Abstract
Exercise is a paradigm of a stress situation. The adaptive response to stressors comprises the activation of the hypothalamic–pituitary–adrenal (HPA) axis and components of the autonomic sympathetic system. An aseptic inflammatory reaction is triggered by exercise, involving the stimulation of the so-called proinflammatory cytokines, such as tumor necrosis factor α (TNFα), interleukin-1 (IL-1), and IL-6. The anti-inflammatory cytokines IL-2, IL-8, and IL-10 increase moderately during resistance exercise. To investigate the effect of a short bout of resistance exercise on components of the stress and inflammatory responses during the exercise period, 17 healthy, young, untrained male volunteers were studied during 3 equal consecutive cycles of resistance exercises of 30 min total duration. Blood sampling was performed at baseline and at the end of each cycle. Lactate, cortisol, catecholamines (epinephrine, norepinephrine), IL-1α, IL-1β, IL-2, IL-6, IL-8, IL-10, epidermal growth factor (EGF), and monocyte chemotactic protein-1 (MCP-1) were measured at all time-points. Circulating levels of catecholamines and lactate increased significantly (P < 0.05) whereas cortisol did not. During the time course of the exercise, circulating levels of TNFα, IL-2, and EGF increased, whereas MCP-1 decreased significantly. IL-1α, IL-1β, IL-6, IL-8, and IL-10 levels did not change significantly. Statistically significant positive linear correlations were found between areas under the curve for increases in levels of IL-2 and TNFα, TNFα and cortisol, as well as epinephrine and norepinephrine. We conclude that acute resistance exercise results in catecholaminergic, but not HPA axis stimulation during exercise, in parallel with a mild inflammatory reaction. The absence of a major inflammatory reaction and of a cortisol increase during acute resistance exercise makes this a good candidate for the exercise of sedentary individuals.
Introduction
Exercise enhances and maintains physical fitness and overall health. Two main types of exercises are endurance and resistance. The former involves primarily aerobic metabolic pathways, whereas the latter relies mainly on anaerobic metabolism, marked by increased creatine phosphate degradation and lactate production. Resistance (or strength) training refers to the use of resistance to muscular contraction in order to build strength, anaerobic endurance, and the size of skeletal muscles. When correctly performed, resistance training can provide significant functional benefits by improving overall health and well-being. According to the American College of Sports Medicine (Haskell et al. Citation2007), the American Heart Association, and the American Diabetes Association (American Diabetes Association Citation2008), resistance exercise is recommended to healthy adults or adults with heart and/or diabetic pathology.
The term “stress” describes the state of a living organism when, under the influence of internal or external stimuli or “stressors”, the dynamic equilibrium of the organism (homeostasis) is threatened (Mastorakos et al. Citation2005). The adaptive response to stressors is multifaceted, including behavioral, systemic, and biochemical adaptations, such as the activation of the hypothalamic–pituitary–adrenal (HPA) axis and components of the autonomic sympathetic system (Mastorakos and Pavlatou Citation2005). Exercise can be considered a stressor because it causes dynamic changes of multiple systems of the organism, such as the cardiorespiratory system, necessitating the adaptation of these systems and resulting in the establishment of a new dynamic equilibrium. Indeed, the two main stress-activated systems, the norepinephrine-sympathetic system (with its endocrine component, the adrenal medulla) and the HPA axis, are activated by exercise and participate in the maintenance of homeostasis (Mastorakos and Pavlatou Citation2005).
However, several authors have described some effects of exercise as an inflammatory response. Aerobic treadmill exercise stimulates interleukin-1 (IL-1) and IL-6 secretion in parallel to the secretion of catecholamines (Papanicolaou et al. Citation1996). Of note, IL-6 has been assigned the term “myokine” because it is secreted directly from the muscle cytosol (Pedersen and Febraio Citation2008). Furthermore, exercise leads to an increase in number of circulating monocytes (Simpson et al. Citation2009) that produce the other proinflammatory cytokine, tumor necrosis factor α (TNFα). In addition, exercise induces an increase in the total white blood cell count, including the individual white cell lines, such as lymphocytes (Mayhew et al. Citation2005) and neutrophils (Ramel et al. Citation2004). The levels of anti-inflammatory cytokines IL-2, IL-8, and IL-10 have been shown to increase moderately during resistance exercise (Petridou et al. Citation2007). Nevertheless, little is known about the acute immune/inflammatory (I/I) response to resistance exercise. In general, most studies have reported that I/I changes after resistance exercise are modest compared with those after intensive and sustained cardiorespiratory endurance exercise (Nieman et al. Citation2004). However, it is possible that during acute resistance exercise, there is mobilization of various components of the I/I response, either locally at the level of the muscles, or generally as a response to the stress of exercise.
The impact of resistance exercise regarding stimulation of the peripheral catecholaminergic systems and the HPA axis, as well as its inflammatory aspects, has not been thoroughly studied as yet. Thus, this study was designed to investigate the effect of a short bout of resistance exercise in healthy volunteers regarding the responses of epinephrine, norepinephrine, and cortisol (stress hormones), as well as those of pro- and anti- inflammatory cytokines, as measured in the circulation.
Material and methods
Participants
Seventeen healthy, young, untrained male volunteers participated in the study. They were recruited among students of university faculties. Informed consent was obtained after all risks, discomforts, and benefits related to the study were explained to the participants. Procedures were in accordance with the Helsinki declaration of 1975, as revised in 1996, and the Institutional Review Board. None of the participants exercised regularly or had undergone any pharmacological or nutritional intervention for 6 months prior to the study. All participants had a stable body weight during the same period, and their fasting plasma glucose concentration was below 5.55 mmol/l. None reported a history of diabetes mellitus, insulin resistance, dyslipidemia, cardiovascular disease, or hypertension. Body weight, height, percentage body fat from skinfold thickness and waist circumference were measured using standard procedures, and body mass index (BMI) was calculated ().
Table I. Anthropometric values of the male subjects (n = 17) who underwent the 30-min resistance exercise, expressed as mean ± SD.
Protocol
The participants visited the laboratory on three occasions. During the first visit they completed a dietary record and had their anthropometric profile measured (). The participants abstained from physical activity, alcohol, and caffeine for 48 h prior to the second and third visits. During the second visit, they had their muscle strength measured in the exercises performed in the resistance exercise protocol. For each exercise, the 1-repetition maximum (RM) was determined. One RM is the maximum weight one can lift in a single repetition for a given exercise (). During the third visit, participants performed the supervised resistance exercise protocol. They exercised on 10 Universal machines (Vita Fitness, Rome, Italy) selected to stress the major muscle groups in the following order: chest press, seated row, leg press, shoulder press, leg extension, leg curls, arm curls, triceps extension, abdominal curls, and lower back extensions. Each subject performed three equal consecutive cycles of resistance exercises. Each cycle was timed to last 10 min. Thus, the resistance exercise protocol lasted 30 min. Between each cycle there was a 2-min resting period. Each cycle comprised 10 sets of resistance exercises in a circuit training fashion (one set of each of the previously described 10 resistance exercises per cycle). Between each set there was a 30-s resting period. Each exercise was performed at 70–75% of RM. Blood sampling was performed at baseline and at the end of each cycle via an antecubital vein cannula (kept patent by flushing with sterile normal, 0.9%, saline), Because venipuncture stress elevates catecholamine levels, baseline samples were collected with the subjects supine 10–15 min after cannulation. Blood was drawn into evacuated tubes containing either Serum Separation Tubes (SST) Gel (for serum analyses) or ethylenediaminetetraacetic acid (EDTA) or heparin (for plasma analysis). The tubes were placed on ice immediately. Blood samples were centrifuged (4°C, 1500g, 15 min), and the resulting serum (from the SST-containing tubes) and plasma (from the EDTA-or heparin-containing tubes) were stored in aliquots at − 75°C until assayed. For lactate determination, 200 μl of blood was immediately added to 400 μl of 5% trichloroacetic acid immediately after drawing and was centrifuged (2500g, 15 min). Samples were thawed once before analysis. All analyses were performed in duplicate.
Assays
To measure blood lactate, an enzymic method with reagents from Sigma (St Louis, MO, USA) was employed. The intra- and inter-assay coefficients of variation (CV) were 2.4 and 3.9%, respectively. Cortisol was measured using an electrochemiluminescence immunoassay (Roche, Basel, Switzerland). The intra- and inter-assay CVs were 1.3 and < 8%, respectively. Catecholamines were measured using high-performance liquid chromatography with electrochemical detection (Chromsystems Diagnostics, Munich, Germany). The intra- and inter-assay CVs were 1.7–11.4% and 3.7–12.7%, respectively. TNFα, monocyte chemotactic protein-1 (MCP-1), IL-1α, IL-1β, IL-2, IL-6, IL-8, IL-10, and epidermal growth factor (EGF) were quantified using the Evidence® biochip array analyzer from Randox (Crumlin, UK). The biochip used consists of a 9 × 9 mm substrate, on which discrete test regions have been constructed. The binding ligands (antibodies) were attached to predefined sites on the chemically modified surface of the biochip. After a simple enzyme-linked immunosorbent assay procedure, each spot was imaged to capture chemiluminescent signals generated on the array. The light signal is captured by a charge-coupled device camera as part of an imaging station and is converted by image-processing software to provide results with reference to calibration curves for each location on the biochip. The intra-assay CV was calculated from one run before analyzing the samples. The inter-assay CV was calculated from the data for two controls, run over the 5 days of sample analysis (n = 30). The intra- and inter-assay CVs were 8.4–11 and 8.9–13%, respectively. Cortisol was measured in serum. Catecholamines were measured in heparin-derived plasma. Cytokines and growth factors were measured in EDTA-derived plasma.
Statistical analyses
Results are reported as mean ± SE. All measured variables were analyzed using one-way ANOVA. Significant main effects were revealed by Fischer's post-hoc test. A time integrated measure of the concentration of each biochemical parameter, evaluated over the protocol exercise period, was calculated as the delta area above basal. The latter was estimated by the trapezoidal rule. The values obtained for each biochemical parameter were correlated with multiple regression analyses, and the appropriate coefficients of correlation were calculated. P < 0.05 was set as the level of significance. Statistical evaluation was performed with the STATISTICA 6 software (STATSOFT).
Results
Changes in circulating stress hormones and lactate during the exercise protocol
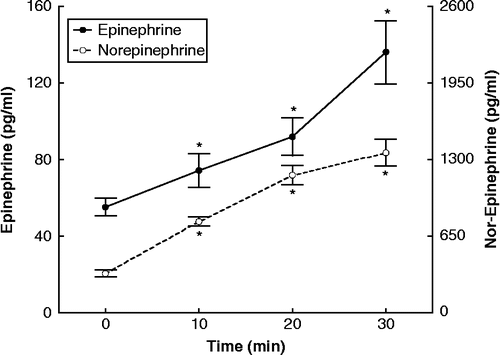
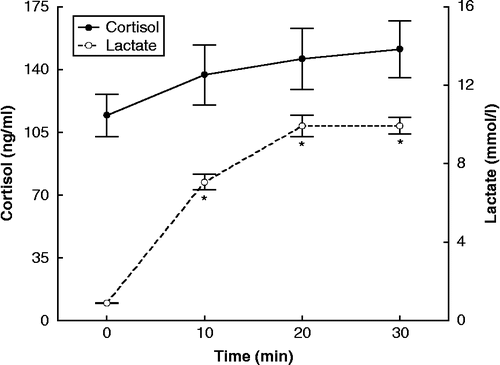
Acute resistance exercise resulted in significant increases in plasma concentrations of catecholamines (epinephrine, F = 10.194, df = 64, p < 0.05; norepinephrine, F = 115.56, df = 64, p < 0.05) () and blood lactate concentration (F = 38.035, df = 64, p < 0.05) (). Changes in serum cortisol concentrations were not significant ().
Changes in plasma concentrations of cytokines and inflammation markers during the exercise protocol
Acute resistance exercise caused significant increases in TNFα (F = 5.9636, df = 64, p < 0.05; ), IL-2 (F = 1.9888, df = 64, p < 0.05; ),and EGF (F = 2.3211, df = 64, p < 0.05; ) as well as a significant decrease in MCP-1 (F = 2.7015, df = 64, p < 0.05; ). IL-1α, IL-1β, IL-6, IL-8, and IL-10 did not exhibit statistically significant changes, during the time-course of the exercise ().
Figure 3. Concentrations of IL-2 and TNFα (assayed in plasma) during exercise, expressed as means ± SE. The asterisk indicates statistically significant (P < 0.05, n = 17) difference from the baseline (0 time) concentration.
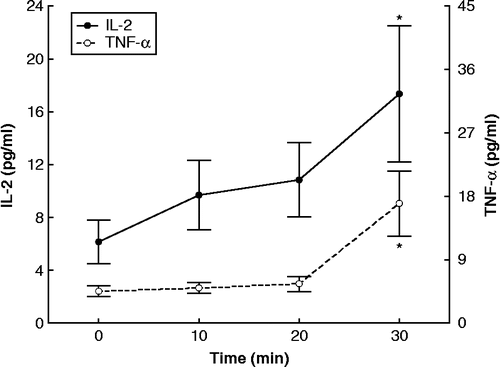
Figure 4. Concentrations of EGF and MCP-1 (assayed in plasma) during exercise, expressed as means ± SE. The asterisk indicates statistically significant (P < 0.05, n = 17) difference from the baseline (0 time) concentration.
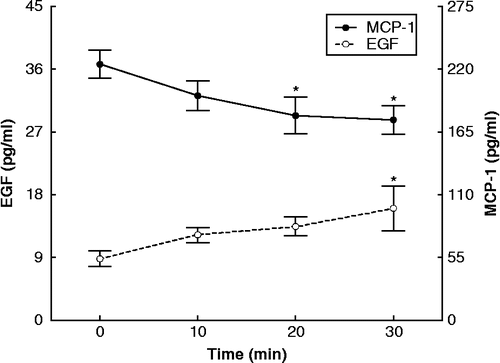
Table II. Plasma concentrations of interleukins that did not exhibit significant changes during acute resistance exercise (n = 17) (mean ± SE).
Correlations among circulating concentrations of stress hormones, cytokines, and inflammation markers during the exercise protocol
Statistically significant (P < 0.05) positive linear correlations were found between areas above basal for IL-2 and TNFα (r = 0.65), TNFα and cortisol (r = 0.59), and between epinephrine and norepinephrine (r = 0.84).
Discussion
In this study, we examined the interaction of components of the I/I response with the main stress hormones (epinephrine, norepinephrine, and cortisol) in the blood of young male volunteers during the time-course of a short bout of resistance exercise. The cellular components of the I/I response consist of circulating nonlymphoid leucocytes, lymphocytes, and local immune accessory cells (Ilias and Mastorakos Citation2002). Macrophages have an important role in muscle repair and remodeling after muscle injury (Tidball Citation2005). Cytokines released from mononuclear cells are important in deploying the I/I response and/or for promoting healing after injury (Smith et al. Citation2008). The proinflammatory cytokines IL-1, IL-6, and TNFα are mainly derived from macrophages and they are responsible for the acute phase response (Suffredini et al. Citation1999). IL-1 and TNFα are released early and act locally on fibroblasts and endothelial cells to induce IL-6 (CitationLord et al. 1991; Tosato and Jones Citation1990). IL-6 is also produced by muscle as a response to muscle contraction (myokine) (Pedersen and Febbraio 2008). All proinflammatory cytokines stimulate potently the HPA axis (Mastorakos et al. Citation2005). During the I/I response, among other actions, norepinephrine activates production of prostaglandins and proinflammatory cytokines (Elenkov and Chrousos Citation2006). Cortisol secreted following the proinflammatory cytokine-induced HPA axis stimulation inhibits these actions (Mastorakos and Pavlatou Citation2005). Thus, a periphera–central immunohormonal regulatory feedback loop is activated.
In this study, we found a significant increase in both epinephrine and norepinephrine levels in plasma during the 30-min resistance exercise bout. This is in accordance with previous reports (Kraemer et al. Citation1987; Kraemer and Ratamess Citation2005; French et al. Citation2007; Goto et al. Citation2008). Acute increases in circulating catecholamine levels are critical to optimal force production and energy liberation during resistance exercise (Kraemer and Ratamess Citation2005). Circulating catecholamine levels increase in submaximal resistance exercise, in parallel with antioxidants and neutrophil counts (Ramel et al. Citation2004). A marked catecholamine increase was demonstrated in different age, sex, and racial groups of men, women, and boys, following different resistance exercise protocols, confirming the consistency of this finding (Walker et al. Citation1992; Fry et al. Citation1998). Epinephrine is produced from chromaffin cells of the adrenal medulla as a part of the “fight or flight response”. Norepinephrine is released by sympathetic (noradrenergic) postganglionic nerve terminals, but also from the adrenal medulla. Proinflammatory cytokines including TNFα stimulate the adrenal medulla (O'Connor et al. Citation2000).
We found that cortisol, the other main stress hormone, did not display a statistically significant change, during the resistance exercise protocol. This is in accordance with some reports (Nieman et al. Citation1995; Hakkinen et al. Citation1998; Kraemer et al. Citation1998Citationb; Castellani et al. 2001; Pullinen et al. Citation2002; Goto et al. Citation2005), in particular those which applied similar experimental protocols (Kraemer et al. Citation1998Citationb; Goto et al. Citation2005). However, most studies on resistance exercise show an increase in cortisol levels (Kraemer et al. Citation1987; Hakkinen and Pakarinen Citation1993; McMurray et al. Citation1995; Ahtiainen et al. Citation2003; Ramel et al. Citation2003; Kokalas et al. Citation2004; Tremblay et al. Citation2004; Cadore et al. Citation2008). In several of these studies, the duration of exercise was longer than in the present one (McMurray et al. 1995; Ramel et al. Citation2003; Kokalas et al. Citation2004). This might be indicative of a late-onset increase in cortisol level. Furthermore, this discrepancy can be attributed to differences in training level, because regularly trained subjects demonstrate a smaller increase in cortisol level (Cadore et al. Citation2008). Differences in age also show conflicting results, with no cortisol increase in both young and older subjects (Hakkinen et al. 1998), or a cortisol increase in both age groups (Kraemer et al. Citation1998aCitation). Previous research has shown that the greatest cortisol response is induced by resistance exercise protocols that evoke the greatest blood lactate response, although significant correlations between these factors have been reported (Kraemer and Ratamess Citation2005). Metabolically demanding protocols high in total performed work, i.e. high volume (>3 sets) of moderate to high intensity (65–75% 1RM) and with short intervals between sets ( ≤ 1 min), elicit the greatest cortisol and lactate response as compared to protocols of higher intensity but lower total volume and longer intervals (Kraemer et al. Citation1987; Hakkinen and Pakarinen Citation1993; Smilios et al. Citation2003; Kraemer and Ratamess Citation2005). Exercise protocols of moderate intensity appear to increase circulating ACTH levels (Risøy et al. Citation2003). In this study, the exercise protocol employed was of moderate intensity, high work-volume and short rest-intervals. Eventually, this load elicited an HPA axis activation, even though the lactate concentration of less than 10 mmol/l concurs with the characterization of the exercise as moderate. The maximum post exercise blood lactate levels have been positively correlated with maximal short-term exercise performance. However, the time point when blood lactate maximizes in each individual is unpredictable. Models to correlate intensity of exercise with blood lactate levels are under development (Beneke et al. Citation2007).
We found an increase in plasma TNFα concentrations, 30 min from the onset of exercise. During exercise (aerobic and resistance), muscles are damaged as demonstrated by markers such as cell-free plasma DNA (Fatouros et al. Citation2006). Inflammatory stimuli induce macrophages, fibroblasts, and endothelial cells present in the muscle to produce proinflammatory cytokines such as TNFα. Increased circulating TNFα levels were reported during acute resistance exercise in middle-aged women (Yang et al. Citation2005). In addition, TNFα mRNA from muscle biopsies increased after resistance exercise (Louis et al. Citation2007; Raue et al. Citation2007). Given that in the past the same authors have shown no TNFα increase in a single muscle fiber, they suggest that the source of TNFα mRNA is most probably the proinflammatory cells in the muscle. The increase in circulating TNFα level in our study was likely facilitated by the low cortisol increase, because among all proinflamatory cytokines, TNFα demonstrates the greatest sensitivity to suppression by cortisol (DeRijk et al. Citation1997). However, cortisol levels correlated positively with TNFα levels. This correlation indicates the physiologic anti-inflammatory response of cortisol to TNFα, even though the former did not increase sufficiently to inhibit the latter. In contrast to TNFα, the other pro-inflammatory cytokines IL-1α, IL-1β, and IL-6 showed no statistically significant changes. This finding is in agreement with the study by Nieman et al. (Citation2004) in which even 2 h of intensive resistance exercise elicited, in homogenized muscle biopsies, only modest increases in IL-6 mRNA, whereas IL-1α and IL-1β mRNA levels did not change. In a similar study involving a single bout of resistance exercise, IL-1α, IL-1β, and IL-6 levels also did not increase, even though other markers of the inflammatory response, such as swelling and soreness of the muscles, were present (Nosaka and Clarkson Citation1996). The absence of IL-6 stimulation in the present study could explain the lack of an HPA axis response (Mastorakos and Pavlatou Citation2005; Mastorakos et al. Citation2005). In the past, we and others have shown that either exogenous or endogenous IL-6 is a potent stimulator of the HPA axis in humans (Mastorakos et al. Citation1993, Citation1994; Crofford et al. Citation1997). In addition to its short-term effects on the hypothalamus, IL-6 can also stimulate pituitary ACTH and adrenal cortisol secretion directly (Mastorakos et al. Citation2005). It is possible that longer observation of the IL-6 and the HPA axis responses might reveal a post-resistance exercise stimulation of both of these biological components of the stress response. Previously, aerobic exercise has been shown to be followed by an increase in plasma IL-6 levels within 20 min of exercise (Papanicolaou et al. Citation1996). However, there is evidence that resistance exercise of moderate intensity may cause various cytokine responses. A resistance exercise protocol similar to ours increased IL-6 level (Izquierdo et al. Citation2009). This enhanced cytokine response to resistance exercise at a given relative exercise intensity occurred with greater accumulated fatigue and metabolic demand measured by blood lactate accumulation, similar to that measured in the present study. Apparently, the magnitude of metabolic demand as reflected by the fatigue experienced during the resistance exercise session influences the cytokine response patterns.
In this study, MCP-1 levels decreased during the 30 min of resistance exercise. The major function of MCP-1 is chemotaxis, but it may also induce adhesion molecule expression, tissue factor secretion, and smooth muscle cell proliferation. It is secreted by a variety of cell and tissue types, including monocytes, and has a role in regulating interactions between muscle and macrophages (Tidball Citation2005). Paulsen et al. (Citation2005) showed an increase of MCP-1, but only after 6 h of resistance exercise; this increase paralleled that of cortisol. MCP-1 is an indicator of inflammation, so the absence of increase in either cortisol or MCP-1 levels in our study attests to mildness of the inflammatory reaction resulting from the resistance exercise protocol. Of interest is that catecholamines, levels of which were increased by resistance exercise, do not seem to increase MCP-1 release (Okutsu et al. Citation2008).
Circulating level of the anti-inflammatory cytokine IL-2 increased significantly with resistance exercise. IL-2 is mainly produced by antigen-stimulated CD4 T cells, but also by CD8 and Treg cells and induces monocyte differentiation in the muscle to dendritic cells, which also produce IL-2 (Olejniczak and Kasprzak Citation2008). Thus, the increase in IL-2 level during exercise might be part of an anti-inflammatory reaction to the exercise-induced inflammation via transformation of monocytes in muscle (Wahl et al. Citation2007). Previous studies of resistance exercise failed to show this IL-2 increase (Nosaka and Clarkson Citation1996; Nieman et al. Citation2004). The increase in level of this anti-inflammatory cytokine correlates with that of TNFα, a pro-inflammatory cytokine, indicating that it might represent a compensatory mechanism to a relatively mild inflammatory reaction. The other anti-inflammatory cytokines, that is IL-8, a chemokine produced by macrophages, epithelial cells and endothelial cells (Hull et al. Citation2001), and IL-10, an anti-inflammatory cytokine produced primarily by monocytes (Grimbaldeston et al. Citation2007), showed no statistically significant changes, in agreement with minor increases reported after 2 h of intensive resistance exercise (Nieman et al. Citation2004). Finally, levels of EGF, produced by platelets and macrophages (Svoboda et al. Citation1992), increased in the present study, although EGF has been usually measured in response to aerobic exercise, where data are conflicting (Konradsen and Nexo Citation1988). In addition, heparin binding EGF-like growth factor secreted by macrophages and muscle, when strongly stimulated, may function to increase muscle cell survival during oxidative stress (Horikawa et al. Citation1999). Our finding may be indicative of the acute phase adaptations to resistance exercise. It is noteworthy that subjects participating in this study were overweight according to BMI measurement. Obese and overweight subjects present different levels of low-grade inflammation (Lemieux et al. Citation2001). The finding that resistance exercise further increases certain inflammatory markers in these overweight subjects underscores its role in the generation of an I/I response.
In conclusion, a short bout of resistance exercise with a regular workload results in catecholaminergic rather than HPA axis stimulation, followed by a mild inflammatory reaction as reflected by increased circulating TNFα level. Our findings suggest that such exercise might be appropriate for the training of sedentary subjects, because in addition to its established health benefits, it does not produce major adverse inflammatory reactions and increased cortisol levels. This suggestion needs further validation, studying resistance exercise in specific groups, such as elderly and obese subjects, and patients with diabetes or rheumatoid arthritis. Interestingly, the ADA has added a short bout of resistance exercise among the recommendations for the management of diabetes. Further study of the complex physiology of resistance exercise, focusing on metabolic pathways, is needed to gain a deeper insight into the adaptive responses.
Acknowledgments
Grants or Fellowships: Funding (reagent expenses) was received from Randox Laboratories, Crumlin, UK (to I.P. and A.M.). The funding sources played no role in the study design, in the collection, analysis, and interpretation of data, in the writing of the report or in the decision to submit the report for publication. Financial disclosures: None.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ahtiainen JP, Pakarinen A, Kraemer WJ, Hakkinen K. 2003. Acute hormonal and neuromuscular responses and recovery to forced vs maximum repetitions multiple resistance exercises. Int J Sports Med. 24:410–418.
- American Diabetes Association. 2008. Standards of medical care in diabetes—2008. Diabetes Care. 31 Suppl. 1: S12–S54.
- Beneke R, Jumah MD, Leithäuser RM. 2007. Modelling the lactate response to short-term all out exercise. Dyn Med. 6:10.
- Cadore EL, Lhullier FL, Brentano MA, da Silva EM, Ambrosini MB, Spinelli R, Silva RF, Kruel LF. 2008. Hormonal responses to resistance exercise in long-term trained and untrained middle-aged men. J Strength Cond Res. 22:1617–1624.
- Castellani JW, Armstrong LE, Kenefick RW, Pasqualicchio AA, Riebe D, Gabaree CL, Maresh CM. 2001. Cortisol and testosterone concentrations in wheelchair athletes during submaximal wheelchair ergometry. Eur J Appl Physiol. 84:42–47.
- Crofford LJ, Kalogeras KT, Mastorakos G, Magiakou MA, Wells J, Kanik KS, Gold PW, Chrousos GP, Wilder RL. 1997. Circadian relationships between interleukin (IL)-6 and hypothalamic–pituitary–adrenal axis hormones: Failure of IL-6 to cause sustained hypercortisolism in patients with early untreated rheumatoid arthritis. J Clin Endocrinol Metab. 82:1279–1283.
- DeRijk R, Michelson D, Karp B, Petrides J, Galliven E, Deuster P, Paciotti G, Gold PW, Sternberg EM. 1997. Exercise and circadian rhythm-induced variations in plasma cortisol differentially regulate interleukin-1 b (IL-1b), IL-6, and tumor necrosis factor-α (TNFα) production in humans: High sensitivity of TNFa and resistance of IL-6. J Clin Endocrinol Metab. 82:2182–2191.
- Elenkov IJ, Chrousos GP. 2006. Stress system—organization, physiology and immunoregulation. Neuroimmunomodulation. 13:257–267.
- Fatouros I, Destouni A, Margonis K, Jamurtas AZ, Vrettou C, Kouretas D, Mastorakos G, Mitrakou A, Taxildaris K, Kanavakis E, Papassotiriou I. 2006. Cell-free plasma DNA as a novel marker of aseptic inflammation severity related to exercise overtraining. Clin Chem. 52:1820–1824.
- French DN, Kraemer WJ, Volek JS, Spiering BA, Judelson DA, Hoffman JR, Maresh CM. 2007. Anticipatory responses of catecholamines on muscle force production. J Appl Physiol. 102:94–102.
- Fry AC, Kraemer WJ, Ramsey LT. 1998. Pituitary-adrenal-gonadal responses to high-intensity resistance exercise overtraining. J Appl Physiol. 85:2352–2359.
- Goto K, Higashiyama M, Ishii N, Takamatsu K. 2005. Prior endurance exercise attenuates growth hormone response to subsequent resistance exercise. Eur J Appl Physiol. 94:333–338.
- Goto K, Takahashi K, Yamamoto M, Takamatsu K. 2008. Hormone and recovery responses to resistance exercise with slow movement. J Physiol Sci. 58:7–14.
- Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. 2007. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 8:1095–1104.
- Hakkinen K, Pakarinen A. 1993. Acute hormonal responses to two different fatiguing heavy-resistance protocols in male athletes. J Appl Physiol. 74:882–887.
- Hakkinen K, Pakarinen A, Newton RU, Kraemer WJ. 1998. Acute hormone responses to heavy resistance lower and upper extremity exercise in young versus old men. Eur J Appl Physiol Occup Physiol. 77:312–319.
- Haskell WL, Lee I-M, Pate RP, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. 2007. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 116:1081–1093.
- Horikawa M, Higashiyama S, Nomura S, Kitamura Y, Ishikawa M, Taniguchi N. 1999. Upregulation of endogenous heparin-binding EGF-like growth factor and its role as a survival factor in skeletal myotubes. FEBS Lett. 459:100–104.
- Hull J, Ackerman H, Isles K, Usen S, Pinder M, Thomson A, Kwiatkowski D. 2001. Unusual haplotypic structure of IL8, a susceptibility locus for a common respiratory virus. Am J Hum Genet. 69:413–419.
- Ilias I, Mastorakos G. 2002. The emerging role of peripheral corticotrophin-releasing hormone (CRH). J Endocrinol Invest. 26:364–371.
- Izquierdo M, Ibañez J, Calbet JA, Navarro-Amezqueta I, González-Izal M, Idoate F, Häkkinen K, Kraemer WJ, Palacios-Sarrasqueta M, Almar M, Gorostiaga EM. 2009. Cytokine and hormone responses to resistance training. Eur J Appl Physiol. 107:397–409.
- Kokalas N, Tsalis G, Tsigilis N, Mougios V. 2004. Hormonal responses to three training protocols in rowing. Eur J Appl Physiol. 92:128–132.
- Konradsen L, Nexo E. 1988. Epidermal growth factor in plasma, serum and urine before and after prolonged exercise. Regul Pept. 21:197–203.
- Kraemer WJ, Ratamess NA. 2005. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 35:339–361.
- Kraemer WJ, Noble BJ, Clark MJ, Culver BW. 1987. Physiologic responses to heavy-resistance exercise with very short rest periods. Int J Sports Med. 8:247–252.
- Kraemer WJ, Hakkinen K, Newton RU, McCormick M, Nindl BC, Volek JS, Gotshalk LA, Fleck SJ, Campbell WW, Gordon SE, Farrell PA, Evans WJ. Acute hormonal responses to heavy resistance exercise in younger and older men. Eur J Appl Physiol Occup Physiol. 1998a; 77:206–211.
- Kraemer WJ, Staron RS, Hagermen FC, Hikida RS, Fry AC, Gordon SE, Nindl BC, Gothshalk LA, Volek JS, Marx JO, Newton RU, Hakkinen K. The effects of short-term resistance training on endocrine function in men and women. Eur J Appl Physiol Occup Physiol. 1998b; 78:69–76.
- Lemieux I, Pascot A, Prud'homme D, Alméras N, Bogaty P, Nadeau A, Bergeron J, Després JP. 2001. Elevated C-reactive protein: Another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol. 21:961–967.
- Lord PC, Wilmoth LM, Mizel SB, McCall CE. 1991. Expression of interleukin-1 alpha and beta genes by human blood polymorphonuclear leukocytes. J Clin Invest. 87:1312–1321.
- Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. 2007. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol. 103:1744–1751.
- Mastorakos G, Pavlatou M. 2005. Exercise as stress model and the interplay between the hypothalamus-pituitary-adrenal and the hypothalamus-pituitary-thyroid axes. Horm Metab Res. 37:577–584.
- Mastorakos G, Chrousos GP, Weber JS. 1993. Recombinant interleukin-6 activates the hypothalamic–pituitary–adrenal axis in humans. J Clin Endocrinol Metab. 77:1690–1694.
- Mastorakos G, Weber JS, Magiakou MA, Gunn H, Chrousos GP. 1994. Hypothalamic–pituitary–adrenal axis activation and stimulation of systemic vasopressin secretion by recombinant interleukin-6 in humans: Potential implications for the syndrome of inappropriate vasopressin secretion. J Clin Endocrinol Metab. 79:934–939.
- Mastorakos G, Pavlatou M, Diamanti-Kandarakis E, Chrousos GP. 2005. Exercise and the stress system. Hormones (Athens). 4:73–89.
- Mayhew DL, Thyfault JP, Koch AJ. 2005. Rest-interval affects leukocyte levels during heavy resistance exercise. J Strength Cond Res. 19:16–22.
- McMurray RG, Eubank TK, Hackney AC. 1995. Nocturnal hormonal responses to resistance exercise. Eur J Appl Physiol Occup Physiol. 72:121–126.
- Nieman DC, Henson DA, Sampson CS, Herring JL, Suttles J, Conley M, Stone MH, Butterworth DE, Davis JM. 1995. The acute immune response to exhaustive resistance exercise. Int J Sports Med. 16:322–328.
- Nieman DC, Davis JM, Brown VA, Henson DA, Dumke CL, Utter AC, Vinci DM, Downs DF, Smith JC, Carson J, Brown A, McAnulty SR, McAnulty LS. 2004. Influence of carbohydrate ingestion on immune changes after 2 h of intensive resistance training. J Appl Physiol. 96:1292–1298.
- Nosaka K, Clarkson PM. 1996. Changes in indicators of inflammation after eccentric exercise of the elbow flexors. Med Sci Sports Exerc. 28:953–961.
- O'Connor TM, O'Halloran DJ, Shanahan F. 2000. The stress response and the hypothalamic–pituitary–adrenal axis: From molecule to melancholia. QJM. 93:323–333.
- Okutsu M, Suzuki K, Ishijima T, Peake J, Higuchi M. 2008. The effects of acute exercise-induced cortisol on CCR2 expression on human monocytes. Brain Behav Immun. 22:1066–1071.
- Olejniczak K, Kasprzak A. 2008. Biological properties of interleukin 2 and its role in pathogenesis of selected diseases—a review. Med Sci Monit. 14:179–189.
- Papanicolaou DA, Petrides JS, Tsigos C, Bina S, Kalogeras KT, Wilder R, Gold PW, Deuster PA, Chrousos GP. 1996. Exercise stimulates interleukin-6 secretion: Inhibition by glucocorticoids and correlation with catecholamines. Am J Physiol. 271:E601–E605.
- Paulsen G, Benestad HB, Strom-Gundersen I, Morkrid L, Lappegard KT, Raastad T. 2005. Delayed leukocytosis and cytokine response to high-force eccentric exercise. Med Sci Sports Exerc. 37:1877–1883.
- Pedersen BK, Febraio MA. 2008. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol Rev. 88:1379–1406.
- Petridou A, Chatzinikolaou A, Fatouros I, Mastorakos G, Mitrakou A, Chandrinou H, Papassotiriou I, Mougios V. 2007. Resistance exercise does not affect the serum concentrations of cell adhesion molecules. Br J Sports Med. 41:76–79.
- Pullinen T, Mero A, Huttunen P, Pakarinen A, Komi PV. 2002. Resistance exercise-induced hormonal responses in men, women, and pubescent boys. Med Sci Sports Exerc. 34:806–813.
- Ramel A, Wagner KH, Elmadfa I. 2003. Acute impact of submaximal resistance exercise on immunological and hormonal parameters in young men. J Sports Sci. 21:1001–1008.
- Ramel A, Wagner KH, Elmadfa I. 2004. Correlations between plasma noradrenaline concentrations, antioxidants, and neutrophil counts after submaximal resistance exercise in men. Br J Sports Med. 38:E22.
- Raue U, Silvka D, Jemiolo B, Hollon C, Trappe S. 2007. Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J Gerontol A Biol Sci Med Sci. 62:1407–1412.
- Risøy BA, Raastad T, Hallén J, Lappegård KT, Baeverfjord K, Kravdal A, Siebke EM, Benestad HB. 2003. Delayed leukocytosis after hard strength and endurance exercise: Aspects of regulatory mechanisms. BMC Physiol. 3:14.
- Simpson RJ, McFarlin BK, McSporran C, Spielmann G, ó Hartaigh B, Guy K. 2009. Toll-like receptor expression on classic and pro-inflammatory blood monocytes after acute exercise in humans. Brain Behav Immun. 23:232–239.
- Smilios I, Pilianidis T, Karamouzis M, Tokmakidis SP. 2003. Hormonal responses after various resistance exercise protocols. Med Sci Sports Exerc. 35:644–654.
- Smith C, Kruger MJ, Smith RM, Myburgh KH. 2008. The inflammatory response to skeletal muscle injury: Illuminating complexities. Sports Med. 38:947–969.
- Suffredini AF, Fantuzzi G, Badolato R, Oppenheim JJ, O'Grady NP. 1999. New insights into the biology of the acute phase response. J Clin Immunol. 19:203–214.
- Svoboda T, Wagner A, Speiser P, Clodi M, Luger A. 1992. Epidermal growth factor plasma concentrations in healthy control persons, acute and chronic stress and during pregnancy. Horm Metab. 24:582–584.
- Tidball JG. 2005. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 288:R345–R353.
- Tosato G, Jones KD. 1990. Interleukin-1 induces interleukin-6 production in peripheral blood monocytes. Blood. 75:1305–1310.
- Tremblay MS, Copeland JL, Van Helder W. 2004. Effect of training status and exercise mode on endogenous steroid hormones in men. J Appl Physiol. 96:531–539.
- Wahl P, Bloch W, Schmidt A. 2007. Exercise has a positive effect on endothelial progenitor cells, which could be necessary for vascular adaptation processes. Int J Sports Med. 28:374–380.
- Walker AJ, Bassett DRJr, Duey WJ, Howley ET, Bond V, Torok DJ, Mancuso P. 1992. Cardiovascular and plasma catecholamine responses to exercise in blacks and whites. Hypertension. 20:542–548.
- Yang SC, Chen CY, Liao YH, Lin FC, Lee WC, Cho YM, Chen MT, Chous CH, Kuo CH. 2005. Interactive effect of an acute bout of resistance exercise and dehydroepiandrosterone administration on glucose tolerance and serum lipids in middle-aged women. Chin J Physiol. 48:23–29.