Abstract
Stress can both impair and enhance memory retrieval. Glucocorticoids mediate impairing effects of stress on memory retrieval. Little is known, however, about factors that facilitate post-stress memory performance. Here, we asked whether stress-induced arousal mediates facilitative stress effects on memory retrieval. Two arousal dimensions were separated: tense arousal, which is characterized by feelings ranging from tension and anxiety to calmness and quietness, and energetic arousal, which is associated with feelings ranging from energy and vigor to states of fatigue and tiredness. Fifty-one men (mean age ± SEM: 24.57 ± 0.61 years) learned emotional and neutral words. Memory for these words was tested 165 min later, after participants were exposed to a psychosocial stress or a non-arousing control condition. Changes in heart rate, self-reported (energetic and tense) arousal, and saliva cortisol in response to the stress/control condition were measured. Overall, stress impaired memory retrieval. However, stressed participants with large increases in both tense and energetic arousal performed comparably to controls. Neither salivary cortisol level nor autonomic arousal predicted memory performance after controlling for changes in energetic and tense arousal. The present data indicate that stress-induced concurrent changes in tense and energetic arousal can compensate for impairing effects of stress on memory retrieval. This finding could help to explain some of the discrepancies in the literature on stress and memory.
Introduction
Stress has multiple effects on physiology and cognition. Typically, acute stress leads to the secretion of glucocorticoids (GCs) from the adrenal cortex, an increase in cardiovascular activity, and an increase in subjective feelings of arousal. These responses facilitate adaptation and prepare the individual to cope successfully with stressful situations. With regard to cognition, it is well established that stress affects memory retrieval (Joels et al. Citation2006; Roozendaal et al. Citation2009). However, the direction of this effect is still a matter of debate. Stress can both facilitate (Domes et al. Citation2002; Nater et al. Citation2007; Buchanan and Tranel Citation2008; Schwabe et al. Citation2009) or impair (Lupien et al. Citation1997; de Quervain et al. Citation1998; Kuhlmann et al. Citation2005; Buchanan et al. Citation2006) retrieval performance. The neurobiological mechanisms mediating these effects are only partly understood. A recent neurobiological model suggests that GCs (with cortisol being the most important GC in humans) and concurrent autonomic arousal interactively mediate impairing effects of stress on memory retrieval (Roozendaal Citation2002; Roozendaal et al. Citation2006). To date, however, it remains unclear which factors account for enhancing effects of stress on retrieval performance. A better knowledge of such factors might improve our understanding of stress effects on memory and could thus prove beneficial for the development of strategies counteracting possible detrimental effects of stress on memory performance.
Stress is typically associated with an increase in emotional arousal (Schlotz et al. Citation2008). Arousal is a state of heightened alertness and responsiveness to sensory inputs which is accompanied by changes in subjective mood and an increase in physiological activity (Thayer Citation1989; Adamantidis and de Lecea Citation2008). Besides physiological measures such as heart rate and blood pressure, self-report has been established as a measure of arousal (Thayer Citation1989). Studies using subjective measures identified two separate dimensions describing a current arousal state: energetic arousal and tense arousal (Thayer Citation1989). Tense arousal is identifiable through feelings that range from tension and anxiety to states of calmness and quietness. Energetic arousal, on the other hand, is characterized by feelings ranging from energy and vigor to states of fatigue and tiredness. Interestingly, energetic and tense arousals are not only distinct with respect to subjective experience but are also associated with different patterns of brain activity (Thayer Citation1989).
It has long been known that moderate levels of arousal can enhance performance in various cognitive tasks (Yerkes and Dodson Citation1908; Revelle and Loftus Citation1992). More recent research suggests that this is particularly true for an increase in energetic arousal. One study showed that energetic arousal facilitates whereas tense arousal is not associated with or even reduces performance in controlled visual and memory search tasks (Matthews and Westerman Citation1994). This finding suggests that stress-induced energetic arousal may mediate facilitative effects of stress on memory retrieval. To date, however, it is unknown whether memory retrieval can be facilitated by high-energetic arousal. In fact, most studies on stress and memory (retrieval) did not pay attention to possible effects of subjective arousal.
Here, we hypothesized that a stress-induced increase in arousal facilitates post-stress memory retrieval. Healthy participants learned a list of emotional and neutral words. Emotionality of the words was varied because previous work revealed that stress effects on memory retrieval are crucially influenced by the affective characteristics of the to-be-remembered word material (Kuhlmann et al. Citation2005). Prior to retention testing for these words, participants were exposed to a psychosocial laboratory stress test or a non-arousing control condition. Changes in subjective and autonomic arousal as well as salivary cortisol concentration were measured before and after the stress/control condition. We hypothesized an enhancing influence of energetic arousal and an inhibitory influence of tense arousal on memory retrieval. In addition, we investigated how arousal effects relate to effects of the stress-induced cortisol secretion on memory retrieval and how these effects are influenced by the emotionality of the to-be-remembered stimulus material.
Methods
Participants
Fifty-one young, healthy male university students between 18 and 31 years of age participated in the present study (mean ± SEM: 24.57 ± 0.61 years). Since estradiol and progestins are known to change the endocrine response to psychosocial stress tests like the Trier Social Stress Test (TSST; Kirschbaum et al. Citation1999), only male participants were included. Participants were excluded from the study if they met any of the following criteria: any acute or chronic disease, smoking of more than five cigarettes per day, familiarity with the TSST, a presence or history of mental illness, use of systemic medication, current participation in another clinical study, body mass index below 18 or above 28, and the presence of a depressive disorder. These criteria were assessed by a physical examination (including among others a screening for cardiovascular or chronic respiratory diseases) and a standardized screening for psychiatric diseases. Presence of a depressive disorder was screened with the German version of the Patient Health Questionnaire (Loewe et al. Citation2002). Participants were asked to refrain from eating meals, drinking coffee or alcohol, and severe physical exercise in the 2 h before the experiment. All participants gave voluntary written informed consent and were compensated for their participation. The study protocol was approved by the Ethical Committee of the State's Medical Association (Landesärztekammer Rheinland-Pfalz, Mainz, Germany).
Procedure
Participants arrived between 13:30 and 15:30 h in our laboratory. If all requirements were met, a word list containing 10 negative, 10 positive, and 10 neutral words (in German) was presented on a piece of paper. Participants were instructed to read the list aloud twice and to rate every word regarding its imageability (difficult to imagine vs. easy to imagine) on a bipolar seven-point rating scale (data not shown). Subjects were not told that memory of these words would be tested later on. Afterwards, the first saliva sample was taken and the participants were randomly assigned to the stress- (n = 33) or the control condition (n = 18). We used a between-subject design to avoid effects of test repetition and to prevent overt rehearsal strategies that might have influenced delayed memory testing.
More participants were assigned to the stress group because it was planned to split this group into subjects with high vs. low stress-induced increases in emotional arousal later on (see statistical analyses section). Electrocardiogram (ECG) electrodes were attached according to a standard lead II configuration. The ECG was used for automated detection of heart rate within the course of the experimental session. In order to standardize activity patterns following word learning, all participants answered questionnaires assessing health and subjective well-being for a duration of 45 min after presentation of the word list. Thereafter, subjects were obliged to restrict themselves to calm and non-arousing activities (e.g. reading newspapers), away from the researchers. The second saliva sample was collected 60 min before the stress or control procedure, respectively. Ten minutes prior to the stress/control procedure, heart rate monitoring was started and participants answered a questionnaire assessing momentary mood Mehrdimensionaler Befindlichkeitsfragebogen (MDBF version A; Steyer et al. Citation1994). In order to avoid influences of orthostatic reactions, all participants were asked to change to a standing position before this. Three minutes before stressor (control procedure) onset, a third saliva sample was collected. The stress or control procedure was started for all participants between 16:00 and 18:00 h (135 min after word learning). This time interval between word learning and the stress/control task was chosen because previous work showed that GCs affect memory consolidation when administered immediately after stimulus encoding but not when administered several hours later (McGaugh Citation1989). The time interval of 135 min used here thus allows for studying isolated effects of stress on memory retrieval. Immediately after the stress/control procedure, a fourth saliva sample was collected. Furthermore, participants were evaluated on three 10-point rating scales of how stressful, anxious, and insecure they felt during the task. Heart rate sampling was stopped 10 min after the stress/control procedure. At this time point, all participants answered again a questionnaire assessing momentary mood (MDBF version B; Steyer et al. Citation1994). Immediately thereafter (165 min after word learning), participants completed a paper- and pencil-based free recall test for the words presented at the beginning of the experimental session. Additional saliva samples were collected 10, 20, 30, 45, and 60 min after cessation of the stress/control procedure. During the stay in our laboratory, participants were not allowed to smoke, eat, or drink anything except water. At the end of the experimental session, all participants were asked to indicate if they expected a memory test for the words presented at the beginning of the experiment. No participant expected a test of memory retrieval for these words.
Stress and control condition
Participants in the stress condition completed the TSST. A detailed protocol of the TSST is described elsewhere (Kirschbaum et al. Citation1993). Briefly, the TSST is a standardized laboratory stressor consisting of a free speech and a mental arithmetic task in front of an audience (a man and a woman) and a video camera. Participants were introduced to the task and were instructed to prepare a presentation in which they had to promote their candidacy for a job. After a 3-min preparation period, they were asked to give a 5-min free speech. Thereafter, participants performed an arithmetic task for 5 min, also standing in front of the audience. Subjects were required to count backwards from 2023 in steps of 17 as fast and accurate as possible; upon a mistake they had to stop and start again from 2023.
Participants in the control condition firstly read aloud a non-arousing popular science newspaper article standing in an empty room. Afterwards, they were asked to do simple paper- and pencil-based arithmetic. Both tasks lasted 5 min. Participants in the control condition were informed that they would not be tape recorded or videotaped.
Word list
Construction of the word list was based on a study by Schwibbe et al. (Citation1994). The authors let university students evaluate 1698 German words regarding their emotional valence on a bipolar seven-point rating-scale ranging from − 3 (negative) to +3 (positive). Ten positive (valence; mean ± SEM: 1.24 ± 0.06), 10 negative ( − 1.50 ± 0.06), and 10 neutral (0.00 ± 0.01) two-syllable nouns from this data pool were selected for the presentation of the word list. There were no differences in word frequency between the three valence categories [univariate analyses of variance (ANOVA): F2,27 = 0.003; p = 0.99; word frequency norms were taken from a German internet data base].
Assessment of physiological and psychological stress responses
Salivary cortisol sampling and biochemical analyses
Saliva was passed from the mouth to Eppendorf tubes (Eppendorf, Hamburg, Germany) by collecting in commercial plastic tubes at 135, 55, and 3 min before as well as 1, 10, 20, 45, and 60 min after the stress/control task. Samples were stored at − 20˚C until analyses. Salivary cortisol was measured with a time-resolved fluorescence immunoassay. The intra-assay coefficient of variation was between 4.0 and 6.7%, and the corresponding inter-assay coefficients of variation were between 7.1 and 9.0%. The lower detection limit of this method is 0.43 nM for a 50-μl saliva sample (Dressendorfer et al. Citation1992).
Assessment of autonomic arousal
Heart rate was derived from a single standard lead II ECG configuration employing a telemetric HP 78100A transmitter and HP 78101A receiver system (Hewlett Packard Corp, Palo Alto, CA, USA). The ECG was sampled at 1 kHz with a 12-bit resolution. Beat detection was performed offline via WinCPRS (Absolute Aliens Oy, Turku, Finland) as was artifact control.
Heart rate measurements were taken continuously 10 min before, during, and 10 min after the stress or control task. The mean pre- and post-task heart rates as well as the mean heart rates during the task was calculated for each participant.
Assessment of subjective arousal and further psychological variables
Mood was assessed 10 min before and 10 min after the stress or control procedure with two parallel versions of a German mood questionnaire (MDBF; Steyer et al. Citation1994). The MDBF measures momentary mood on three bipolar dimensions: (1) wakefulness–sleepiness; (2) calmness–restlessness; and (3) pleasant–unpleasant mood. This 3D conceptualization of mood has frequently been confirmed (Thayer Citation1989; Schimmack and Reisenzein Citation2002). Here, we follow the terminology suggested by Thayer (Citation1989) who used the terms energetic arousal and tense arousal to describe the two mood dimensions associated with activity. We employed the MDBF-wakefulness vs. sleepiness-scale to measure energetic arousal and the MDBF-calmness vs. restlessness-scale to measure tense arousal.
In addition, all participants were rated on three scales ranging from 0 (not at all) to 10 (very much) of how stressed, anxious, and insecure they felt during task participation.
Data analyses
Kolmogorov–Smirnov tests revealed that all variables tested were normally distributed. We used methods based on the general linear model (GLM) in order to analyze effects of stress-induced arousal on memory retrieval. The GLM approach allows for investigating interacting influences of categorical and continuous variables on a dependent variable in one single analysis. Here, we calculated a GLM with the within-subject factor word valence (positive, negative, and neutral) and the between-subject factors change in energetic arousal, change in tense arousal, change in heart rate, and the maximum increase in cortisol concentration to test for significant influences of these variables on memory retrieval. The GLM included main effects for all mentioned variables. Moreover, the interaction term change in energetic arousal × change in tense arousal was entered into the model. The latter was done because past research suggests that energetic arousal and tense arousal may interactively predict cognitive performance (Matthews and Westerman Citation1994). All variables were centered before entering into the GLM. Next, further illustrative analyses were conducted in order to disentangle significant interaction effects revealed by the GLM analysis. Specifically, a median split on change in energetic arousal and change in tense arousal within the stress group was used to create four new subgroups representing different combinations of changes in tense and energetic arousal. An ANOVA was used to compare memory performances between these four groups and the control group. The change in energetic arousal, tense arousal, and feelings of pleasantness in response to the stress or control task was calculated by subtracting post-task measurements (MDBF-version B) from pre-task measurements (MDBF-version A). The change in heart rate was expressed (1) as the increase in heart rate from pre-TSST to the highest heart rate during the TSST and (2) as the increase in heart rate from pre-TSST to post-TSST. The maximum increase in salivary cortisol concentration was expressed as the individual increase in cortisol from the last measurement before the TSST or control task to the highest individual cortisol value after the respective task. Since both measures of heart rate were highly correlated, they were included in separate analyses. Cortisol, heart rate data, and further subjective reactions to the stress or control task were analyzed by means of one-way and two-way mixed design ANOVAs. Follow-up tests of ANOVA effects were done using the Tukey's honest significant difference (HSD) correction and only corrected p-values are shown. In the case of ANOVAs with repeated measurements, the Greenhouse–Geisser correction was employed, where appropriate. Only corrected p-values and df are shown. Pearson product–moment correlations were calculated to assess associations among variables. A p-value ≤ 0.05 two-tailed was considered significant. Data are presented as mean ± SEM.
Results
Subjective and physiological stress responses
Psychological measures
Subjective responses to the stress and the control task are summarized in . Ratings of wakefulness (F1,49 = 1.62; p = 0.21), calmness (F1,49 = 2.00; p = 0.16), and pleasant mood (F1,49 = 0.15; p = 0.70) were comparable between the stress and the control group at baseline, i.e. 10 min before the stress/control procedure (). Compared to the control group, the stress group showed a stronger increase in restlessness, i.e. tense arousal (F1,49 = 52.36; p < 0.0001; η2 = 0.52), and a stronger decrease in pleasant mood (F1,49 = 10.22; p = 0.002; η2 = 0.17). Wakefulness, i.e. energetic arousal, however, increased comparably in both groups (F1,49 = 0.01; p = 0.94), probably due to the fact that both groups were exposed to a cognitive task. Moreover, participants in the stress group felt more stressed, insecure, and anxious during the TSST than participants in the control group during the control task (all p < 0.001). Next, we analyzed associations among measures of arousal and further subjective responses to the TSST. Results of these analyses are reported in . While the change in tense arousal was positively correlated with perceived stress, anxiety, insecurity, and the decline in mood, no such associations were found for the change in energetic arousal.
Table I. Subjective and heart rate responses to the stress and control task.
Table II. Pearson product–moment correlations among measures of arousal and further subjective reactions to the TSST (n = 33).
Heart rate
Heart rate data are summarized in . A two group (stress group and control group) by four time (pre-task, reading, arithmetic, and post-task) mixed design ANOVA indicated that participants in the stress group showed a stronger increase in heart rate than participants in the control group (group by time interaction: F1.8,89.4 = 17.98; p < 0.0001; η2 = 0.27). Heart rates were comparable between both groups before (F1,49 = 1.06; p = 0.31) and after (F1,49 = 0.97; p = 0.33) the task. Moreover, no associations were found among subjective arousal measures and autonomic arousal as measured by the change in heart rate (maximum change and change pre- to post-TSST). We furthermore asked if changes in energetic and tense arousal would be associated with different heart rate response patterns to the TSST. To this end, we ran a GLM with repeated measurements on heart rate data within the stress group and included the independent variables change in energetic arousal, change in tense arousal, and the interaction term between these variables. The critical time by change in energetic arousal by change in tense arousal interaction was not significant (F2,52 = 0.53; p = 0.56), indicating that tense and energetic arousal were not associated with different heart rate response patterns to the TSST.
Saliva cortisol concentration
Stress and control participants showed comparable cortisol values before stressor/control procedure onset (all p>0.20; ). However, groups differed regarding their cortisol responses to the stress or control procedure, respectively. A two group (stress group and control group) by nine time (timepoint of measurement 1–9) mixed design ANOVA revealed that cortisol responses were higher in the stress group as than the control group (group by time interaction: F2.2,107.2 = 15.24; p < 0.0001; η2 = 0.24). By analogy to heart rate data, a GLM analysis within the stress group revealed that stress-induced changes in energetic and tense arousal were not associated with different cortisol response patterns to the TSST (time by change in energetic arousal by change in tense arousal interaction: F2,61 = 2.23; p = 0.12).
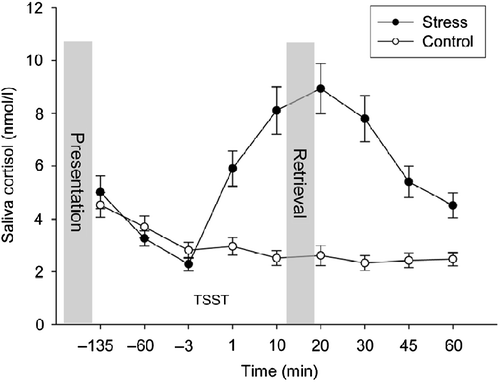
Figure 1 Salivary cortisol response to the stress and the control task. Salivary cortisol concentration increased in the stress group (n = 33) but not in the control group (n = 18) (ANOVA, time by group interaction, p < 0.0001); presentation, presentation of the word list; and retrieval, free recall of words learned 165 min earlier. Data are presented as mean ± SEM.
Memory performance
Effects of stress on memory retrieval
Stress prior to retention testing reduced retrieval performance (F1,49 = 8.07; p = 0.007; η2 = 0.14; ). Although this effect appeared to be especially pronounced for negative words, the referring group by word valence interaction did not reach statistical significance (F2,98 = 1.78; p = 0.17).
Table III. Memory performance in percentage for positive, negative, neutral words, and in total in the stress group (n = 33) and the control group (n = 18).
Influences of arousal, salivary cortisol, and word valence on memory retrieval
A GLM including the independent variables word valence (positive, negative, and neutral) change in energetic arousal, change in tense arousal, change in heart rate, and the maximum increase in cortisol concentration was calculated within the stress group. This analysis revealed a significant main-effect of the factor change in energetic arousal (F1,27 = 4.64; p = 0.04; η2 = 0.15), a significant change in energetic arousal by change in tense arousal interaction (F2,27 = 4.50; p = 0.04; η2 = 0.14), and a significant change in tense arousal by word valence (F2,54 = 3.26; p < 0.05; η2 = 0.11) interaction. These effects are analyzed in detail in the following paragraphs. None of the other effects reached significance (see ). For reasons of comparability with previous studies, a separate GLM was calculated within the stress group that included the factors word valence (positive, negative, and neutral) and maximum increase in saliva cortisol only. This analysis revealed that the stress-related cortisol secretion tended to predict post-stress memory retrieval (F1,31 = 3.32; p = 0.07; η2 = 0.10). There was no interaction between the increase in salivary cortisol concentration and the factor word valence (F2,62 = 0.43; p = 0.66). Correlational analyses indicated a positive association among the maximum increase in cortisol secretion and overall memory performance (r = 0.31, p = 0.07). However, after controlling for changes in energetic and tense arousal, the association between increase in salivary cortisol and memory retrieval no longer tended to be significant (F1,28 = 1.05; p = 0.32). We furthermore analyzed if absolute levels of energetic or tense arousal measured before and after the TSST would predict post-stress memory retrieval. No such associations were found (all p>0.18).
Table IV. Influences of tested independent variables on memory retrieval of positive, negative, and neutral words within the stress group as revealed by GLM analysis.
Change in energetic arousal by change in tense arousal interaction
The significant change in energetic arousal by change in tense arousal interaction indicated that both variables predicted memory performance interactively. We, therefore, analyzed the interaction among these variables and not the significant effect of change in energetic arousal alone. As reported above, energetic arousal changed comparably in both the stress and the control groups. It was thus analyzed if effects of changes in self-reported arousal on memory retrieval differed among stressed and non-stressed participants. A GLM including the variables word valence (positive, negative, and neutral), group (stress, control) change in energetic arousal, change in tense arousal as well as the interaction term between these variables, and the factor group was included as independent variables into the analysis. This analysis revealed a significant main effect of the factor group (F1,43 = 5.16; p < 0.03; η2 = 0.11) and a significant group by change in energetic arousal by change in tense arousal interaction (F2,43 = 3.20; p = 0.05; η2 = 0.13). The significant interaction indicated that (i) only the combination of change in energetic arousal and change in tense arousal predicted memory performance and (ii) this influence differed between the stress and the control groups. As already reported, the change in energetic arousal by change in tense arousal interaction was significant in the stress group. However, an additional analysis showed that neither the main effects change in energetic arousal (F1,14 = 0.35; p = 0.57) nor change in tense arousal (F1,14 = 0.05; p = 0.82) nor the interaction term between these variables reached significance in the control group (F1,14 = 1.79; p = 0.20), indicating that self-reported arousal did not affect memory performance in the control group.
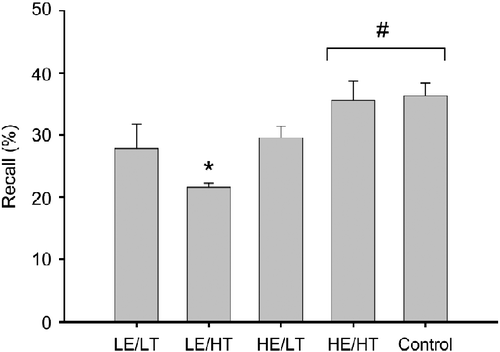
Next, further illustrative analyses were conducted in order to analyze the significant two-way interaction within the stress group in more detail. Specifically, a median split on change in energetic arousal and change in tense arousal within the stress group was used to create four new groups representing different combinations of changes in energetic arousal and tense arousal (data shown as mean ± SEM): (1) low energetic arousal (0.00 ± 0.57)/low tense arousal (2.00 ± 1.00), (2) low energetic arousal ( − 1.00 ± 0.27)/high tense arousal (9.88 ± 0.67), (3) high-energetic arousal (4.50 ± 0.60)/low tense arousal (4.30 ± 4.5), and (4) high-energetic arousal (3.00 ± 0.44)/high tense arousal (7.89 ± 0.89). Next, a five-group (high-energetic arousal/low tense arousal, low energetic arousal/high tense arousal, high-energetic arousal/high tense arousal, low energetic arousal/low tense arousal, and control group) ANOVA on the number of words retrieved was calculated (). This analysis indicated significant group differences (main effect group: F4,46 = 5.53; p = 0.001; η2 = 0.33). Tukey's HSD-corrected follow-up tests revealed that participants in the stress group with low change in energetic arousal but high change in tense arousal performed worse than participants with high change in energetic arousal and high change in tense arousal (p = 0.009) and participants in the control group (p = 0.007). No other pairwise comparisons were significant after alpha error correction. A contrast analysis, however, indicated that the memory performance of participants in the stress group with high changes in energetic arousal and tense arousal was comparable to that of participants in the control group and that both groups differed from the other three groups in the stress condition (F1,46 = 16.46; p < 0.001; η2 = 0.26). Furthermore, participants in the stress group with low change in energetic arousal but high change in tense arousal performed worse than the participants in the other four groups (F1,46 = 11.37; p = 0.002; η2 = 0.20).
Figure 2 The x-axis represents subgroups of participants with different combinations of high and low changes in energetic and tense arousal in response to the TSST as well as the control group. LE/LT, low change in energetic arousal and low change in tense arousal (n = 8); LE/HT, low change in energetic arousal and high change in tense arousal (n = 8); HE/LT, high change in energetic arousal and low change in tense arousal (n = 8); HE/HT, high change in energetic arousal and high change in tense arousal (n = 9); and control = no stress group (n = 18). Contrast analyses based on ANOVAs revealed that both HE/HT and control participants performed better than the other three groups (#p ≤ 0.05) and that LE/HT showed lowest memory performance of all groups (*p ≤ 0.05). Recall %, percentage of words retrieved from a list of words learned 165 min before. Data are presented as mean ± SEM.
Change in tense arousal by word valence interaction
The significant change in tense arousal by word valence interaction indicated that the impact of stress-induced change in tense arousal on memory retrieval was influenced by word valence. Additional analyses showed that change in tense arousal tended to be correlated with negative words (r = 0.31; p = 0.08), but was not significantly associated with positive (r = − 0.11; p = 0.54) or neutral words (r = − 0.14; p = 0.45).
Discussion
Here, we asked whether stress-induced changes in arousal facilitate post-stress memory retrieval. Although we did not find a memory enhancement by arousal, our data show that stress-induced arousal can compensate for impairing effects of stress on memory performance. We will discuss this result in more detail in the following paragraphs.
As hypothesized, stress-induced arousal was associated with post-stress memory retrieval. A multifaceted picture of arousal effects on memory performance emerged. Overall, stress impaired post-stress memory retrieval. However, within the stress group, participants with high stress-related changes in both energetic arousal and tense arousal showed best memory retrieval; they performed similar to control participants. This suggests that concurrent increases in energetic and tense arousal may compensate for the impairing effects of stress on memory retrieval. Overall, this finding is in agreement with previous studies that found facilitative influences of heightened arousal and alertness on cognitive performance in attentional and vigilance tasks and tests of declarative memory (Revelle and Loftus Citation1992; Aston-Jones Citation2005). Specifically, the facilitative influence of energetic arousal on memory performance was expected. Studies on sleep deprivation showed that increased subjective feelings of sleepiness are associated with impaired cognitive performance (Thomas et al. Citation2000; Matsumoto et al. Citation2002), and research on individual differences revealed that heightened energetic arousal predicts high performance in sustained attention, visual or memory search, and letter transformation tasks (Matthews et al. Citation1990; Matthews and Davies Citation2001). Unexpectedly, however, an increase in energetic arousal alone did not affect memory performance in the control group. Moreover, only a combination of high change in energetic arousal and tense arousal was associated with unimpaired memory performance in the stress group. The latter finding is of particular interest. Based on previous work (Matthews et al. Citation1990; Matthews and Westerman Citation1994), we expected that a high change in tense arousal would impair memory retrieval. In support of this assumption and in contrast to unimpaired memory performance in participants with concurrent high increase in energetic and tense arousal, memory performance was worst in participants with high stress-induced change in tense arousal but low change in energetic arousal. This complex pattern of arousal effects suggests that the neural and peripheral systems underlying both arousal states interact and that this interaction determines effects of arousal on post-stress memory retrieval.
Stress-induced tense arousal was accompanied by states of heightened anxiety and fearfulness, which are known to induce activation in limbic brain areas such as the amygdala (Davidson Citation2003; Wang et al. Citation2005). This finding is in line with the evidence that tense arousal is associated with activation in limbic brain structures (Thayer Citation1989). Ample evidence suggests that impairing effects of GCs on memory retrieval require noradrenergic activation within the amygdala (Okuda et al. Citation2004; Kuhlmann and Wolf Citation2006; Roozendaal et al. Citation2006; de Quervain et al. Citation2007). It is thus tempting to speculate that activation of limbic brain areas by isolated tense arousal mediated impairing effects of stress on memory retrieval. In contrast, energetic arousal was suggested to be associated with general mobilization of physiological and cognitive capabilities that may have a neurophysiologic correlate in heightened activation of the brainstem reticular formation (Thayer Citation1989). More recent research on neurobiological mechanisms of wakefulness and arousal has identified several brain regions, such as the locus coeruleus and different hypothalamic nuclei, as well as noradrenergic, cholinergic, dopaminergic, and serotonergic transmitter systems that are involved in the regulation of wakefulness and arousal (Jones Citation2003; Aston-Jones Citation2005). Brain activation induced by energetic arousal might modulate activation in the amygdala (or in brain areas connected to the amygdala) induced by tense arousal, compensating for impairing effects of isolated tense arousal on memory retrieval. However, further studies are needed to test this hypothesis directly.
Numerous studies suggest that effects of arousal on cognitive performance are nonlinear, following an inverted-U relationship (Diamond et al. Citation2007). Our finding of impairing as well as protective effects of stress-induced arousal on memory retrieval is in line with this literature. Recently, it was shown that norepinephrine and dopamine, which are interactively involved in the control of stress-induced arousal, have inverted U-shaped influences on prefrontal cortex (PFC) physiology and cognition (Vijayraghavan et al. Citation2007; Arnsten Citation2009). This brain region is a key player in cognitive control and is involved in many cognitive domains, including episodic memory (Gilboa Citation2004). The PFC could therefore be a crucial moderator of nonlinear effects of subjective arousal on cognitive performance and memory retrieval. Future studies will focus on the validity of this theory.
The complex interactive arousal effect found in the present study might explain some of the discrepancies in the literature on effects of cortisol and arousal on post-stress memory retrieval. It is well established that emotional arousal induced by affective stimuli (de Quervain et al. Citation2007) or psychosocial stress (Kuhlmann et al. Citation2005) is a prerequisite for impairing effects of cortisol on memory retrieval. However, some authors found better memory retrieval in high-cortisol responders than low responders to a laboratory stressor (Domes et al. Citation2002; Nater et al. Citation2007; Schwabe et al. Citation2009). Our results suggest that the strength and combination of stress-induced change in energetic and tense arousal critically affect the direction of stress effects on memory retrieval. Under conditions of isolated increase in tense arousal or relatively specific activation of affective systems (de Quervain et al. Citation2007), GCs might interact with arousal-induced activity in the limbic system and impair post-stress memory retrieval. In contrast, a combination of high increase in energetic and tense arousal might override these impairing effects and lead to unimpaired or even facilitated memory performance. This could offer a parsimonious explanation for unexpected positive effects of GCs on post-stress memory performance reported previously (Domes et al. Citation2002; Nater et al. Citation2007; Schwabe et al. Citation2009).
Our data indicate that the pattern of stress-induced change in subjective arousal is a better predictor of individual differences in post-stress memory retrieval than the absolute level of arousal at a specific point in time. A predisposition to react to stress with high increase in tense arousal but low increase in energetic arousal might thus represent a vulnerability factor that leads to impaired memory retrieval in stressful situations. In contrast, individuals with high change in energetic as well as tense arousal might be protected against such detrimental effects of stress. This finding might have relevance for the development of therapeutic approaches against detrimental effects of stress on memory and cognition.
We found no association between post-stress memory performance and a measure of autonomic arousal, i.e. heart rate. This could be due to the fact that we measured heart rate only until 10 min after the TSST and assessed subjective arousal only thereafter. However, the dissociation between subjective and autonomic arousal measures might also be due to the multifaceted structure of the arousal construct. Past research showed that a generalized arousal component can be found that accounts for a substantial amount of behavioral variance in single forms of arousal such as sexual behavior or fear (Garey et al. Citation2003; Pfaff et al. Citation2008). Moreover, data suggest that self-report may be a better indicator of generalized arousal than single physiological measures (Thayer Citation1989). In an early study, Thayer investigated associations between physiological (heart rate, finger blood volume, and skin conductance) and psychological arousal reactions to a laboratory stress task (Thayer Citation1970). He found that inter-correlations between the physiological functions were very low. However, after combining physiological measures to form a single general arousal index, self-report measures correlated substantially with this general arousal index. Our data suggest that self-report measures of arousal might prove particularly beneficial as predictors of memory performance because they represent a generalized arousal component.
Previous work showed that the affective characteristics of the to-be-remembered stimuli mediate effects of stress on memory performance (Buchanan Citation2007; Schwabe et al. Citation2008). In the present study, the general effect of stress on memory retrieval was not influenced by the valence of the learned words. Earlier studies indicated that stimulus arousal has a stronger impact on stress-related memory phenomena than stimulus valence (Buchanan and Lovallo Citation2001; Cahill et al. Citation2003; Kuhlmann et al. Citation2005; Buchanan and Tranel Citation2008). Thus, the absence of a valence effect in the present study might be due to the fact that we tested memory for stimuli that differed along the valence but not the arousal dimension. However, we found some evidence that arousal effects on memory performance are modulated by the affective characteristics of the presented stimulus material. Within the stress group, the change in restlessness tended to correlate positively with retrieval of negative words, whereas no association was found with retrieval of positive or neutral words. It is well established that the match between affective characteristics of the to-be-remembered stimuli and the mood state at retrieval affect memory performance (Lewis et al. Citation2005; Buchanan Citation2007). Here, the change in tense arousal was correlated with a decline in pleasantness. Mood congruency effects may thus have mediated the facilitative effects of tense arousal on memory retrieval.
In sum, the present findings demonstrate that stress-induced arousal as measured by self-report predicts post-stress memory retrieval. Importantly, our data suggest that a certain pattern of combined high change in energetic arousal and high change in tense arousal compensates for impairing effects of stress on memory performance. This finding may help to explain some of the discrepancies in the literature on stress effects upon memory retrieval. Moreover, it may prove beneficial for the development of new strategies against detrimental effects of stress on memory performance, as in stressful working environments or stressful testing situations.
Acknowledgement
This work was part of the International Research Training Group “Psychoneuroendocrinology of Stress” (GRK 1389/1) funded by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adamantidis A, de Lecea L. 2008. Physiological arousal: A role for hypothalamic systems. Cell Mol Life Sci. 65:1475–1488.
- Arnsten AF. 2009. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 10:410–422.
- Aston-Jones G. 2005. Brain structures and receptors involved in alertness. Sleep Med. 6 Suppl 1: S3–S7.
- Buchanan TW. 2007. Retrieval of emotional memories. Psychol Bull. 133:761–779.
- Buchanan TW, Lovallo WR. 2001. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 26:307–317.
- Buchanan TW, Tranel D. 2008. Stress and emotional memory retrieval: Effects of sex and cortisol response. Neurobiol Learn Mem. 89:134–141.
- Buchanan TW, Tranel D, Adolphs R. 2006. Impaired memory retrieval correlates with individual differences in cortisol response but not autonomic response. Learn Mem. 13:382–387.
- Cahill L, Gorski L, Le K. 2003. Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learn Mem. 10:270–274.
- Davidson RJ. 2003. Darwin and the neural bases of emotion and affective style. Ann NY Acad Sci. 1000:316–336.
- de Quervain DJ, Roozendaal B, McGaugh JL. 1998. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 394:787–790.
- de Quervain DJ, Aerni A, Roozendaal B. 2007. Preventive effect of beta-adrenoceptor blockade on glucocorticoid-induced memory retrieval deficits. Am J Psychiatry. 164:967–969.
- Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. 2007. The temporal dynamics model of emotional memory processing: A synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes–Dodson law. Neural Plast. 2007:60803.
- Domes G, Heinrichs M, Reichwald U, Hautzinger M. 2002. Hypothalamic–pituitary–adrenal axis reactivity to psychological stress and memory in middle-aged women: High responders exhibit enhanced declarative memory performance. Psychoneuroendocrinology. 27:843–853.
- Dressendorfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. 1992. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol. 43:683–692.
- Garey J, Goodwillie A, Frohlich J, Morgan M, Gustafsson JA, Smithies O, Korach KS, Ogawa S, Pfaff DW. 2003. Genetic contributions to generalized arousal of brain and behavior. Proc Natl Acad Sci USA. 100:11019–11022.
- Gilboa A. 2004. Autobiographical and episodic memory—one and the same? Evidence from prefrontal activation in neuroimaging studies. Neuropsychologia. 42:1336–1349.
- Joels M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. 2006. Learning under stress: How does it work?. Trends Cogn Sci. 10:152–158.
- Jones BE. 2003. Arousal systems. Front Biosci. 8:s438–s451.
- Kirschbaum C, Pirke KM, Hellhammer DH. 1993. The “Trier Social Stress Test”—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 28:76–81.
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. 1999. Impact of gender, menstrual cycle phase, oral contraceptives on the activity of the hypothalamus–pituitary–adrenal axis. Psychosom Med. 61:154–162.
- Kuhlmann S, Wolf OT. 2006. A non-arousing test situation abolishes the impairing effects of cortisol on delayed memory retrieval in healthy women. Neurosci Lett. 399:268–272.
- Kuhlmann S, Piel M, Wolf OT. 2005. Impaired memory retrieval after psychosocial stress in healthy young men. J Neurosci. 25:2977–2982.
- Lewis PA, Critchley HD, Smith AP, Dolan RJ. 2005. Brain mechanisms for mood congruent memory facilitation. Neuroimage. 25:1214–1223.
- Loewe B, Spitzer RL, Zipfel S, Herzog W. 2002. Gesundheitsfragebogen für patienten (PHQ-D). Karlsruhe: Pfizer.
- Lupien SJ, Gaudreau S, Tchiteya BM, Maheu F, Sharma S, Nair NP, Hauger RL, McEwen BS, Meaney MJ. 1997. Stress-induced declarative memory impairment in healthy elderly subjects: Relationship to cortisol reactivity. J Clin Endocrinol Metab. 82:2070–2075.
- Matsumoto Y, Mishima K, Satoh K, Shimizu T, Hishikawa Y. 2002. Physical activity increases the dissociation between subjective sleepiness and objective performance levels during extended wakefulness in human. Neurosci Lett. 326:133–136.
- Matthews G, Davies DR. 2001. Individual differences in energetic arousal and sustained attention: A dual task study. Pers Individ Dif. 31:575–589.
- Matthews G, Westerman GJ. 1994. Energy and tension as predictors of controlled visual and memory search. Pers Individ Dif. 17:617–626.
- Matthews G, Davies DR, Lees JL. 1990. Arousal, extraversion, and individual differences in resource availability. J Pers Soc Psychol. 59:150–168.
- McGaugh JL. 1989. Involvement of hormonal and neuromodulatory systems in the regulation of memory storage. Annu Rev Neurosci. 12:255–287.
- Nater UM, Moor C, Okere U, Stallkamp R, Martin M, Ehlert U, Kliegel M. 2007. Performance on a declarative memory task is better in high than low cortisol responders to psychosocial stress. Psychoneuroendocrinology. 32:758–763.
- Okuda S, Roozendaal B, McGaugh JL. 2004. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci USA. 101:853–858.
- Pfaff D, Ribeiro A, Matthews J, Kow LM. 2008. Concepts and mechanisms of generalized central nervous system arousal. Ann NY Acad Sci. 1129:11–25.
- Revelle W, Loftus DA. 1992. The implications of arousal effects for the study of affect and memory. In: Christianson SA, editor. The handbook of emotion and memory. Hillsdale, NJ: Erlbaum.
- Roozendaal B. 2002. Stress and memory: Opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem. 78:578–595.
- Roozendaal B, Okuda S, de Quervain DJ, McGaugh JL. 2006. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 138:901–910.
- Roozendaal B, McEwen BS, Chattarji S. 2009. Stress, memory and the amygdala. Nat Rev Neurosci. 10:423–433.
- Schimmack U, Reisenzein R. 2002. Experiencing activation: Energetic arousal and tense arousal are not mixtures of valence and activation. Emotion. 2:412–417.
- Schlotz W, Kumsta R, Layes I, Entringer S, Jones A, Wust S. 2008. Covariance between psychological and endocrine responses to pharmacological challenge and psychosocial stress: A question of timing. Psychosom Med. 70:787–796.
- Schwabe L, Boehringer A, Chatterjee M, Schachinger H. 2008. Effects of pre-learning stress on memory for neutral, positive and negative words: Different roles of cortisol and autonomic arousal. Neurobiol Learn Mem. 90:44–53.
- Schwabe L, Romer S, Richter S, Dockendorf S, Bilak B, Schachinger H. 2009. Stress effects on declarative memory retrieval are blocked by a beta-adrenoceptor antagonist in humans. Psychoneuroendocrinology. 34:446–454.
- Schwibbe M, Räder G, Schwibbe G, Borchard M, Geiken-Pophanken G. 1994. Zum emotionalen Gehalt von Substantiven, Adjektiven und Verben. In: Hager W, Hasselhorn M, editors. Handbuch deutschsprachiger Wortnormen. Göttingen: Hogrefe. 272–284.
- Steyer R, Schwenkmezger P, Notz P, Eid M. 1994. Testtheoretische Analysen des Mehrdimensionalen Befindlichkeitsfragebogens (MDBF). Diagnostica. 40:320–329.
- Thayer RE. 1970. Activation states as assessed by verbal report and four psychophysiological variables. Psychophysiology. 7:86–94.
- Thayer RE. 1989. The biopsychology of mood and arousal. New York: Oxford Unversity Press, Inc.
- Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Wagner H, Thorne D, Popp K, Rowland L, Welsh A, Balwinski S, Redmond D. 2000. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 9:335–352.
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. 2007. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 10:376–384.
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA. 2005. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci USA. 102:17804–17809.
- Yerkes RM, Dodson JD. 1908. The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurol Psychol. 18:459–482.