in our model and this was attenuated by Sutherlandia treatment. Apparently normal protein turnover in skeletal muscle under healthy conditions does not seem to be linked with caspase-3 mediated protein breakdown; however, in catabolic conditions the excessive myofibrillar protein breakdown can be blocked by the caspase inhibitor, Ac-DEVD-CHO. The PI3-Kinase/Akt pathway is also linked to caspase-3. Activation of caspase-3 is associated with a suppressed activity of this kinase (Lee et al. Citation2004). Thus, when PI3-Kinase/Akt activity is low, both the apoptotic and ubiquitin–proteasome proteolysis pathways are activated, suggesting that the PI3-Kinase/Akt participates in the inhibition of caspase-3 (Argilés et al. Citation2008), as we have also seen here in response to regular, brief episodes of psychological stress.
Apart from its roles in protein anabolism and catabolism, the PI3-Kinase/Akt signaling pathway can also inhibit apoptosis by preventing the nuclear localization of the transcription factor, p53. Akt binds to and phosphorylates MDM2 on Ser166 and Ser186, thereby inducing its nuclear import or increasing its ubiquitin ligase activity (Mayo and Donner Citation2001). MDM2 is greatly involved in the inactivation of p53 and, therefore, any increase in nuclear MDM2 activity will result in inactivation of the pro-apoptotic transcriptional function of p53. Restraint stress caused a significant increase in p53 phosphorylation in our model, which was counteracted by Sutherlandia treatment. This is also supported by the results of Ferreira and co-workers (Citation2007) who demonstrated elevated p53 protein content in parallel with the upregulation of Bax (pro-apoptotic protein) in soleus muscle under simulated weightlessness conditions, suggesting the involvement of p53 in skeletal muscle apoptosis, despite its multinucleated cellular morphology.
Interestingly, no significant differences in MURF-1 and atrogin-1 were observed between the groups (). This might be attributed to the levels of MURF-1 and atrogin-1 returning to baseline after four weeks in our model, as also shown in a model of denervation-induced atrophy (Sasheck et al. Citation2007).
In conclusion, the present study demonstrated for the first time that S. frutescens has stress-relieving properties which counteract the detrimental effects of intermittent restraint on signaling events in rat skeletal muscle. Although literature indicates that most of the observed effects on signaling molecules in our model of restraint stress in skeletal muscle can be induced by glucocorticoids, there is no direct evidence in the literature for the phosphorylation p53 and cleavage of caspase-3 by glucocorticoids. Therefore, although the current data and the literature suggest that the majority of this effect may be mediated by reducing circulating glucocorticoid, this does not rule out potential localized effects of some components of the herb.
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V, Edgerton VR. 1997. Apoptosis: A mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol. 273:C579–C587.
- Allen DL, Roy RR, Edgerton VR. 1999. Myonuclear domains in muscle adaptation and disease. Muscle Nerve. 22 10: 1350–1360.
- Argilés JM, López-Soriano FJ, Busquets S. Apoptosis signaling is essential and precedes protein degradation in wasting skeletal muscle during catabolic conditions. Int J Biochem Cell Biol. 2008 doi: 101016/jbiocel200802001.
- Attaix D, Aurousseau E, Combaret L, Kee A, Larbaud D, Ralliere C, Souweine B, Taillandier D, Tilignac T. 1998. Ubiquitin–proteasome dependent proteolysis in skeletal muscle. Reprod Nutr Dev. 38:153–165.
- Bates SH, Jones RB, Bailey CJ. 2000. Insulin-like effect of pinitol. Br J Pharmacol. 130:1944–1948.
- Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA. 2001. Identification of ubiquitin ligases for skeletal muscle atrophy. Science. 294:1704–1708.
- Borisov AB, Carlson BM. 2000. Cell death in denervated skeletal muscle is distinct from classical apoptosis. Anat Rec. 258:305–318.
- Borisov AB, Dedkov EL, Carlson BM. 2001. Interrelations of myogenic response, progressive atrophy of muscle fibers, and cell death in denervated skeletal muscle. Anat Rec. 264:203–218.
- Bradford MM. 1976. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 71:248–254.
- Brooks N, Cloutier GJ, Cadena SM, Layne JE, Nelsen CA, Freed AM, Roubenoff R, Castaneda-Sceppa C. 2008. Resistance training and timed essential amino acids protect against the loss of muscle mass and strength during 28 days of bed rest and energy deficit. J Appl Physiol. 105 1: 241–248.
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 96:857–868.
- Bullock GR, Carter EE, Elliot P, Peters RF, Simpson P, White AM. 1972. Relative changes in the function of muscle ribosome and mitochondria during the early phase of steroid-induced catabolism. Biochem J. 127:881–892.
- Childs TE, Spangenburg EE, Vyas DR, Booth FW. 2003. Temporal alterations in protein signaling cascades during recovery form muscle atrophy. Am J Physiol. 285:C391–C398.
- Combaret L, Taillandier D, Dardevet D, Bechet D, Ralliere C, Claustre A, Grizard J, Attaix D. 2004. Glucocorticoids regulated mRNA levels for subunits of the 19 S regulatory complex of the 26 S proteasome in fast-twich skeletal muscles. Biochem J. 378:239–246.
- Di Giovanni S, Molon A, Broccolini A, Melcon G, Mirabella M, Hoffman EP, Servidei S. 2004. Constitutive activation of MAPK cascade in acute quadriplegic myopathy. Ann Neurol. 55:195–206.
- Durieux A-C, Amirouche A, Banzet S, Koulmann N, Bonnefoy R, Pasdeloup M, Mouret C, Bigard X, Peinnequin A, Freyssenet D. 2008. Ectopic expression of myostatin induces atrophy of adult skeletal muscle by decreasing muscle gene expression. Endocrinology. 148 7: 3140–3147.
- Epel ES, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD, Bell J, Ickovics JR. 2000. Stress and body shape: Stress-induced cortisol secretions is consistently greater among women with central fat. Psychosom Med. 62 5: 623–632.
- Ferreira R, Vitorina R, Neuparth MJ, Appell H-J, Amado F, Duarte JA. 2007. Cellular patterns of the atrophic response in murine soleus and gastrocnemius muscles submitted to simulated weightlessness. Eur J Appl Physiol. 101:331–340.
- Fitts RH, Riley DR, Widrick JJ. 2001. Functional and structural adaptations of skeletal muscle to microgravity. J Exp Biol. 204:3201–3208.
- Gayan-Ramirez G, Vanderhoydonc F, Verhoeven G, Decramer M. 1999. Acute treatment with corticosteroids decreases IGF-1 and IGF-2 expression in the rat diaphragm and gastrocnemius. Am J Resp Crit Care Med. 159:283–289.
- Gericke N, Albrecht CF, van Wyk BE, Mayeng B, Mutwa C, Hutchings A. 2001. Sutherlandia frutescens. Aust J Med Herb. 13:9–15.
- Gilson H, Schakman O, Combaret L, Lause P, Grobet L, Attaix D, Ketelslegers JM, Thissen JP. 2007. Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Endocrinology. 148:452–460.
- Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. 2001. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA. 98:14440–14445.
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. 2006. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 11:859–871.
- Hickson RC, Czerwinski SM, Wegrzyn LE. 1995. Glutamine prevents downregulation of myosin heavy chain synthesis and muscle atrophy from glucocorticoids. Am J Physiol. 268:E730–E734.
- Jin H, Wu Z, Tian T, Gu Y. 2001. Apoptosis in atrophic skeletal muscle induced by brachial pelxus injury in rats. J Trauma. 50:31–35.
- Johnson Q, Syce J, Nell H, Rudeen K, Folk WR. 2007. A randomized, double-blind, placebo-controlled trial of Lessertia frutescens in healthy adults. PLoS Clin Trials. 2 4: 1–7.
- Johnston GAR. 2005GABAA Receptor channel pharmacology. Curr Pharm Designs. 11:1867–1885.
- Kandarian SC, Jackman RW. 2006. Intracellular signaling during skeletal muscle atrophy. Muscle and Nerve. 33:155–165.
- Kayali AG, Young VR, Goodman MN. 1987. Sensitivity of myofibrillar proteins due to glucocorticoid-induced muscle proteolysis. AM J Physiol. 252:E621–E626.
- Kewalramani G, Puthanveetil P, Kim MS, Wang F, Lee V, Hau N, Behesthi E, Ng N, Abrahani A, Rodrigues B. 2008. Acute dexamethasone-induced increase in cardiac lipoprotein lipase requires activation of both Akt and stress kinases. Am J Physiol Endocrinol Metab. 295 1: E137–E147.
- Lee SW, Dai G, Hu Z, Wang X, Du J, Mitch WE. 2004. Regulation of muscle protein degradation: Coordinated control of apoptotic and ubiquitin–proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol. 15:1537–1545.
- Li Y-P, Chen Y, John J, Moylan J, Jin B, Mann DL, Reid MB. 2005. TNF-α acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin 1/MAFbx in skeletal muscel. FASEB J. 19:362–370.
- Lundberg U. 1999. Stress responses in low-status jobs and their relationship to health risks: Musculoskeletal disorders. Ann NY Acad Sci. 896:162–167.
- Mammucari C, Giulia M, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. 2007. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 6:458–471.
- Mayo LD, Donner DB. 2001. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci USA. 98:11598–11603.
- Ma K, Mallidis C, Bhasin S, Mahabadi V, Artaza J, Gonzalez-Cadavid N, Arias J, Salehian B. 2003. Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am J Physiol. 285:E363–E371.
- McCann L, Hughes CM, Adair CG, Cardwell C. 2009. Assessing job satisfaction and stress among pharmacists in Northern Ireland. Pharm World Sci. 31 2: 188–194.
- Mills E, Cooper C, Seely D, Kanfer I. 2005. African herbal medicines in the treatment of HIV: Hypoxis and Sutherlandia. An overview of evidence and pharmacology. Nutr J. 19:1–6.
- Orth DN, Kovacs WJ. 1998. The adrenal cortex. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR, editors. Williams textbook of endocrinology. Philadelphia: WB Saunders. 517–564.
- Pellegrino MA, D'Antona G, Bortolotto S, Boschi F, Pastoris O, Botttinelli R, Polla B, Reggiani C. 2004. Clenbuterol antagonizes glucocorticoid-induced atrophy and fiber type transformation in mice. Exp Physiol. 89:89–100.
- Prevoo D, Smith C, Swart P, Swart AC. 2004. The effect of Sutherlandia frutescens on steroidogenesis: Confirming indigenous wisdom. Endocrine Res. 30 4: 745–751.
- Sacheck JM, Hyatt J-PK, Rafaello A, Jagoe RT, Roy RR, Edgerton R, Lecker SH, Goldberg AL. 2007. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 21:140–155.
- Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A. 2004. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 117:399–412.
- Siu PM, Always SE. Mitochondria-associated apoptotic signaling in denervated rat skeletal muscle. J Physiol. 2005a; 565:309–323.
- Siu PM, Always SE. Id2 and p53 participate in apoptosis during unloading-induced muscle atrophy. Am J Physiol Cell Physiol. 2005b; 288:C1058–C1073.
- Smith C, Myburgh K. 2004. Treatment with Sutherlandia frutescens ssp. Microphylla alters the corticosterone response to chronic intermittent immobilization stress in rats. S Afr J Sci. 100:229–232.
- Song G, Ouyang G, Bao S. 2005. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 9 1: 59–71.
- Sun L, Trausch-Azar JS, Muglia LJ, Schwartz AL. 2008. Glucocorticoids differentially regulate degradation of MyoD and Id1 by N-terminal ubiquitination to promote muscle protein catabolism. Proc Natl Acad Sci. 105 9: 339–3344.
- Taillandier D, Combaret L, Pouch MN, Samuels SE, Bechet D, Attaix D. 2004. The role of ubiquitin–proteasome-dependent proteolysis in the remodeling of skeletal muscle. Proc Nutr Soc. 63:357–361.
- Tai J, Cheung E, Hasman D. 2004. In vitro culture studies of Sutherlandia frutescens on human tumor cell lines. J Ethnopharmacol. 93:9–19.
- Tilly JL, Kowalski KI, Johnson AI, Hsueh JW. 1991. Involvement of apoptosis in ovarian follicular atresia a post-ovalutory regression. Endocrinology. 129:2799–2801.
- Tracey KJ. 2002. Lethal weight loss: The focus shift to signal transduction. Sci STKE. 130:PE21.
- Tran H, Brunet A, Griffith EC, Greenberg ME. 2003. The many forks in FOXO's road. Sci STKE. 2003:RE5.
- Uchikawa K, Takahashi H, Hase K, Masakado Y, Liu M. 2008. Strenous exercise-induced alterations on muscle fiber cross-sectional area and fiber-type distribution in steroid myopathy rats. Am J Phys Med Rehabil. 87 2: 126–133.
- Williamson D, Gallagher P, Harber M, Hollon C, Trappe S. 2003. Miotogen-activated protein kinase (MAPK) pathway activation: Effects of age and acute exercise on human skeletal muscle. J Physiol. 547:977–987.
- Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. 2008. Sarcopenic obesity: A new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. 18 5: 388–395.
- Zamecnik J, Vesely D, Jakubicka B, Cibula A, Pitha J, Schutzner J, Mazanec R. Atrophy of type II fibers in myasthenia gravis muscle in thymectomized patients: steroid-induced change with prognostic impact. J Cell Mol Med. 2008 Doi: 10.1111/j.1582-4934.2008.00431.x.
- Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. 2007. FoxO3 coordinalely activates protein degradation by autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 6:472–483.