Abstract
A comprehensive review of published and previously unpublished studies was performed to evaluate the neonicotinoid insecticides for evidence of developmental neurotoxicity (DNT). These insecticides have favorable safety profiles, due to their preferential affinity for nicotinic receptor (nAChR) subtypes in insects, poor penetration of the mammalian blood–brain barrier, and low application rates. Nevertheless, examination of this issue is warranted, due to their insecticidal mode of action and potential exposure with agricultural and residential uses. This review identified in vitro, in vivo, and epidemiology studies in the literature and studies performed in rats in accordance with GLP standards and EPA guidelines with imidacloprid, acetamiprid, thiacloprid, clothianidin, thiamethoxam, and dinotefuran, which are all the neonicotinoids currently registered in major markets. For the guideline-based studies, treatment was administered via the diet or gavage to primiparous female rats at three dose levels, plus a vehicle control (≥20/dose level), from gestation day 0 or 6 to lactation day 21. F1 males and females were evaluated using measures of motor activity, acoustic startle response, cognition, brain morphometry, and neuropathology. The principal effects in F1 animals were associated with decreased body weight (delayed sexual maturation, decreased brain weight, and morphometric measurements) and acute toxicity (decreased activity during exposure) at high doses, without neuropathology or impaired cognition. No common effects were identified among the neonicotinoids that were consistent with DNT or the neurodevelopmental effects associated with nicotine. Findings at high doses were associated with evidence of systemic toxicity, which indicates that these insecticides do not selectively affect the developing nervous system.
Introduction
The neonicotinoids are a commercially important class of insecticides, with increased usage in recent years due to their favorable safety profiles, the restrictions placed on other insecticides and their utility for resistance management (Jeschke et al. Citation2011; Simon-Delso et al. Citation2015). Representatives of this class are used to control insect pests in a variety of agricultural, commercial, residential, and veterinary settings, with insecticidal activity attributed to the activation of post-synaptic nicotinic acetylcholine receptors (nAChR) in insects (Buckingham et al. Citation1997; Nagata et al. Citation1998). Neonicotinoids registered in major markets are acetamiprid, imidacloprid, thiacloprid, clothianidin, thiamethoxam, and dinotefuran. These six neonicotinoids have distinct structural features compared with nicotine that result in enhanced selectivity for insect nAChRs (see for a comparison of structures). Also, the amino nitrogen atom in nicotine is ionized, while in the neonicotinoids, the corresponding nitrogen atom is not ionized but bears a partial positive charge (Yamamoto et al. Citation1998). Given their mode of insecticidal activity and that nicotine is neurotoxic and generally recognized as a developmental neurotoxicant (Levin & Slotkin Citation1998; Ernst et al. Citation2001), it is important to understand the neurotoxic potential of neonicotinoids in humans at all life stages and ensure the information used for risk assessments is suited to protect infants and children.
Table 1. Neonicotinoid insecticides examined in guideline developmental neurotoxicity studies and structural comparison to nicotineTable Footnotea.
Yamamoto and Casida (Citation1999) provide the most complete set of published mammalian toxicology information for the neonicotinoids, with chapters on imidacloprid, acetamiprid, thiamethoxam, and nitenpyram. These resource and studies cited by Chao & Casida (Citation1997) and Sheets (Citation2014) report that some neonicotinoids produce transient signs of nicotinic activity (e.g. tremors) in rats and mice following acute oral administration; however, there are considerable differences in activity across the class, with no evidence of nicotinic activity in rodents treated with dinotefuran at any dose level (Akayama & Minamida Citation1999). For reference, the expression of “nicotinic signs” following exposure to nicotine varies by species but generally includes ataxia, tremor, and seizures, followed by evidence of CNS depression, muscular weakness, paralysis, or respiratory failure (Schep et al. Citation2009). Previous reviews with a limited number of neonicotinoids (Chao & Casida Citation1997; Yamamoto Citation1999; Sheets Citation2002) indicate that these compounds are not developmental or reproductive toxicants, but this is the first critical review to evaluate the neonicotinoids for evidence of developmental neurotoxicity.
The specificity of the neonicotinoid insecticides for the nAChR subtype that occurs in insects, combined with poor penetration of the mammalian blood–brain barrier, rapid metabolism, and low application rates (Chao & Casida Citation1997; Tomizawa & Casida Citation1999 Citation2003 Citation2005; Yamamoto et al. Citation1995) contributes to high margins of human safety associated with commercial uses (Sheets Citation2002). By comparison, nicotine is more toxic to mammals than insects, due to a higher affinity for mammalian nAChRs and greater penetration across the blood–brain barrier in mammals (Chao & Casida Citation1997). Demonstrated differences in nAChR subtype specificity are illustrated by the comparison of LD50 values and receptor binding data in insects and mammals (). Based on acute LD50 values, nicotine is 5-fold more toxic to the rat (53 mg/kg) than the housefly (272 mg/kg), whereas imidacloprid is 20-fold less toxic to the rat (450 mg/kg) than the housefly (22 mg/kg) (Yamamoto Citation1999). Accordingly, imidacloprid binds avidly to the insect nAChR, and relatively poorly to the mammalian nAChRs, including the major neuronal subtypes α4β2 and α7, while nicotine binds poorly to the insect nAChR and more avidly to the mammalian nAChRs (Matsuda et al. Citation2001).
Table 2. Comparative acute toxicity and nicotinic receptor binding of substrates in insects and mammals.
A cursory literature search indicates limited published information that pertains to investigating the neonicotinoid insecticides for evidence of DNT. Examples include evidence of nicotinic activity (i.e. receptor-mediated Ca2+ influx) with imidacloprid and acetamiprid in primary cell cultures of cerebellar granule cells from neonatal rats (Kimura-Kuroda et al. Citation2012) and findings in offspring from pregnant rats after a single intraperitoneal dose of imidacloprid (337 mg/kg, 0.75 × LD50) on gestation day (GD) 9 (Abou-Donia et al. Citation2008). In addition, there is a complement of previously unpublished DNT studies performed in accordance with EPA (Citation1998) guidelines for all six neonicotinoid insecticides that are currently registered for commercial uses in major markets. Finally, there is a considerable body of published studies that implicate nicotine as a developmental neurotoxicant for reference (Slikker et al. Citation2005; Slotkin Citation1998 Citation2004).
The present review provides a critical evaluation of all the available in vitro and in vivo studies that tested neonicotinoid insecticides for DNT in mammals or other relevant test systems, as well as epidemiology studies that evaluated the relationship between evidence of exposure to neonicotinoids during pregnancy, infancy and/or early childhood, and neurodevelopmental outcomes. This includes findings identified in a comprehensive literature review for the entire class and previously unpublished studies performed in accordance with EPA (Citation1998) guidelines. These guideline studies were performed to complement developmental toxicity and two-generation reproduction studies for each neonicotinoid insecticide, in order to satisfy EPA requirements to investigate potential effects on the developing nervous system and determine whether an additional safety factor is needed to protect infants and children under the US Food Quality Protection Act (FQPA Citation1996). With limited information available in the literature, publishing these guideline DNT studies will significantly add to the body of information that is available to the broader scientific community. The collective results are then examined to determine whether the neonicotinoid insecticides produce evidence of DNT under conditions that are relevant to human health.
Methods
A comprehensive review of the literature was performed to identify all the published studies that might be relevant to the subject of the neonicotinoid insecticides and developmental neurotoxicity. The resulting collection of in vitro, in vivo, and epidemiology studies was then evaluated for relevance to the subject using established inclusion criteria. Previously unpublished DNT studies with the neonicotinoids that were performed in rats in accordance with EPA guidelines (EPA Citation1998) were also identified. Finally, the published and guideline-based studies that satisfied inclusion criteria were evaluated using criteria that have been developed to assess human relevance.
Literature review
A comprehensive literature search using PubMed was performed on 24 September 2014 and again on 16 June 2015 to gather the relevant studies that examined neonicotinoids for effects on the developing nervous system. This search used the following terms for development, neurotoxicity, and neonicotinoids, respectively: (1) offspring OR neonatal OR in utero OR development OR developmental OR pregnancy OR pregnant OR gestational OR newborn OR prenatal OR perinatal OR teratology OR fetus OR fetal OR age-dependent OR age dependent OR age sensitivity) AND (2) brain OR neuron OR nervous OR neurotoxic∗ OR neurolog∗ OR neurobehavior∗ OR neurodevelopment OR developmental neurotoxic∗ OR motor OR cognition OR cognitive OR behavior OR receptor OR neurotransmitter OR cerebellum OR hippocampus OR striatum OR cortex) AND (3) neonicotinoid OR imidacloprid OR acetamiprid OR clothianidin OR thiacloprid OR dinotefuran OR thiamethoxam. This search identified 236 citations that were screened for relevance to this review. A search performed on 25 June 2015 using ToxNet identified 284 citations that were also screened for subject relevance.
Additional MEDLINE (PubMed) searches were performed on 24 September 2014 and again on 16 June 2015 with the following terms and results: developmental neurotoxicity AND imidacloprid (OR acetamiprid) – one citation; developmental neurotoxicity AND thiacloprid (OR clothianidin, thiamethoxam, dinotefuran) – 0 citations; neurotoxicity AND neonicotinoid – seven citations; neurodevelopmental toxicity AND neonicotinoid – one citation; neurodevelopmental toxicity AND (imidacloprid, acetamiprid, thiacloprid, clothianidin, thiamethoxam, OR dinotefuran) – 0 citations; chloronicotinyl insecticides AND developmental neurotoxicity – 0 citations; brain development AND neonicotinoid – eight citations. A broader search of nervous system AND neonicotinoid provided 52 citations, while nervous system AND neonicotinoid NOT insect – identified 11 citations. Reference lists in recent articles on the subject were also cross-checked for relevant papers that may have been missed by the aforementioned searches. A search performed using ToxNet on 25 June 2015 identified no additional citations that pertained to the subject.
Inclusion criteria
In vivo studies
Studies from the literature reviews were included if exposure involved a single neonicotinoid insecticide that was delivered during gestation and/or lactation and the study measured neurodevelopmental endpoints. Inclusion criteria used by the EPA (Citation2012) were applied to determine whether the study warranted inclusion and detailed evaluation for reliability and relevance to human health. Those criteria consisted of the following: results are presented in English as a full article or as an EPA (including joint review with PMRA) evaluation, with data (i.e. not a secondary source or only an abstract); exposure to a single neonicotinoid, not a mixture; the test article characterization, chemical concentration, and dose levels are clearly reported; the duration of exposure during gestation and/or lactation before weaning is clearly defined; studies with exposure continuing after weaning (e.g. one- or multi-generation studies) are included; treatment group(s) is compared with appropriate concurrent controls. If testing a commercial formulation, this would include a group treated with the formulation minus the active ingredient; the nature of the test organism and sample size are defined; and findings are considered relevant to assess human health risks for DNT outcomes, including functional, neurologic, morphologic or pharmacologic changes.
In vitro studies
In vitro studies were included if published in English and the investigators link the findings to DNT outcomes with exposure to a single neonicotinoid.
Epidemiology studies
The search criteria identified studies that evaluated the relationship between exposure to neonicotinoids during pregnancy, infancy and/or early childhood, and neurodevelopmental outcomes. Studies that ascertained pesticide exposure by questionnaires, environmental monitoring, or biomarkers were eligible for inclusion, provided that exposure to specific neonicotinoids or to neonicotinoids in general was measured or queried directly.
Guideline studies
A search for guideline DNT studies was performed by identifying all the neonicotinoid insecticides that are registered for commercial uses in the US, where this study is conditionally required for registration, and by contacting the principal registrants, as these studies were not published. Reviews of the associated study reports performed by the EPA or jointly with Health Canada Pesticide Management Regulatory Agency (PMRA) were used, along with determinations from the study scientists, to identify laboratory-specific study design and procedures and findings that were attributed to treatment.
Criteria to evaluate suitability to support human health risk assessment
In vitro studies
Standardized criteria based on EPA (Citation2012) were used to evaluate the utility of in vitro studies for identifying a potential hazard or understanding a mode of action relevant to DNT. These criteria included adequate descriptions of the test system/test method, purity, composition, and origin of the test substance, dose-concentrations tested, solubility, impurities and pH, the presence or absence of metabolic activation, appropriate negative and positive controls for reference, and a clear description of statistical methods used to analyze the data.
In vivo studies
The following standardized criteria were used as guiding principles to evaluate the reliability and suitability of studies to identify potential neurodevelopmental effects on human health. These are consistent with criteria used by others to review neurodevelopmental toxicology in the peer-reviewed literature (Adams Citation2010; Maurissen Citation2010; Neurotoxicology and Teratology Guide for Authors 2015) and those articulated by EPA’s Office of Pesticide Program Guidance for considering and using open literature toxicity studies to support human health risk assessment (EPA Citation2012).
Litter of origin is accounted for in study design and statistical analyses; maternal influences with exposure during gestation or lactation may exert significant impact on the offspring (DeSesso et al. Citation2009; Holson & Pearce Citation1992; Holson et al. Citation2008).
A minimum of six animals per treatment condition is needed to provide minimal confidence in the results (Chapin et al. Citation2008, p. 235). For reference, the DNT guideline requires a minimum 10 per sex for neurobehavioral endpoints (EPA Citation1998).
Route of administration should be relevant to human exposure conditions.
Dose–response evaluations include more than two dose levels; studies with only one dose level are considered to have very limited utility.
Studies conducted only at dose levels associated with significant systemic toxicity or lethality, or designed to compare LD50s, were considered to be of low utility.
The time of testing needs to be balanced across dose groups and other factors if data are pooled in the statistical analyses (Maurissen Citation2010). For example, if sex is a factor in the analysis, the time of testing should be balanced with respect to sex.
Contemporary historical control data from the same laboratory using identical methods can be used to assess reliability of the methodology, variability inherent in the measure, and whether the difference from control represents a treatment effect (EPA Citation2014; Raffaele et al. Citation2008).
Epidemiology studies
The suitability of the epidemiology studies is based on the following questions developed by EPA Office of Pesticide Programs (EPA Citation2012; Li et al. Citation2012):
Was the study conducted primarily in a hypothesis-generating or a hypothesis-testing mode? Studies with no specific a priori hypothesis are more likely to generate false-positive results.
Were the methods used for assessing exposure and outcomes valid, reliable and adequate? Was a wide range of exposures sampled?
Was appropriate information collected on both cases and controls for potentially confounding factors, such as sociodemographic, behavioral, and dietary factors, and were they appropriately controlled in the analyses of the data?
Did the study sample the population or individuals of interest? Was selection bias minimized and generalizability optimized? How does the study population relate to the universe of potentially exposed populations?
Did the study have adequate statistical power to detect meaningful differences for outcomes between the different groups of exposed and unexposed or less exposed individuals, while controlling for important confounding factors?
Results
The numbers of publications identified in the literature using various search terms are provided in the Methods. Only six of these publications, which report in vivo or in vitro studies with imidacloprid, acetamiprid, and clothianidin, satisfied the inclusion criteria used to identify the relevant literature (). The conformance of these studies with criteria used to establish human relevance is shown in , with the results and experimental design summarized in . This review provides a summary of key results of published studies and a discussion of their strengths and limitations, relative to an evaluation of developmental neurotoxicity and human relevance. The reader is referred to the original articles for the actual data. Publications that did not meet inclusion criteria include reports of studies that examined neurotoxicity in insects or adult vertebrates or developmental toxicity but without any neurodevelopmental endpoints. Two epidemiology studies that are relevant to this subject were also identified. Yang et al. (Citation2014) did not satisfy the inclusion criteria that pertain to exposure but it was included to provide additional perspective.
Table 3. Inclusion criteria used to evaluate publications in this review.
Table 4. Criteria used to evaluate the strength and relevance of studies included in this review.
Table 5. Results of published studies that examined neonicotinoid insecticides for developmental neurotoxicity.
Publications: in vitro DNT studies
Kimura-Kuroda et al. (Citation2012) examined the effects of imidacloprid (>98% pure), acetamiprid (>98% pure), and nicotine (>99% pure) on primary cell cultures of cerebellar granule cells that were collected from newborn rats and cultured for 14 d. All three compounds were reported to produce an excitatory Ca2+ influx at concentrations greater than 1 μM in small neurons that expressed mRNA of α3-, α4- and α7-nAChR subunits that was inhibited by nAChR antagonists (α-bungarotoxin, dihydro-β-erythroidine, and mecamylamine). This excitatory response to acetamiprid and imidacloprid was shown to occur at lower concentrations than predicted based on their binding affinities (Tomizawa & Casida Citation2005). The firing patterns, proportion of excited neurons, and peak excitatory Ca2+ influxes with acetamiprid and imidacloprid differed from nicotine, which was attributed to different effects on nAChRs by the neonicotinoids.
These results suggest that acetamiprid and imidacloprid express greater potency for nAChRs that are present in the neonatal rat than expected based on binding affinities for nAChRs expressed in adult tissues (). However, this model system has severe limitations to assess the potential for neonicotinoids to produce acute neurotoxicity or DNT in vivo. For example, the neurons had reached an advanced stage of maturation, since proliferation, migration, and differentiation had already occurred (Bal-Price et al. Citation2010) and metabolic degradation and a blood–brain barrier are critical components that do not exist in this system. Also, the method used to examine nAChR activity was not specific to a single mode of action, which may explain inhibition with a variety of nAChR antagonists and the similar reaction of the cells to nicotine, acetamiprid, and imidacloprid at equimolar concentrations, despite considerable differences in relative potency (). Further, the antagonism of the Ca2+ influx by probes that selectively affect either α7 or α4β2 receptors had the same effect on nicotine and both neonicotinoids at equimolar concentrations, which the authors acknowledge, was unexpected. For example, the effect of all three chemicals was completely blocked by the homomeric nAChR antagonist α-bungarotoxin, suggesting an α7-mediated effect, whereas the heteromeric nAChR antagonist dihydro-β-erythroidine (DhβE) also blocked their effects completely. Finally, alternative (non-cholinergic) reactions and pathways may have been involved, as this system used an indirect measurement of a cholinergic reaction (by excluding acetylcholine as a positive control). In any case, evidence of nAChR activity as a molecular-initiating event in this model system must be evaluated in the context of in vivo results to support DNT as an adverse outcome (Bal-Price et al. Citation2015).
Publications: in vivo DNT studies
Abou-Donia et al. (Citation2008) investigated the effects of imidacloprid (∼99.5% pure) administered as a single intraperitoneal (i.p.) dose in corn oil to pregnant Sprague–Dawley rats on gestation day (GD) 9, with groups consisting of five vehicle control and five treated animals (). The treated group received 337 mg imidacloprid/kg body weight, which was cited as 75% of the acute oral LD50 in rats; however, the relative potency by intraperitoneal versus oral administration is unknown. Inclined plane, beam-walking, and forepaw grip performance were evaluated on post-natal day (PND) 30, with motor cortex, septal hippocampus, and cerebellum examined for histopathology.
Differences from control in F1 (first-generation offspring) animals that were attributed to treatment included deficits in beam-walk time without a difference in beam-walk score. Deficits in the inclined plane test and grip time were also noted, along with increased plasma cholinesterase activity (F1 males only), and brain region-specific acetylcholinesterase activity in F1 males and females. Glial fibrillary acidic protein (GFAP) immunostaining was increased in motor cortex (layer III), CA1 layer, CA3 layer, and dentate gyrus of the hippocampus of F1 animals, compared with vehicle control, without evidence of histopathology in any brain region examined.
Key aspects of the experimental design and analysis present challenges with respect to data reliability and human relevance, which is consistent with the author’s conclusion that the findings require additional investigation for support. This includes the use of a small sample size and a single high dose administered by a route of uncertain relevance to human exposures. The reported differences from control, including beam-walk time and inclined plane performance, were also minimal, with littermates treated as independent subjects (N = 20 pups versus N = 5 litters), which can severely inflate alpha levels (i.e. the false declaration of an effect) (Holson et al. Citation2008). Further, having only one dose group to compare with controls precludes establishing a dose–response relationship. The exposure window and corresponding effects observed also raise questions that pertain to the experimental design and known biology. In particular, GFAP immunostaining, which represents an active glial process, was increased in specific brain regions ∼50 d after a single dose. The mechanism that would mediate such a response is unclear, especially since only rudimentary elements of the CNS (i.e. neural plate and early neural folds; DeSesso Citation2012) are present when the dose was administered.
Tanaka (Citation2012a,Citationb) reports two studies with clothianidin (>99% pure)-treated CD-1 mice (). In Tanaka (Citation2012a), parental (P)-females received clothianidin in the diet at 0, 20, 60, or 180 ppm during gestation and lactation, and all groups received control diet after weaning. In Tanaka (Citation2012b), P-generation male and female mice received clothianidin at constant dietary levels of 0, 30, 60, or 120 ppm from 5 weeks of age until the F1 offspring were 11 weeks old. Measures in F1 animals in both studies included surface righting, negative geotaxis, cliff avoidance, swimming behavior, olfactory orientation, cognitive function (in a Biel multiple-T water maze), exploratory behavior (3 and 8 weeks old; 10-min session), and spontaneous behavior (9–10 weeks old; 120 min session with twelve 10-min intervals). Both studies used the same equipment and measures of exploratory and spontaneous behavior: the total distance (cm), number of horizontal activities, movement time(s), number of rearings, rearing time(s), average time of movement(s), average speed (cm/s), and average time of rearing(s). One pup/sex/litter was tested for all neurobehavioral measures, except for the spontaneous behavior and Biel maze, where all pups were reportedly tested. Multiple comparisons between treated and controls were conducted for each 10-min interval using the Steel–Dwass test. In addition, the longitudinal pattern over the 120-min session was statistically analyzed. Furthermore, a number of the differences ascribed to treatment were only supported by trend tests; not by the statistical analyses cited in the methods. The results from both studies are compared below to evaluate the consistency of effects on neurodevelopmental endpoints, with a more detailed and comprehensive comparison of results provided in the discussion.
Clothianidin had no effect on reproduction or body weight in P-generation males or females in either study at any dietary level. Further, measures of cognition in the Biel maze were not affected in F1 males or females in either study at any dose. Increased body weight was reported in F1-animals at PND 4–21 (Tanaka Citation2012a) or PND 4–7 (Tanaka Citation2012b), but these differences from control were not dose-related. Further, the group mean body weight of control F1 males (9.80 g) and females (9.34 g) on PND 21 in Tanaka (Citation2012a) was notably greater than control F1 males (8.28 g) and females (8.00 g) in Tanaka (Citation2012b). Treatment-related changes in some neurodevelopmental landmarks were noted during lactation, and movement parameters showed some differences at 3, 8, and/or 9–10 weeks of age; however, relatively few parameters were altered considering the large number of group comparisons that were analyzed for each endpoint, time point, and/or time interval. In addition, the pattern of statistical differences from control was somewhat inconsistent across these two studies, in terms of the endpoints that were involved or the sex and age associated with the finding, and differences from control occurred most often at the low- or mid-dose, not the high dose (). Therefore, it is unclear whether these differences in outcome indicate inconsistent results or are due to differences in study design.
These studies satisfy criteria used to judge data reliability and human relevance; however, comparison of results for control and treated animals between the two studies suggests variable data and modest differences for a few of multiple comparisons that were not consistently dose-related. Furthermore, the differences in neurodevelopmental parameters in treated F1 mice typically represented increases in performance, relative to control, that may be confounded by unexplained differences in body weight (i.e. higher body weights in treated groups) during lactation. A more detailed evaluation of these studies is provided in the Discussion section.
Ozdemir et al. (Citation2014) examined clothianidin (purity not reported) for potential effects on learning and memory in infant Wistar rats and the expression of related genes in the hippocampus. Doses of 0, 2, 8, and 24 mg/kg clothianidin were administered once daily by gavage on PND 7 through PND 97 to six F1 males per litter (4 litters per dose level). Separate groups of six adult males received the same complement of daily doses to establish whether findings were specific to developmental exposure. Cognitive function was evaluated using the Morris water maze, and mRNA expression levels of N-methyl d-aspartate 1 (GRIN 1), muscarinic receptor M1, synaptophysin (SYP), and growth-associated protein 43 (GAP-43) were measured in the hippocampus on PND 97. Acquisition (learning) was measured as the time required for animals to locate the submerged platform on each of five consecutive days, with memory consolidation measured in a probe trial on the following day, based on the time spent in the correct quadrant with the platform removed. The treatment had no effect on acquisition (learning phase) of the Morris water maze or gene expression at any dose. However, a significant reduction in time the high-dose animals spent in the target quadrant for the probe test suggests a treatment effect on the consolidation of memory. By comparison, clothianidin had no effect on performance in the Morris water maze in males that were treated only as adults for a similar duration.
This study evaluated multiple dose levels to establish a dose–response relationship and testing clothianidin for effects in separate groups of males treated for 3 months helps to support this finding being associated with exposure during development. However, this study does not satisfy important criteria used to judge data reliability and human relevance, including an adequate sample size, balancing testing across treatment groups to account for diurnal variation, consideration of the litter (rather than the individual pups) as the experimental unit and characterization of the test article. The use of different vehicles (corn oil or DMSO) to treat separate groups of adult males at the same doses also raises questions concerning the suitability (e.g. solubility) of water as a vehicle in the developmental study.
Crosby et al. (Citation2015) examined imidacloprid (purity not reported) for developmental neurobehavioral effects in zebrafish (Danio rerio) as a model organism. Zebrafish larvae were exposed via immersion in aqueous solutions containing 45 or 60 μM imidacloprid or 45 or 60 μM nicotine from 4 h through 5 d post-fertilization. Neurobehavioral testing was conducted on day 6 post-fertilization (larvae) or at 1.5 mo old (adolescent) or 3 mo (adult) of age. The battery of tests included activity measure, sensorimotor response and habituation in a tap-elicited startle test, novel tank swimming, and shoaling behavior. Both imidacloprid and nicotine decreased larval activity during dark phases (a time when control activity is higher compared to light phases). Persistent neurobehavioral changes were noted in adolescent (n = 15–18/dose group) and adult (n = 30–34/group) zebrafish. Developmental exposure to imidacloprid significantly increased the startle response in the adolescent but not the adult. Nicotine had similar effects, except that the startle response was increased only at the low (45 μM) dose for the adolescent fish, not at the high dose or adults at either dose. Developmental exposure to imidacloprid decreased novel tank exploration in both the adolescent and the adult, whereas decreased novel tank exploration following nicotine exposure was noted only for the adult. Neither treatment affected shoaling behavior or habituation to the startle response at either dose or age.
These results indicate persistent effects in zebrafish associated with exposure to imidacloprid via immersion during the first 5 d of development and that these effects are somewhat consistent with the findings in zebrafish associated with developmental exposure to nicotine. This study satisfies criteria used to judge data reliability, including multiple (two) dose levels, adequate sample size, and balancing testing across treatment groups. The purity of the test material was not stated, but high purity is expected based on the source of the material cited. No measures of body growth of the zebrafish were provided. Such information would help determine whether the reported findings were associated with a treatment-related effect on growth and development. There was no mention as to whether the tests were performed in a “blinded” fashion to avoid potential bias, although the tests were largely automated. The human relevance for the dosage and route of exposure (i.e. immersion of the larvae) in this screening model is uncertain and it is unclear how the findings from zebrafish relate to mammalian/human neuronal processes. For example, Papke et al. (Citation2012) report differences in the response of nAChRs in zebrafish to some nicotinic compounds, compared with mammals. Crosby et al. (Citation2015) acknowledge the need to extend the research from zebrafish to mammals.
Publications: epidemiology studies
Yang et al. (Citation2014) examined whether residential proximity to pesticide applications during early pregnancy was associated with an increased risk of anencephaly, spina bifida, or other anatomical anomalies (e.g. cleft palate), based on population-based data and information from maternal interviews for the San Joaquin Valley of California (1997–2006). This study was hypothesis generating in nature, with a “sizable” number of comparisons made. Interviews were conducted with mothers of 71% of eligible cases (n = 763) and 69% of controls (n = 974). Seventy-three cases of anencephaly and 123 cases of spina bifida were identified in the study population, with imidacloprid the only neonicotinoid among 257 chemicals and 52 chemical groups that were identified. The study established that there was a general lack of association between proximity to agricultural pesticide use and risks of selected birth defects, as the associated odds ratios for the birth defects examined may have arisen by chance alone. This paper failed to satisfy the inclusion criteria because pesticide exposure was based on residential proximity to pesticide use during early pregnancy, not evidence of actual exposure to any particular pesticide or chemical class.
Keil et al. (Citation2014) examined whether there was an association between autism spectrum disorder (ASD) and the use of products that contain approximately 9% imidacloprid as a flea and tick treatment on household pets from 3 months prior to conception through breastfeeding, as recalled by the mother when the children averaged 3.6–3.8 years of age. The dataset included information from 262 typically developing controls and 407 children assigned the status of ASD by study personnel. Children previously identified as having ASD were recruited from an administrative database of the California Department of Developmental Services, which contract 21 Regional Centers to coordinate services for persons with developmental disabilities, health and service providers, other research studies, and self-referrals. Exposure and confounder data were collected through a phone interview with the mother, with no biomonitoring information to establish or estimate exposure.
The adjusted odds ratio (OR) with 95% confidence interval (CI) was 1.3 (0.79–2.2). More specifically, the OR was 0.69 (CI 0.27–1.8) for occasional users (less than once a month during pregnancy) and 2.0 (CI 1.0–3.9) for mothers identified as consistent (at least once a month during pregnancy) users of these products. The models were adjusted for the child’s sex, regional center of birth, age, maternal education, race/ethnicity and parity, and pet ownership during the prenatal period. Keil et al. (Citation2014) caution that based on sensitivity analysis, the apparently higher ORs for exposure among consistent users could be a result of recall bias if mothers of children with ASD disproportionately report consistent (rather than occasional) use. Further, the paper cautions that analysis of subgroups (e.g. occasional versus consistent users) can result in loss of precision, due to the smaller numbers of individuals. Accordingly, Keil et al. (Citation2014) conclude the association between ASD and prenatal imidacloprid exposure in this study was weak and could result from exposure misclassification alone.
Guideline DNT studies
Guideline DNT studies with imidacloprid, acetamiprid, clothianidin, thiacloprid, thiamethoxam, and dinotefuran are included in this review. The only neonicotinoid insecticide with active registrations that is not included is nitenpyram, which has limited registrations only outside the US. The structures of the six neonicotinoids and purity of the test materials used in DNT studies are shown in .
Compliance
These studies were conducted in accordance with EPA OCSPP guideline 870.6300 (EPA Citation1998) and US (40 CFR Part 160) and OECD (ENV/MC/CHEM (98) 17) Good Laboratory Practice (GLP) guidelines. The principal elements of the experimental design are shown in , with differences in test procedures among the four laboratories that performed the studies listed in . Certain differences in experimental design, compared with this EPA guideline, were applied to these studies, to assist interpretation and to support compliance with anticipated requirements for OECD TG 426 (OECD Citation2007), which was not finalized when several of these studies were performed. These differences in design included (1) exposure from GD 0 or 6 – PND 21 (rather than GD 6 – PND 10); (2) the sample size for neuropathology and brain morphometry was 10 F1 animals/sex/dose, representing 20 litters at each age (rather than 6/sex/dose, representing 12 litters at each age); and (3) additional brain morphometry measurements were taken.
Table 6. Principal elements of EPA and OECD guideline developmental neurotoxicity studyTable Footnotea.
Table 7. Laboratory-specific test procedures for guideline developmental neurotoxicity studies.
Test material
Technical-grade test materials (95.5–99.5% purity) were provided by the Sponsors () and the identity of the active ingredient and stability under the conditions of storage were verified by appropriate analytical methods. Acetamiprid was formulated in corn oil and administered once daily by gavage to P-generation females, while other test materials were mixed in the diet for ad libitum consumption. Homogeneity and stability in corn oil or the diet were verified, and dose or dietary levels were periodically analyzed to verify the concentration of test substance given to the animals at each level and to determine the associated dose levels during gestation and lactation.
Study design
Test materials were administered at a constant concentration (ppm) in the diet or a constant dosage (mg/kg/d) administered by gavage from GD 0 or GD 6 through day 21 of lactation and postnatal development (). By convention, the day on which insemination was evident is designated GD 0 and the day of parturition is PND 0. Treatment via the diet or by gavage of the dam is used to model dietary exposure in humans during pregnancy and lactation, with the offspring exposed to test substance and/or toxicologically active metabolite(s) that passes through the placenta, into the milk, and (with dietary administration) by consuming progressively greater amounts of treated feed from ∼PND 15 to PND 21. Pharmacokinetic data, evidence of offspring toxicity, or changes in biomarkers provide evidence the offspring were exposed through the milk to the test substance or active metabolite(s).
P-females were randomly assigned to one of the three dose levels or a vehicle (corn oil or blank diet, as appropriate) control group (20–30/dose level). On specific days after parturition, F1 animals from each litter were randomly selected to provide the minimum number of males and females needed for neurobehavioral and neuropathologic assessments (). Details regarding the test procedures and associated measures, as well as requirements to verify the suitability of the various measures to identify treatment-related effects with reference chemicals, are provided in the test guideline (EPA Citation1998). Additional details of the procedures for neuropathology and brain measurements are also provided in Supplementary materials (Appendix I). In accordance with the test guideline (EPA Citation1998), the high dose was selected with the objective to induce evidence of toxicity in the dam or pups without precluding a meaningful evaluation of the results, with the mid- and low-doses selected to induce less toxicity than the high dose and no evidence of toxicity, respectively. Also in accordance with EPA guidelines, tissues from control and high-dose F1 males and females were examined for neuropathology and brain morphometry, with step-down analysis of lower dose groups if there was evidence of a treatment effect at the high dose. Additional details that are specific to the individual studies are provided in , .
Statistical analysis
The statistical analyses that were used in the guideline studies for each neonicotinoid are summarized in . In all cases, the litter was the experimental unit of analysis. In general, body weight, motor activity, acoustic startle response, and maze testing results were analyzed separately for each sex and age, using two-way repeated-measures analysis of variance (RANOVA) and/or one-way ANOVA, with post-hoc tests including Dunnett’s or Student’s t-test. For thiamethoxam, Y-maze performance data were initially transformed using Freeman and Tukey’s double arcsine transformation prior to one-way ANOVA for each intersession and total session measure. For acetamiprid, behavioral data for both sexes were combined (1 male or female per litter) and analyzed using a three-way RANOVA mixed model approach. Approaches used to analyze continuous data with unequal variance included Kruskal–Wallis followed by Dunn’s or Fisher's-Exact Test. For passive avoidance results with imidacloprid, latency-to-cross and trials-to-criterion data were analyzed using Kruskal–Wallis, Wilcoxon, and Fisher's exact tests. The incidence of neuropathology findings was analyzed using Fisher's exact or Kolmogorov–Smirnov tests. Brain morphometry data were analyzed using ANOVA (essentially t-test for two groups). For thiamethoxam, morphometry data were analyzed using an analysis of covariance, with final body weight as a covariate.
Table 8. Statistical analyses used in guideline developmental neurotoxicity studies.
Findings in guideline studies
The following analysis is based on reviews of the study reports and supporting data conducted by EPA or through joint review with Health Canada PMRA (). This approach provides the reader with a consistent and objective evaluation of the available information for all six neonicotinoids, performed by an authoritative body with the responsibility to evaluate and apply these results for hazard identification and risk assessments. For additional details, the reader is referred to the associated data evaluation records prepared by these Agencies (cited below). The interpretation of the scientists who performed the studies and/or analyzed the results is also included to provide additional information and perspective.
Table 9. Summary of results for guideline developmental neurotoxicity studiesTable Footnotea.
Decreased body weight associated with decreased weight gain during gestation or lactation was the most common treatment-related finding in P-females (); however, it should be understood that the evaluation of P-females is limited in this study, with the emphasis on evaluating the F1 generation for evidence of DNT. For F1 males and females, decreased body weight and weight gain during lactation were also the most common findings, with birth weight decreased only with the high dose of acetamiprid and thiamethoxam. None of the neonicotinoids affected reproduction or produced clinical signs in P-females or F1-animals at any dose. Further, there was no effect on habituation (motor activity or the acoustic startle response) or evidence of cognitive deficits or neuropathology in F1 males or females in any of these studies.
Acetamiprid
Acetamiprid (>99% purity) was administered daily by gavage to P-females on GD 6 to LD 21 at dose levels of 0, 2.5, 10, or 45 mg/kg/d. The NOAEL for P-females and F1 offspring was 10 mg/kg/d, based on the findings at the high dose of 45 mg/kg/d (EPA Citation2013a). Body weight was decreased (4–5%; p < 0.05) from GD 9 to 20 in P-females at 45 mg/kg/d, with a mean body weight loss of 3 g from GD 6 to 9, compared with a mean 13 g gain in controls. For the entire gestation period, mean body weight gain in pregnant females at 45 mg/kg/d was 15% lower (p < 0.05) than the control group. Food consumption was decreased 40% (p < 0.05) during the first week of exposure (GD 6–12) and 18% (p < 0.05) from GD 6 to 20.
Treatment-related effects in high-dose F1 animals included decreased post-natal survival (PND 0–1; 3 dams had total litter loss on PND 1), decreased birth weight (−7% in F1 males (NS) and −9% in F1 females; p < 0.05), decreased body weight (−5 to −10% in F1 males and females) and decreased body weight gain (−9 to −21%, primarily in F1 males) after weaning to PND 72, and decreased acoustic startle response in F1 males on PND 20 (−42%) and PND 60 (−53%). There were no treatment-related macroscopic or microscopic findings for brain, spinal cord or peripheral nerves or for brain morphometry measurements. EPA (Citation2008) deferred drawing conclusions for the learning and memory and motor activity data (see below), but has since concluded “the toxicology database is complete for acetamiprid and acceptable guideline studies for developmental, reproductive toxicity, neurotoxicity (including DNT) and immunotoxicity are available” (EPA Citation2013b).
The acoustic startle response test in the DNT guideline is primarily designed to evaluate habituation (EPA Citation1998), which is a primitive learning process in which the organism decreases its response to redundant, non-significant stimuli (Davis Citation1984). On both PND 20 and PND 60, there was no effect on acoustic startle habituation (no significant interaction of dose with trial; p > 0.05). On PND 20, there was a significant dose (p = 0.003) effect for peak startle amplitude, but no significant dose × sex interaction. In post-hoc comparisons with control, only the high dose was decreased significantly when males and females were combined (p = 0.002) or when males were analyzed alone (p = 0.023). On PND 60, males and females were analyzed separately, due to a significant dose × sex interaction (p = 0.038). There was a significant dose effect in males (p = 0.046) but not females, with high-dose males significantly different from control (p = 0.015).
There were numerical decreases in startle amplitude at 10 mg/kg on PND 20 (−27%) and PND 60 (−40%) that EPA did not consider were treatment related. This interpretation was based on the differences from control not being statistically significant and the group means for treated males being near the 50th percentile or within the inter-quartile range (IQR) of the historical control (HC) data (19–22 studies using identical test procedures), which indicates a startle response consistent with controls for the laboratory. Furthermore, these non-statistically significant differences in startle amplitude at 10 mg/kg/d are within the variability for control animals of 30% at weaning and 51% as adults for peak startle amplitude published by EPA scientists for smaller-scale studies (Supplementary Table 1). Benchmark dose–response levels of 25% and 40% (lower than EPA’s average CV (SD × 100/mean)) approximate the benchmark dose levels (1 SD) of 12.6 and 13.1 mg/kg/d, respectively, for PND 20 and 60 (PROAST software Version 38.9; RIVM Citation2014) using best fit and lowest values from Exponential and Hills models. This analysis provides additional support for the NOAEL of 10 mg/kg/d.
As mentioned above, EPA (Citation2008) indicated that further evaluation of the motor activity and Biel maze tests would rely on a retrospective analysis for comparison of the variability of results in this early study (one of the first submitted to EPA) with other DNT study results. EPA’s initial concerns were related to variability and control behavior for motor activity on PND 13 and 17 and variability in learning and memory data. It is now recognized that there is high biological variability for motor activity data in this study design, especially at PND 13, when the animals have limited mobility and sensory function, as well as PND 17, when sensorimotor skills and function are rapidly developing (Makris et al. Citation2009). The mean CVs (males and females) for the acetamiprid DNT study were 73, 70, 51, and 36% for motor activity for PND 13, 17, 21, and 61, respectively (EPA Citation2008 Citation2013a). These values are within the range (73–87; 46–75; 29–55; 13–38%) and comparable with the means ± SD (83 ± 3; 59 ± 12; 40 ± 13; 25 ± 9%) for the CV values of motor activity control data from five other neonicotinoid studies (EPA Citation2002a Citationb Citation2007 Citation2013b; Health Canada PMRA Citation2002).
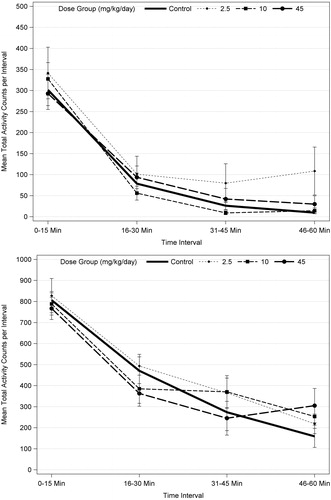
Repeated measures ANOVA analysis of motor activity with male and female data combined increased the sample size from 10 to 20 per dose group and resulted in a significant effect over intra-session time (p < 0.001) at PND 17, 21, and 61, which reflects habituation. There were no statistically significant dose effects or dose interactions with time at any age, indicating no difference in habituation for any dose group (). These results support the determination that acetamiprid did not affect motor activity at any age or dose level.
Figure 1. Motor activity for PND 21 (top) and PND 60 (bottom) F1 male rats (n = 10/sex/dose level) exposed to acetamiprid via gavage administration to the dam (GD 6 to LD 21). There were no statistically significant results based ANOVA for each trial for males or females, separately, or when data were combined for males and females and analyzed using repeated measures ANOVA and Dunnett’s (see text).
The Biel Maze test used in this study is one of the most challenging learning and memory tests used in studies performed in accordance with EPA (Citation1998) guidelines, as it involves sequential (Path A) and reverse (Path B) learning through a multiple-T water maze. EPA (Citation2008) noted non-statistically significant increases in the number of errors for trials 5–10 in Path B only in high-dose F1 males on PND 22, but not on PND 62 (), and no effect in females at either age. On PND 22, there was a significant effect of trial (p < 0.001) for both latency and the number of errors for Path A, Path B (path reversal), and Path A-probe (see for details), indicating learning in all groups. However, there were no significant dose × trial interactions (p = 0.146–0.993), indicating no effect of acetamiprid on learning (). There was a marginally significant overall dose effect (p = 0.053) for the number of errors for Path A, with only the high dose level marginally significant (p = 0.060; Dunnett’s). Graphical presentation of the data indicates that the most consistent learning behavior occurs during Path B (), after the animal was introduced to the maze in Path A, and that there is no consistent dose-related pattern of effects on Path B.
Figure 2. Mean (±SEM) number of errors per trial on Biel water maze (8 unit T-test) in F1 male offspring (10/sex) on PND 22 (top) and PND 62 (bottom), following developmental exposures to acetamiprid. Both males and females were tested for 7 d with four swim trials on a straight channel on the first day (no effect; data not shown); 2 trials/day on Path A for 2 d (A1–A4), 2 trials/d on path B (reverse of path A; B5–B10) for 3 d; and two trials on path A (A11–A12) on the last day (probe test). A different set of animals was tested on PND 22 and PND 62. The number of errors (deviation from correct channel with all four paws) and the time required for the animal to escape (data not shown) were recorded. EPA (2008) identified possible trend in errors only in high-dose males at PND 22, but also noted there were no effects on latency. There was no statistical significance at either age when males and females were analyzed separately (ANOVA and Dunnett’s for each trial; α = 0.05). When males and females were combined (n = 20 litters/dose group) and analyzed using repeated measures ANOVA with trials as the repeated measures, there was a significant effect of trials (p < 0.018 or p < 0.001) but no significant dose effects or dose interactions with sex or trial for each age (α = 0.05).
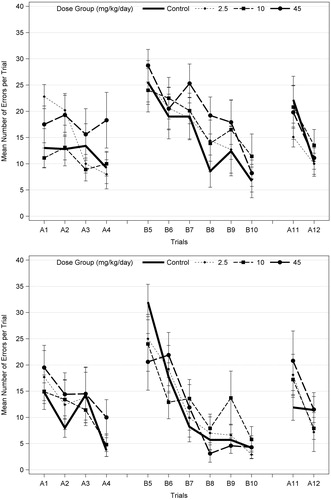
In summary, the high dose of 45 mg/kg/d acetamiprid decreased body weight gain in P-females and F1 animals, decreased the acoustic startle response in F1 males and was associated with a marginally significant increase in the number of errors in the Biel maze in F1 males just after weaning. The biological significance of this numerical increase is uncertain, because it was not seen in F1 males for Path B or Path A-probe or in females for either path. EPA concluded the study is acceptable and established a NOAEL of 10 mg/kg/d for P-females and F1 offspring (EPA Citation2013a).
Imidacloprid
Imidacloprid (98.4% purity) was administered on GD 0 to LD 21 at constant dietary levels of 0, 100, 250, or 750 ppm. Treatment-related effects in P-females and F1 animals were evident at 750 ppm but not at lower dietary levels (EPA Citation2002a). Based on these results, the EPA selected the dose equivalents of 55 and 20 mg/kg/d for the LOAEL (750 ppm) and NOAEL (250 ppm), respectively, pending morphometric measurements for caudate-putamen in F1 females at 250 ppm ().
In high-dose P-females, feed consumption was decreased during the third week of gestation (−9%; NS) and the first week of lactation (−14%, p < 0.05), with an associated decrease in body weight gain during LD 0–7 (−33%, p < 0.05). Treatment-related effects in high-dose F1 animals consisted of decreased body weight in males and females, averaging 11–13% less than control from PND 4–21, decreased motor activity (NS; ANOVA/Dunnett’s) on PND 17 (−31 to −38% in males and females) and PND 21 (−37% in females only). EPA (Citation2002a) also considered decreased caudate-putamen width (−1.9%; p = 0.03; Chi-square/Fisher’s exact test) in term females (PND 72), compared with controls, a treatment effect. Litter parameters, including litter size and birth weight, clinical signs, acoustic startle response, or measures of cognition were not affected at any dose, and there was no evidence of neuropathology in high-dose F1 males or females.
The modest (−1.9%; 70 μm) difference in caudate-putamen width in high-dose F1 females on PND 72, compared with controls, was considered a treatment effect, based on statistical significance and a non-statistical (−5.4%; p = 0.07) difference from control on PND 11, which suggested a consistent pattern (EPA Citation2002a). Measures at the mid-dose were requested to assist interpretation; however, shrinkage associated with the continued storage of those tissues in formalin (Garman et al. Citation2015; Tsuji & Crofton Citation2012) precluded morphometric comparison with tissues from control and high-dose animals. Those tissues were not processed and measured along with control and high-dose tissues because the study scientists considered the differences in caudate-putamen width at the high dose spurious and unrelated to treatment, given the minimal difference from control and the unusually high measure for the controls. In support of this interpretation, the group mean for the female concurrent control group at PND 72 set the upper limit value for 19 studies in the historical control database (3.21–3.75 mm), while the group mean for 750 ppm treated females (3.68 mm) was above the average for historical controls at PND 72 (). The lack of effect in high-dose males on PND 11 (p = 0.55) or on PND 72 (p = 0.25) also supports the conclusion that this difference in females was unrelated to treatment.
Table 10. Caudate-putamen width (mm) in F1 rats treated with imidaclopridTable Footnotea.
The study scientists attributed the decreased motor activity for high-dose F1 males (PND 17) and females (PND 17 and 21) to acute toxicity associated with the pups consuming high doses of imidacloprid in the treated feed, in addition to the milk. This interpretation is supported by the pattern of occurrence in both sexes at the highest dietary level, specifically during late lactation when the pups are consuming the treated feed, with no effect on habituation at any age or any effect on any measure of activity on PND 13 or PND 60.
In summary, the high dose of 750 ppm imidacloprid decreased body weight and weight gain in P-females and F1 animals and reduced motor activity in F1 males and females during the period of peak exposure associated with consuming the treated diet before weaning. There were no treatment-related clinical signs, and no effect on the acoustic startle response or cognition at any dose level or evidence of neuropathology at the high dose. The evidence supports the difference in caudate-putamen width in high-dose F1 females, compared with controls, was a spurious finding but measures at lower doses are not available to assist the interpretation.
Thiacloprid
Thiacloprid (99.2% purity) was administered on GD 0 to LD 21 at constant dietary levels of 0, 50, 300, or 500 ppm. The EPA (Citation2002b) determined the NOAEL for P-females and F1 animals was 50 ppm (4.4 mg/kg/d), based on findings at 300 and 500 ppm (25.6 and 40.8 mg/kg/d, respectively). Body weight gain and food consumption were reduced in P-females during the first week of exposure (GD 0–6) at 300 and 500 ppm and LD 1–4 at 500 ppm. In F1 males and females, body weight was decreased on PND 7–21 and after weaning at 300 and 500 ppm, with delayed preputial separation at both dietary levels and delayed vaginal opening at the high dose (EPA Citation2002b).
Decreased body weight in mid- and high-dose F1 males and females was evident beginning on PND 7, with statistical differences from control in both sexes on PND 11 (−9 to −15%) at 300 ppm and on PND 21 (−13 to −15%) at 500 ppm. After treatment was discontinued, body weight remained less than control at both doses, with a mean weight 8–10% less than control on PND 37 and 3–7% less than control on PND 64. Associated with the decreased body weight and weight gain during growth and development, preputial separation was delayed at the mid- and high-dose an average 1.5 d, compared with controls (PND 48.2 versus 46.7), and vaginal patency was delayed an average 1.3 d (PND 34.7 versus 33.4) at the high dose.
The EPA (Citation2002b) noted “altered performance” in the passive avoidance task in F1 females at 300 and 500 ppm, including a significant increase in Trial 2 latency for the first test session and an adverse effect on retention (). For brain morphometry measurements in high-dose F1 males, measures of the corpus striatum (−4%) and corpus callosum (−14%) were significantly (p < 0.05) less than control on PND 11 and measures of corpus striatum (−4%) and dentate gyrus (−5%) were significantly less than control at term, with non-significant differences from control in high-dose F1 females (corpus callosum (−3%) on PND 11 and corpus striatum (−2%) at term) () cited as evidence to support a pattern of effects (EPA Citation2002b). Offspring survival, autonomic function, M-water maze performance, brain weight, and qualitative histopathology were not affected by treatment in F1 males or females at any dietary level.
Table 11. Trials-to-criterion and latency (sec) for passive avoidance in F1 Sprague–Dawley rats treated with thiaclopridTable Footnotea.
Table 12. Brain measurements (μm) in F1 rats treated with thiaclopridTable Footnotea.
For motor activity, both the mean number of movements and the time spent in movement were comparable with control F1 animals on all test days at all dietary levels (EPA Citation2002b); however, the EPA noted a “suggestive” effect that involved a higher number of movements for test intervals 2–4 (of six intervals), compared with controls, for 300 and 500 ppm males and females on PND 17 and 21. EPA (Citation2002b) also considered non-statistical (p > 0.05, ANOVA/Dunnett’s) increases in the acoustic startle response for multiple testing blocks in PND 60 F1 males (300 and 500 ppm) and females (500 ppm) suggestive evidence of a treatment effect. The results show the mean response amplitude for males on PND 60 was 34% and 27% greater than control at the mid- and high-doses, respectively, while the mean response amplitude for high-dose females was 15% greater than control. The relationship between the motor activity and acoustic startle findings and treatment is unclear, as the differences from control were modest, inconsistent by sex and were generally not dose-related. For these reasons, the study scientists considered these differences from control were spurious and unrelated to treatment.
For passive avoidance, the neurobiological or toxicological significance of increased latency for Trial 2 of acquisition in F1 females is also unclear, as it would indicate more rapid, not impaired, acquisition and there was no difference in the number of trials-to-criterion for acquisition or retention at any dose. For this reason, and because males showed no similar difference from control, the study scientists considered this a spurious finding that was unrelated to treatment. For retention, the study scientists did not consider the numerically lower latency to cross for Trial 1 that EPA (Citation2002b) noted for treated males was an effect, because it was not statistically significant or dose related and there was no other indication of a difference in performance in males to support this as a treatment effect.
Statistical and non-statistical differences in brain measurements noted above were generally modest (4–5%) and group means for treated animals (with only one exception) were within the historical control range (). The 14% lower measure of corpus callosum in PND 11 males was statistically significant but was not accompanied by a difference at PND 67–78 or in females at either age. This relatively large difference from control on PND 11 is attributed to greater variability in brain measures at that age, when the brain is relatively small and growing rapidly, with less distinct surface markers for reference or myelination to help achieve consistent section homology (Bolon et al. Citation2006; Garman et al. Citation2001; Kaufmann & Groters Citation2006).
In summary, dietary levels of 300 and 500 ppm thiacloprid decreased maternal body weight gain and decreased body weight in F1 animals on PND 7–21 and after weaning, with delayed preputial separation at both doses and delayed vaginal opening at the high dose. EPA (Citation2002b) noted “suggestive effects” on motor activity and acoustic startle and “altered performance” for passive avoidance at these doses, with differences from control for certain brain measurements in high-dose F1 males on PND 11 (corpus striatum and corpus callosum) or at term (corpus striatum and dentate gyrus), concluding the study is acceptable with an overall NOAEL of 50 ppm (4.4 mg/kg/d) for P-females and F1 offspring. The study scientists associated the delayed sexual maturation with decreased body weight during lactation and after weaning and considered the other findings represented spurious differences from control that were unrelated to treatment.
Clothianidin
Clothianidin (95.5–95.9% purity) was administered on GD 0 to LD 21 at constant dietary levels of 0, 150, 500, or 1750 ppm. The NOAEL for P-females was 500 ppm (43 mg/kg/d) based on decreased body weight, body weight gain, and food consumption at the highest dietary level of 1750 ppm (PMRA Citation2002). The NOAEL for F1 animals was 150 ppm (13 mg/kg/d) based on decreased body weight, body weight gain, motor activity, and the acoustic startle response in females at 500 ppm ().
Food consumption was significantly reduced during gestation and lactation at 1750 ppm, with P-female body weight reduced as much as 8% (p < 0.05) during both gestation and lactation. Body weight gain was markedly reduced, compared with controls, shortly after the introduction of treated feed on GD 0 (−63%, GD 0–3, p < 0.01) and during early lactation (−67%, PND 4–7, p < 0.05), whereas body weight gain was increased (+215%, p < 0.01) on PND 14–22 of lactation, such that overall lactational body weight gain was similar to controls (PMRA Citation2002).
Body weight gain from PND 0 to 21 was decreased in F1 males and females by 18% (p < 0.01) at 1750 ppm and by 7% (p < 0.05) in 500 ppm females, with a maximum 16% (p < 0.05) decrease in body weight at the high dose before weaning. Body weight was also decreased 6–7% (p < 0.05) on PND 13–21 in F1 females at 500 ppm. After weaning, body weight remained significantly less than control in 1750 ppm F1 males and females on PND 23–37 (from −3 to −15%) and in F1 males on PND 72 (−4%), and body weight gain was reduced 21% (p < 0.05) in F1 females on PND 65–72, compared to controls. A statistically significant decrease in the mean percent of high-dose F1 animals achieving criterion performance for surface righting on PND 3 was considered equivocal, and the onset of pinna unfolding, response to an acoustic stimulus, preputial separation or vaginal patency was not delayed at any dietary level (PMRA Citation2002). There were no treatment-related clinical signs; however, five high-dose F1 animals were found dead on PND 24–26, in spite of receiving only untreated feed since weaning on PND 21.
For motor activity, there was no effect on habituation or statistically significant difference in total session activity count or time spent in movement (PMRA Citation2002). However, on PND 21 there were non-statistical decreases in total motor activity (count) in F1 females at 500 ppm (−21%) and in 1750 ppm F1 males (−24%) and females (−10%), as well as decreased time (s) spent in movement for females at 500 ppm (−24%) and 1750 ppm (−19%) and for F1 males at 1750 ppm (−27%). The results for the number of movements are shown in to illustrate these findings. Finally, there were statistically significant decreases in activity counts or time spent in movement in one-to-four of 12 5-min testing blocks on PND 21 for 1750 ppm males and females and the time spent in movement (−36%) in 500 ppm females for testing block 4. PMRA (Citation2002) noted the interpretation of motor activity data is “made difficult due to the large coefficients of variation on PND 13, 17, and 21, which is a likely reason for lack of significance for some testing blocks”.
Table 13. Number of movements (counts) in F1 animals treated with clothianidinTable Footnotea.
PMRA (Citation2002) attributed differences in activity count for F1 females at 500 ppm to treatment, based on a similar reduction in time spent in movement. The study scientists also concluded motor activity was decreased in 1750 ppm males and females, but concluded the differences in activity count for F1 females at 500 ppm were not a treatment effect since statistical significance was limited to only one test interval (block 4) and not the total session, there was no difference in F1 males at 500 ppm and the difference from control was generally not dose related.
The mean peak acoustic startle response was 48% (p < 0.01) less than controls on PND 22 in 1750 ppm F1 females and 27% (NS) less than control in 500 ppm F1 females; the difference from control in 500 ppm females was statistically significant for only block 2 (−36%, p < 0.05) of five 10-trial blocks. The values for control females at this age were high, relative to historical control; however, PMRA (Citation2002) noted historical control data were only available for two studies when this review was performed.
PMRA (Citation2002) considered increased thickness of the hippocampal gyrus (+9%, p < 0.05) and cerebellum height (+10%, p < 0.05) on PND 11 and decreased thickness of the hippocampal gyrus (−5%; p < 0.05) on PND 82–86 for 1750 ppm F1 females represented treatment-related effects. No morphometric findings were noted in 1750 ppm F1 males or 500 ppm F1 females at either age. The study scientists considered the aforementioned differences in 1750 ppm females spurious and unrelated to treatment, based on a lack of consistency (e.g. increased hippocampal gyrus and cerebellum measures on PND 11 and decreased hippocampal gyrus without a difference in cerebellum measures at term). An increased cerebellum height in high-dose F1 females on PND 11 (+10%) was also considered an isolated and spurious finding because there was no difference from control for males on PND 11 or either sex at term. Moreover, as noted for thiacloprid, it is especially difficult to achieve highly standardized coronal sections of the cerebellum in rats as young as PND 11 (Garman et al. Citation2001); consequently, the Society of Toxicologic Pathologists recommends that morphometric measures be conducted at weaning, rather than PND 11 (Bolon et al. Citation2006 Citation2011).
In summary, the high dose of 1750 ppm clothianidin produced treatment-related effects on body weight, body weight gain, and food consumption in P-females and decreased body weight, body weight gain, motor activity, and acoustic startle in F1 males and females. In F1 females, transient and slight decreases in body weight, body weight gain, and the acoustic startle response were evident at 500 ppm. Increased measures of the hippocampal gyrus and cerebellum at PND 11 and decreased thickness of the hippocampal gyrus at term, relative to control groups, are considered to represent spurious findings that are unrelated to treatment.
Thiamethoxam
Thiamethoxam (98.8% purity) was administered on GD 6 to LD 21 at constant dietary levels of 0, 50, 400, or 4000 ppm. The NOAEL for P-females and F1 animals was 400 ppm (34.5 mg/kg/d), based on treatment-related effects at the highest dietary level of 4000 ppm (298.7 mg/kg/d) (EPA Citation2007). Reduced body weight, body weight gain, and food consumption were evident in high-dose P-females during gestation and lactation, with a maximum 5% and 7% lower body weight during gestation and lactation, respectively, compared with controls. In high-dose F1 males and females, body weight gain during lactation was 13–20% (p < 0.05) less than control, with body weight less than controls at birth (−7 to −9%, p < 0.01) and during lactation (−9 to −12%, p < 0.01) and after weaning. The mean terminal body weight for high-dose animals selected for brain morphometry was 12–15% less than controls on PND 11 and 8–9% less than controls on PND 62 and absolute brain weight for F1 high-dose males and females was 4–5% less than controls (p < 0.05) on PND 11 and PND 62 (EPA Citation2007). Preputial separation in high-dose males was also delayed by an average 1.5 d, compared with controls (p < 0.01).
Associated with decreased brain weight at the high dose, brain measurements were generally reduced compared to control values. On PND 11, the cerebellum molecular layer thickness (−12%, p < 0.01) and cerebellum length (−7%, p < 0.05) were reduced in high-dose F1 males and thalamus width was reduced (−6%, p < 0.01) in high-dose F1 females, compared to controls. Several other brain measurements averaged 3–5% less than control on PND 11, but the differences from control were not statistically significant. On PND 62, high-dose F1 males had significant reductions in dorsal cortex (−6 to −11%, p < 0.01), piriform cortex (−9%, p < 0.05) and corpus callosum thicknesses (−20%, p < 0.01), thalamus height (−11%, p < 0.01) and width (−7%, p < 0.01), thalamus cortex overall width (−5%, p < 0.05), and hippocampus width (−9%, p < 0.01), compared with controls. On PND 62, high-dose F1 females had significant reduction in dorsal cortex thickness (−6 to −9%, p < 0.01), thalamus width (−5 to −8%, p < 0.01), thalamus/cortex overall width (−7%, p < 0.01), and hippocampus width (−6%, p < 0.01), compared with controls (EPA Citation2007). At lower doses, brain weight and morphometry were not different from control in males or females at either age. Moreover, there were no effects on functional or neurobehavioral parameters, as assessed by the functional observational battery, motor activity, acoustic startle response or learning, and memory tests at any dose (EPA Citation2007).
The study scientists concluded that the delay in the onset of preputial separation was secondary to reduced body weight during early growth and development. They also considered the differences in absolute brain weight and brain morphometry measures in the high-dose F1 animals were secondary to lower body weight during development, not a selective effect on the developing brain. To evaluate the relationship further, brain weight data were analyzed relative to terminal body weight (ANCOVA) and morphometric measurements were adjusted based on terminal body weight (ANCOVA) to determine whether the differences were related to decreased offspring growth resulting from poor nutrition (Supplementary Table 2). When brain weight is adjusted for body weight, there is no statistically significant difference from control for any dose group. Similarly, there were no statistically significant differences for any brain measurement on PND 11, when adjusted for body weight. For the PND 62 measurements, small decreases in dorsal cortex thickness, thalamus width and/or hippocampus width, relative to controls, fell within the historical control range, while the concurrent control values were high, compared with the historical controls (Supplementary Table 3). The few statistically significant differences in PND 62 brain measurements that remained after adjustment for terminal body weight were also considered to be secondary to decreased body weight during brain growth and development.
In summary, the high dose of 4000 ppm thiamethoxam produced decreased food consumption and body weight in P-females throughout gestation and lactation, and significant decreases in F1 offspring body weights at birth, during lactation and associated growth and development. As a result, absolute brain weights and brain measurements were lower in the F1 offspring at 4000 ppm, compared to control, and the males had a 1.5-d delay in onset of preputial separation. The reduced brain weight and size in the F1 offspring is consistent with prior studies, which show large deficits in maternal nutrition reduced both body weight and absolute brain weight of the offspring (Garman et al. Citation2001; West & Kemper Citation1976).
Dinotefuran
Dinotefuran (99.5% purity) was administered on GD 6 to LD 21 at constant dietary levels of 0, 1000, 3000, or 10 000 ppm. There were no treatment-related effects in the P-females or F1 males or females at any dietary level (EPA Citation2013b); therefore, a NOAEL for all toxicological effects in this study was established as 10 000 ppm (784 mg/kg/d). A transient decrease in body weight gain (−45%, p < 0.01) from GD 6 to 9 in the P-female 10 000 ppm dose group was not considered adverse, since it was not sustained (e.g. no effect on GD 9–12, 12–15, 15–18, and 18–20) and there was no difference in body weight among dose groups during gestation, lactation, or post-weaning.
Discussion
This review identified six studies reported in the literature that tested three neonicotinoid insecticides for evidence of DNT, as well as previously unpublished DNT studies conducted in accordance with EPA (Citation1998) guidelines for all six neonicotinoid insecticides that are registered for commercial uses in major markets. The published DNT studies used an assortment of in vivo and in vitro test systems and study designs with various research objectives. By comparison, the guideline-based studies are standardized and designed for use to identify evidence of DNT and for risk assessments, with a relatively large sample size, complement of tests and selection of doses administered by a route that is relevant to human circumstances of exposure with commercial uses (Raffaele et al. Citation2010). The guideline-based studies were not specifically tailored for the neonicotinoids, but the study design lends itself to identify evidence of DNT by any potential mode of action. The consistent study design used in these studies also serves to facilitate comparisons of findings across the class, in spite of the studies being performed at four different laboratories. Since clothianidin is a principal metabolite of thiamethoxam, evaluating the results from these two independent studies offers an excellent opportunity for comparison.
Following a brief summary of DNT effects with nicotine below, the results of epidemiology and DNT studies with neonicotinoid insecticides are discussed in terms of biological significance, human relevance, and consistency of effects across the different neonicotinoids and in comparison with the effects of nicotine.
Evidence of developmental neurotoxicity with nicotine
Potential concern that the neonicotinoid insecticides could affect the developing nervous system is associated with their nicotinic mode of insecticidal action and recognition of nicotine as a likely developmental neurotoxicant in humans and laboratory animals. A detailed review of the evidence from individual DNT studies with nicotine is beyond the scope of this paper; therefore, the findings cited here are primarily taken from reviews on the subject (Abbott & Winzer-Serhan Citation2012; Bruin et al. Citation2010; Dwyer et al. Citation2008 Citation2009; Pauly & Slotkin Citation2008; Slikker et al. Citation2005; Slotkin Citation1998 Citation2004; Tiesler & Heinrich Citation2014).
In humans, the adverse effects associated with cigarette smoking during pregnancy on fetal and postnatal development have been the subject of many epidemiology studies. Adverse health consequences associated with smoking during pregnancy include low birth weight, sudden infant death syndrome, attention deficit hyperactivity disorder (ADHD), and various cognitive/behavioral disorders. However, components in cigarette smoke other than nicotine may contribute to these effects. For example, some studies using nicotine replacement (e.g. transdermal patches) during pregnancy indicate nicotine per se does not adversely affect newborn body weight (Clark & Nakad Citation2011; Wisborg et al. Citation2000).
Animal studies have also described adverse developmental effects following exposure to nicotine during gestation and/or lactation. In these studies, nicotine was generally administered to pregnant rats or mice via minipumps, repeated subcutaneous injections, or the drinking water, to achieve and maintain dose levels approximating moderate-to-high tobacco usage. The results from these studies suggest that developmental nicotine exposure impacts a variety of functions in the developing fetus/neonate, including altered growth, control of respiratory and/or cardiovascular systems, motor activity levels, and/or cognitive function. Nicotine is believed to interfere with neuronal development, with adverse effects on various brain regions, including neocortex, hippocampus, cerebellum, and/or the limbic system (Dwyer et al. Citation2009). Developmental exposure to nicotine has been associated with many biochemical alterations in the brain, including decreased DNA synthesis, increased ornithine decarboxylase and c-fos activity, and changes in neurotransmitter levels and adenylyl cyclase activity (see reviews by Dwyer et al. Citation2009; Slikker et al. Citation2005). Quantitative morphometric and structural abnormalities have also been shown in various brain structures, including the hippocampus, cerebral cortex and nucleus accumbens, following gestational exposure to nicotine. In several brain regions, including the nucleus accumbens, Muhammad et al. (Citation2012) noted changes in the number of dendritic branch bifurcations, dendritic length, and/or spine density of distal dendrites of the offspring (PND 21) of rats exposed to nicotine prenatally. Roy et al. (Citation2002) noted increased numbers of glia in the hippocampus and somatosensory cortex of the offspring (PND 21–30) of rats exposed to nicotine during gestation. These investigators also noted decreased neuronal cell size and reductions in the thickness of the CA1, CA3, and dentate gyrus layers in the hippocampus. In both studies, these findings were noted in the absence of any effect of nicotine on offspring body weight.
Comparison of nicotine and neonicotinoid modes of action
Binding of nicotine to mammalian nAChRs during development is a necessary step leading to developmental neurotoxicity (Slikker et al. Citation2005). One likely consequence of nAChR stimulation during gestational/neonatal CNS development is the premature onset of cell differentiation, resulting in neuronal death and/or structural alterations in regional brain areas. In contrast to nicotine, the neonicotinoid insecticides exhibit nicotinic activity (e.g. tremors) in adult rats only at high dose levels, due to their low affinity for nAChR subtypes in vertebrates and poor penetration of the blood–brain barrier (Chao & Casida Citation1997; Tomizawa & Casida Citation2005; Yamamoto et al. Citation1995) (). Based on the differences in binding affinity to nAChR subtypes of different species, the toxicological properties of nicotine in insects and mammals are substantially different from the neonicotinoids.
In accord with such differences, the Insecticide Resistance Action Committee (IRAC Citation2015) Mode of Action Classification Scheme makes the following distinctions in sub-classes of insecticides acting as nAChR agonists (). Regarding the assignment of nicotine and other chemicals to sub-groups (4B, 4C, and 4D) separate from the neonicotinoids (4A), the IRAC made the following comment: “Although these compounds are believed to have the same target site, current evidence indicates that the risk of metabolic cross-resistance between subgroups is low” (IRAC Citation2015). The IRAC sub-grouping of nicotine separately from the neonicotinoids, along with the literature studies that highlight the large inter-species differences in affinity for binding to nAChR’s of mammals or insects (), point to very different biological and toxicological properties of the neonicotinoids, compared to nicotine.
Table 14. IRAC classification of chemicals interacting with the nAChRTable Footnotea.
Sulfoxaflor and the butenolides are sub-grouped separately from either nicotine or the neonicotinoids; accordingly, chemicals from these two subgroups (4C and 4D) are not evaluated in detail in this review. Sulfoxaflor is distinguished from the other sub-groups by the manner in which it interacts with the (α1)2β1δγ nAChR expressed in the muscle of the fetal rat, which produces forelimb flexure, hind-limb rotation, bent clavicles and decreased neonatal survival as a result of sustained muscle contraction (Rasoulpour et al. Citation2012). In the rat, the fetal nAChR (α1)2β1γδ is replaced shortly after birth with the adult form (α1)2β1δɛ, which does not appear to interact with sulfoxaflor. Given the fundamental qualitative differences in sulfoxaflor agonism on the rat versus the human muscle nAChR, this mode of action is not relevant to, or at least very unlikely to occur, in humans (Ellis-Hutchings et al. Citation2014). Therefore, sulfoxaflor is a developmental toxicant in the rat but it does not result in neurotoxicity in the adult rat or other mammalian species, due to its specificity in interaction with the fetal nAChR.
Neonicotinoid epidemiology studies
Two epidemiology studies were identified that examined the potential relationship between maternally reported history of residential proximity to pesticide applications during pregnancy and neurodevelopmental defects (Yang et al. Citation2014) or maternally reported history of using imidacloprid products for flea and tick treatment on pets and ASD (Keil et al. Citation2014). Yang et al. (Citation2014) showed a general lack of association between proximity to agricultural pesticide use and risks of selected birth defects. Keil et al. (Citation2014) reported a “weak imprecise positive association” (the lower bound estimates for the 95% CI was less than or equal to 1) between product use during pregnancy and increased risk of ASD that could result from exposure misclassification alone, due to potential bias of mothers with autistic children and reliance on recall from ∼4 years prior to the survey. Exposure estimates associated with post-application contact with cats and dogs treated with products containing 10% imidacloprid, similar to the conditions experienced by users in the study by Keil et al. (Citation2014), are 3.4–6.6 μg/kg/d (Lunchick Citation2010; Supplementary Appendix II), which indicate very low risk associated with such uses. In fact, these exposure estimates are ∼3000–6000-fold lower than the NOAEL of 20 mg/kg/d for the DNT study with imidacloprid in rats and 1400–2700-fold lower than the NOEL of 9.3 mg/kg/d that is used for intermediate-term human risk assessments by EPA (Citation2012b).
Comparison of neonicotinoid and nicotine: in vitro study
Kimura-Kuroda et al. (Citation2012) reported excitatory Ca2+ influx with nicotine, imidacloprid, and acetamiprid that was possibly associated with nAChR activation in primary cultures of cerebellar granule cells from newborn rats at 1–100 μM. Differences in firing patterns, proportion of excited neurons, and peak excitatory Ca2+ influxes that were evident with imidacloprid and acetamiprid, relative to nicotine, were attributed to different effects on nAChRs. It is unclear whether evidence of Ca2+ influx in this system is associated with nAChR activity or whether this would lead to DNT, due to the transient nature of the exposure and advanced stage of cell maturation. Moreover, systems that limit access to sensitive tissues in vivo (e.g. metabolic degradation and the blood–brain barrier) did not exist in this test system; therefore, such findings identify a potential molecular-initiating event in vitro, but do not necessarily represent an adverse outcome for DNT. Previously, these neonicotinoids have been shown to express marked differences in nAChR activity and lower potency than nicotine, which was not the case in this study. Therefore, these findings should be confirmed and must be interpreted in the context of the results from in vivo studies.
Comparison of neonicotinoids and nicotine: in vivo DNT studies
General indices of growth (P-females and F1 animals)
Nicotine has anorexic properties in mature animals that have been associated with central cholinergic-linked metabolic processes (Jo et al. Citation2002). Several studies have shown decreased body weight gain with post-natal exposure to doses of nicotine as low as 0.5 mg/kg/d (Abbott & Winzer-Serhan Citation2012). The effect of prenatal nicotine exposure on pup birth weight is not as clearly defined (Winzer-Serhan Citation2008), although a more recent study in rats suggests that prenatal nicotine reduces birth weight (Schneider et al. Citation2011). While evidence supports nicotine having a specific, direct effect on processes that mediate body weight gain, decreased birth weight and decreased body weight gain commonly represent generalized non-specific effects or are associated with reduced feed consumption with treatments administered at high dietary concentrations.
Decreased body weight gain, body weight, and/or food consumption occurred in P-females at the high dose in five of the six guideline DNT studies, with decreased body weight gain and food consumption also occurring with clothianidin at the next lower dietary level. With dinotefuran, a transient decrease in body weight gain in high-dose P-females was attributed to palatability at an approximate limit dose of 10 000 ppm in the diet, rather than an adverse or toxic effect. Decreased body weight and body weight gain were also the most common effects in F1 animals. Sustained decreases in body weight during development were associated with delayed sexual maturation in high-dose males (thiacloprid and thiamethoxam) and females (thiacloprid). By comparison, Tanaka (Citation2012b) reported increased body weight and weight gain in F1 mice treated with clothianidin during lactation and evidence of accelerated development at all dose levels. The differences from control in this and a related study (Tanaka Citation2012a) show a possible treatment-related increase in weight gain in treated mice during early development and maturation, but the observed differences were not dose related and therefore may not be robust or represent a treatment-related effect.
Neuropathology and brain morphometry (F1 animals)
An investigation of neonicotinoids for DNT should include a thorough microscopic examination of the brain, including regions associated with morphologic effects following exposure to nicotine during development or that contain cholinergic neurons. In this context, exposure to nicotine during gestation has been reported to produce structural abnormalities in the hippocampus and somatosensory cortex in rats (Roy et al. Citation2002) and the cerebellum, hippocampus, basal ganglia, and thalamus are rich in nAChR (Court et al. Citation2000). Abou-Donia et al. (Citation2006) have also reported that exposure of P-females to 3.3 mg/kg/d nicotine via minipump on GD 4–20 decreased surviving Purkinje neurons in the cerebellum of F1 animals and increased GFAP immunostaining in the cerebellum (white mater and granular cell layer) on PND 90.
As per EPA (Citation1998) guidelines, the guideline DNT studies with the neonicotinoids included a rigorous microscopic evaluation of brain, spinal cord, peripheral nerves, and other neural tissues for neuropathology, along with gross and microscopic measurements of several brain regions. The results from these studies identified no evidence of neuropathology in any neural tissue, including brain regions cited for morphologic changes following developmental exposure to nicotine (Roy et al. Citation2002) or that are rich in nAChR (Court et al. Citation2000).
Abou-Donia et al. (Citation2008) reported increased GFAP immunostaining by 16–45% in specific areas of the motor cortex and hippocampus of rats on PND 30, without evidence of histopathology, following a single high-dose of imidacloprid (337 mg/kg; 75% of the acute oral LD50 value) delivered i.p. on GD 9. However, the authors note that these findings require additional investigation to support, due to the limited scope of this study. Moreover, it is difficult to devise a mode of action to explain the occurrence of an active glial response in specific brain regions 7 weeks after a single dose that was administered when only rudimentary elements of the CNS existed (implantation of the blastocyst on GD 6). In terms of human relevance, i.p. administration of a high dose in corn oil has unknown relevance to human exposure circumstances, as this route bypasses first-pass catabolism in the gut and liver and the absorption kinetics are unknown. The guideline DNT study with imidacloprid administered at dietary levels equivalent to 55 mg/kg/d during gestation and 80 mg/kg/d during lactation also reported no evidence of neuropathology in cerebral cortex or hippocampus (EPA Citation2002a). GFAP activity was not measured in this study, but there was no evidence of inflammation (e.g. microglia) associated with activated astrocytes on PND 11 or PND 72.
While brain weight and associated brain tissue are typically conserved in adult rats that experience decreased food consumption and body weight with various treatments, decreased maternal nutrition during gestation and lactation has been shown to decrease offspring body weight and brain measurements independent of chemical exposure (Garman et al. Citation2001; West & Kemper Citation1976). Where large deficits in maternal nutrition reduce offspring body weight and absolute brain weight, the brains of undernourished pups may never achieve the age-appropriate developmental size of controls (Garman et al. Citation2001). Moreover, in such cases, the effect may be greater on brain regions that mature after birth (e.g. cerebral cortex, cerebellum, and hippocampus) than on subcortical structures that develop in utero (Garman et al. Citation2001). Garman et al. (Citation2001) noted the need for additional published results on brain weight adjusted for body weight in DNT studies for a wide array of compounds to allow a systematic determination of whether alterations in these measurements are predictive of neurotoxicity or are instead an indicator of malnutrition.
The DNT study with thiamethoxam provides a useful example of compound-induced decreases in birth weight, lactational body weight, absolute brain weight, and brain size that appears to be secondary to lower maternal nutrition. Among the studies cited in this review, the high dose of thiamethoxam had the greatest effect on body weight in F1 animals during development, which was associated with a decrease in brain weight and several brain measurements (EPA Citation2007). With thiamethoxam, the pattern of a consistent decrease in multiple brain measurements (without adjustment for body weight), the decrease in absolute brain weight and F1 offspring body weight, and the 15–20% decrease in P-female food consumption throughout gestation and lactation and no specific indicators of DNT, supports the conclusion that decreased absolute brain weight and brain measures at the high dose resulted from malnutrition during gestation and lactation, rather than a direct effect on the developing brain.
Differences in microscopic brain measurements are more problematic to interpret in the absence of neuropathology, as a decrease in such measurements may result from non-specific malnutrition during development (e.g. as seen with thiamethoxam) or represent biological variability or variability associated with methods for selection of homologous sections and morphometry measures, rather than a specific effect on brain development. There is higher biological variability in younger animals (e.g. PND 11) and curved brain structures (e.g. hippocampus and cerebellum). In addition, variability can be associated with methods for processing tissues and defining anatomic landmarks as anchor points for selection of homologous sections and morphometry measurements (Garman et al. Citation2015). To assist interpretation, it is useful to determine whether differences from control are dose related or consistent by age and gender and to compare measures for control and treated animals to historical control values that are suited for comparison. The interpretation is also more problematic when measures are only available for control and high-dose animals, as measures for other dose groups would otherwise help illustrate biological variability among groups of 6–10 rats per sex within the same study and a dose-response relationship. As in this review, it is also useful to compare positive and negative findings among representatives of the same chemical class or mode of action to determine whether there is a consistent pattern of effects, but it is important to verify whether the measures from different studies are suited for comparison.
Treatment with imidacloprid was associated with a slight (−1.9%, p ≤ 0.05) decrease in caudate-putamen thickness in high-dose females on PND 72, with a numerically lower (−5.4%) but non-statistical difference from control in high-dose females on PND 11 (EPA Citation2002a). A decrease in the same measurement at both ages may be considered consistent over time; however, disruption of morphologic development would alter growth and differentiation, which would be expected to produce a greater effect on tissue size later in development (Bolon et al. Citation2006; Garman et al. Citation2001). In the present case, one would expect a greater (not lesser) effect on PND 72 than PND 11, since this measure of the caudate-putamen increased by 35% from PND 11 (2.77 mm) to PND 72 (3.75 mm) in control females. Measures at lower doses are not available to determine whether this difference was dose related or to establish a clear NOAEL, as the tissues from lower dose groups were not measured, due to shrinkage from continued storage of those tissues in formalin (Garman et al. Citation2015; Tsuji & Crofton Citation2012). To avoid this situation, the timely processing of all tissues reserved for morphometry to paraffin or slides at all dose levels is now standard laboratory practice. In the present case, it is relevant to note measures for high-dose males were not different from control at either age and the modest differences from control in high-dose females were associated with a particularly high group mean for the concurrent control group (i.e. this control group set the upper limit for 19 DNT studies that constituted the laboratory HC database), while the group mean for high-dose females was well within the HC range. Therefore, it is reasonable to accept the study scientists’ conclusion that this finding in one sex is not a robust or treatment-related effect.
With thiacloprid, statistical differences in measures of corpus striatum and corpus callosum in high-dose F1 males on PND 11 and corpus striatum and dentate gyrus in high-dose F1 males at term were attributed to treatment (EPA Citation2002b). As with imidacloprid, measures were not taken at lower doses to assist interpretation and establish a clear NOAEL. However, there were no similar differences in females at either age and the magnitude of the difference from control did not increase with further growth and development of the tissues.
The only evidence of potential overlap among the morphometric findings involves the measures of caudate-putamen and corpus striatum with imidacloprid (EPA Citation2002a) and thiacloprid (EPA Citation2002b). However, precise comparisons cannot be made, due to differences in the measurements that were taken. With imidacloprid, the caudate-putamen width was measured at the level of the optic chiasm, whereas with thiacloprid the corpus striatum was measured as the diagonal width of the caudate-putamen and globus pallidus combined at the level of the septal nuclei and anterior commissure. Further, this finding was evident in females with imidacloprid and in males with thiacloprid, and in both cases, the differences were slight (−1.9% to −5.4% for imidacloprid and −4% for thiacloprid) and the finding with imidacloprid was associated with an exceptionally high mean value for the concurrent female control group ().
Thus, there are no reports of neuropathology with any of the neonicotinoids evaluated, which includes brain regions identified as rich in nAChR (Court et al. Citation2000), regions reported to express structural abnormalities following developmental exposure to nicotine (Roy et al. Citation2002) and regions that showed an excitatory response to neonicotinoids in primary cell cultures (Kimura-Kuroda et al. Citation2012). Among the brain measurements used to investigate DNT, differences from control were either associated with decreased body weight during development, including lower birth weight (thiamethoxam), or were evident in only one sex for a given neonicotinoid (imidacloprid and thiacloprid) or inconsistent among representatives of the class. Moreover, we are not aware of any gender-specific brain lesion associated with exposure to a neuroactive chemical during sexually-immature stages of development. For the guideline study with imidacloprid (EPA Citation2002a), the differences in caudate-putamen and corpus striatum measurements are likely spurious and unrelated to treatment, given the biological variability and unusually high group mean value for female controls.
Clinical signs and motor function (P-females and F1 generation)
The expression of acute “nicotinic signs” with nicotine varies to some extent by species. In humans, nicotinic signs generally consist of an excitatory phase that may include nausea and vomiting, excessive salivation, sweating, hypertension, tachycardia, ataxia, tremor, and seizures, and a later phase with hypotension and bradycardia, central nervous system depression, coma, muscular weakness, paralysis, or respiratory failure (Schep et al. Citation2009).
None of the DNT studies in this review reported nicotinic signs of toxicity in P- or F1 animals treated with a neonicotinoid insecticide. With imidacloprid, tremor has been reported to occur in adult rats and mice at high doses administered by gavage, but not at lower doses or with dietary administration (Sheets Citation2014), as the target tissue concentrations required to produce such effects are only achieved under such circumstances. In the guideline DNT studies, the neonicotinoids were generally administered via the diet. Although acetamiprid was administered by gavage to the P-females in the guideline DNT study, nicotinic signs were not evident in the P-females or F1 animals at any dose. The lack of nicotinic signs in these studies reflects the low activity of the neonicotinoids toward nAChR subtypes in mammals, poor penetration of the blood–brain barrier and the low tissue levels associated with sustained exposures, which are generally relevant to human circumstances of exposure.
Evidence of neurologic or behavioral effects with neonicotinoid insecticides includes slight deficits associated with beam-walk time, inclined plane test and grip time in 30-d-old rats treated with a single intraperitoneal dose of 337 mg/kg imidacloprid on GD 9 (Abou-Donia et al. Citation2008). However, additional investigation is required to determine whether these findings are reproducible and represent treatment related effects, based on the widely accepted criteria used to judge data reliability. A more thorough investigation, as suggested by the study authors, has not been reported and other studies in rats did not use the same tests or administer the treatment at a similar dose or route. For reference, deficits in motor coordination were not evident in the guideline DNT study with imidacloprid, at dietary levels that achieved a minimum 55 and 80 mg/kg/d during gestation and lactation, respectively (EPA Citation2002a). However, subtle deficits in motor function may have contributed to decreased activity in high-dose F1 males and females on PND 17 and 21 when they were exposed to high doses of imidacloprid via the diet.
Motor activity
Motor activity reflects the general status or condition of the animal and is sensitive to a variety of neurotoxic and non-neurotoxic mechanisms (Reiter Citation1983), including disturbances of sensory, motor, reactivity, excitability, cognitive, motivational states, perturbation of regulatory states, or general malaise. As such, automated measures of motor activity performed under well-controlled conditions are suited to screen chemicals for toxicity but not to characterize the underlying cause unless there is additional information to assist interpretation. For this reason, an automated measure of activity is included in neurotoxicity screening studies with pesticidal active ingredients (OCSPP 870.6200 and OECD TG 424) using adult rats, in combination with detailed clinical observations, a functional observational battery (FOB) and neuropathology.
Mixed results have been reported regarding the effect of gestational and/or postnatal exposure to nicotine on motor activity. While many studies report increased activity or no effect in rodents exposed prenatally to nicotine, others have reported decreased activity (see review by LeSage et al. Citation2006). Schneider et al. (Citation2012) speculated that repeated daily testing is necessary to detect an increase in activity following gestational exposure to nicotine (Supplementary Table 4), presumably to allow the animals to habituate to the activity chambers. However, Tizabi et al. (Citation2000) did not detect an increase in activity on the fourth consecutive day of testing to high daily doses of nicotine. Furthermore, Thomas et al. (Citation2000) and Huang et al. (Citation2007) both report changes in activity without prior experience in the chamber, but the effects were not consistent (an increase and decrease, respectively) (Supplementary Table 4).
The reason(s) for these mixed results with nicotine is unknown. Contributing factors could involve differences among the studies in the daily nicotine dose, route of administration (including gradual increments of dose levels), period of exposure, control for hypoxia and weight loss, postnatal time point of testing, and/or some aspect of the testing procedures (LeSage et al. Citation2006; Schneider et al. Citation2012). For most studies, motor activity was tested after exposure to nicotine was discontinued, looking for persistent or latent effects. While a transient decrease in activity during the exposure period is a sensitive measure of acute toxicity with many chemical agents (Reiter Citation1983), a persistent change in activity, hyperactivity or decreased habituation after discontinuation of exposure are more clearly associated with DNT (Norton Citation1976), especially if such effects occur at doses that do not produce maternal toxicity.
Motor activity is measured in guideline-compliant (EPA Citation1998) DNT studies during the period of exposure on PND 13, 17, and 21, as well as on PND 60 to test for latent or persistent effects (Tyl et al. Citation2008). The effects noted in the guideline studies with neonicotinoids consisted of decreased motor activity at the highest dietary level with imidacloprid on PND 17 and 21 (EPA Citation2002a) and with clothianidin on PND 21 (PMRA Citation2002), without an effect on habituation in either case (). Decreased activity in F1 animals during the peak period of exposure to the test substance is consistent with the nature of the effect (e.g. decreased activity without an effect on habituation) and the sensitivity of this assay to detect acute toxic effects. With dietary exposure during lactation, F1 animals receive substantial dosages (mg/kg/d) of the test substance by consuming the treated diet during the last several days before weaning on PND 21, in addition to exposure through the milk.
Tanaka (Citation2012a,Citationb) evaluated mice treated during development with clothianidin for effects on eight activity parameters measured for 10 min each at 3 and 8 weeks of age and for 120 min (statistically analyzed for each 10-min interval) at 9–10 weeks of age (). In Tanaka (Citation2012a), dietary exposure to clothianidin (0, 20, 60, and 180 ppm) during gestation and lactation did not result in any statistically significant difference from control for any of the movement parameters measured at 3 or 8 weeks of age, with the exception of decreased average time of rearing at 8 weeks in mid-dose F1 females only (). Tanaka (Citation2012a) reported an increased average speed of movement (cm/s) in 3-week-old F1 males based on a trend test, but there were no statistical differences based on multiple comparisons between treated and control groups. These isolated findings are not consistent with an adverse effect on motor activity, considering the lack of a consistent pattern across dose levels, sex and time, and the small number of statistical differences out of many analyzed parameters. In 9–10 week old mid-dose F1 males, several inter-related activity parameters were statistically increased in one or two of the 10-min intervals during the last 30 min of the 120-min session. However, there was no significant difference from control in the longitudinal pattern for any of the eight activity parameters at any dose level (). There were also no statistically significant differences in the low- or high-dose male groups or any of the female F1 groups at 9 – 10 weeks of age.
In Tanaka (Citation2012b), mice received dietary levels of 0, 30, 60, and 120 ppm clothianidin from 5 weeks of age (P generation) through gestation, lactation and post-lactation, to 11 weeks of age. At 3 weeks, there was an increased trend for the number of rearings in F1 female mice but no statistical differences in pairwise comparisons and no effects in any of the eight parameters for males including inter-related measures of rearing time, average time of rearing and movement time (). In the F1 mice at 8 weeks, mid-dose males showed a statistically decreased average rearing time, whereas males had a significantly increased trend for movement time. Other measures in treated F1 male mice had no differences from control or significant trends, and none of the parameters was affected in F1 females at 8 weeks. At 9–10 weeks of age, all movement variables measured were parallel in a longitudinal pattern, except for movement time in females. Three statistically significant decreases in one parameter or one treatment group (low or mid-dose) were reported for an isolated 10-min interval (e.g. significantly lower average speed (cm/s) in females only at 70 min), but the overall pattern across the entire 120 min session showed no effect on any of these parameters (). Although Tanaka (Citation2012b) considered the findings to represent adverse effects, the pattern was not consistent when considering the negative and positive results for the complement of movement parameters measured across age, sex, dose, and test intervals.
In summary, the effects of clothianidin on motor activity reported by Tanaka (Citation2012a,Citationb) were inconsistent within each study and across both studies, without a coherent pattern of increased or decreased activity measures in treated mice. The author attributes the different results across studies to differences in the two study designs, but the findings are not consistent across endpoints or sex, nor are they consistently dose related. Further, the statistical analysis did not address the multiplicity problem associated with the large number of statistical comparisons (e.g. dose levels, sex, parameters, age of testing, and test intervals), which inflates the chance of Type I (false positive) errors (Holson et al. Citation2008; Maurissen Citation2010). In the guideline DNT study, dietary exposure to clothianidin from GD 6 to PND 21 was associated with decreased activity on PND 21 in F1 male and female rats at 142 mg/kg/d (both sexes) and females at 42.9 mg/kg/d (PMRA Citation2002). This profile of effects is consistent with decreased motor activity in high-dose F1 rats on PND 17 and 21 in the guideline DNT study with imidacloprid. The occurrence of a similar effect during a period of high exposure before weaning is also consistent with the interpretation that this represents an acute toxic effect, which is a very common outcome in acute neurotoxicity studies with a variety of treatments, including imidacloprid (Sheets Citation2014).
Acoustic startle
Sustained exposure to nicotine during gestation has been reported to increase the startle response in F1 rats on PND 14, 18, and 70 (Lacy et al. Citation2011). The exposure used in this study was designed to simulate tobacco use during pregnancy, with the administration of 0.05 mg/kg nicotine three times daily on GD 8–21 via IV catheterization to the dam. While the test procedure investigated the effects of nicotine on pre-pulse inhibition, the effect on the startle response can be derived from trials without a pre-pulse stimulus. However, these results cannot be used to evaluate the effects of nicotine on startle habituation, since the variable pre-pulse conditions that were delivered among the trials may impede habituation (Koch Citation1999).
The startle response was increased in adolescent (1.5 months) zebrafish at 45 μM but not at 60 μM nicotine and there was no effect in adults (3 months) at either concentration (Crosby et al. Citation2015). Habituation of the startle response was not affected at either age. Similarly, developmental exposure of zebrafish to imidacloprid at 45 or 60 μM increased the startle response in adolescents and not in adults, without affecting habituation of the response at either age (Crosby et al. Citation2015).
For guideline-compliant studies, the acoustic startle response test is designed and primarily included to evaluate the effects of treatment on habituation (EPA Citation1998), a primitive learning process in which the organism decreases its response to redundant, non-significant stimuli (Davis Citation1984). None of the neonicotinoids affected startle habituation in weanling or adult F1 animals at any dose level. Acetamiprid and clothianidin decreased total session startle response amplitude in F1 males and/or females without affecting habituation, but the pattern of effects with these two treatments differed. With acetamiprid, differences from control were evident in males only, on both PND 20 and 60, whereas with clothianidin, the effect was greater in females, with differences from control in both sexes only on PND 22. These results also differ from the reported effect of nicotine to increase the startle response following gestational exposure in rats (Lacy et al. Citation2011) or the reported increase in startle response in adolescent (but not adult) zebrafish exposed to nicotine or imidacloprid during early development (Crosby et al. Citation2015). For both acetamiprid (EPA Citation2008) and clothianidin (PMRA Citation2002), the effects on startle amplitude in guideline DNT studies occurred only at doses that decreased body weight, body weight gain, and pup survival.
Cognition (learning and memory)
There is wide-spread concern for reduced academic achievement and impaired intellectual abilities in the children of mothers who smoke during pregnancy (Clifford et al. Citation2012) but the results from investigations to determine whether there is a clear relationship are inconclusive (Tiesler & Heinrich Citation2014). The results from animal studies with nicotine are mixed, potentially due to differences in the model used (species, gender, nicotine dose, and critical period of exposure) and/or the task used to assess performance. For example, prenatal exposure of rats to nicotine has been associated with deficits in a spontaneous alteration task or radial arm maze performance (Levin & Slotkin Citation1998; Levin et al. Citation1993). These reports note the treatment-related effects with nicotine are more apparent with more complex tasks. While some investigators have demonstrated persistent cognitive deficits from prenatal nicotine exposure based on findings from two-way active avoidance tasks (Vaglenova et al. Citation2008), others report no effect of prenatal nicotine exposure in the acquisition of choice accuracy in a radial arm maze (Cutler et al. Citation1996). Eppolito and Smith (Citation2006) evaluated the effects of nicotine in the Morris water maze of young-adult offspring of rat dams continuously infused with nicotine (∼1 mg/kg/d) from GD 4 to PND 11. At PND 60, swim speed was significantly reduced in F1 males but not in females. The distance and the latency for Path A were significantly increased in F1 females (not in males) when collapsed across all 5 d of testing, but not for individual days. The longer latencies in nicotine-exposed females, which appeared to decrease after test days 2 and 3, were considered a deficit in reference memory. Abbott and Winzer-Serhan (2012) reviewed the collective results for these and other animal studies and concluded that developmental exposure to nicotine has only a minor effect on cognitive performance.
Male rats dosed daily by gavage with 24 mg/kg of clothianidin from PND 7 until PND 97 spent significantly less time than controls in the target quadrant of the Morris water maze (). On one hand, the occurrence of this difference at the high dose suggests a treatment effect on consolidation of memory and the absence of this finding in a separate group of males that were treated for a similar duration beginning as adults suggests an effect associated with exposure during development. On the other hand, the deficiencies in this study that pertain to data reliability and human relevance () should be considered in evaluating the weight-of-evidence for clothianidin having an effect on cognition.
Three other DNT studies have investigated clothianidin for effects on cognition and determined it had no effect. Each of these studies administered clothianidin in the diet, while Ozdemir et al (Citation2014) treated the F1 males by gavage. Treatment of mice at dietary levels as high as 29 and 100 mg clothianidin/kg/d during gestation and lactation, respectively, did not affect performance at 7 weeks of age in a Biel-type water maze (Tanaka Citation2012a,Citationb), which involves learning to escape a relatively challenging multiple-T water maze. In the guideline study, dietary exposure to clothianidin from GD 6 to PND 21 did not affect acquisition, retention or other measures of performance in a passive avoidance conditioning test performed after weaning or a water maze task at PND 62–63 at any dose, including the high dose of 142–299 mg/kg/d administered via the diet (PMRA Citation2002; ). Therefore, the finding reported at 24 mg/kg/d with clothianidin (Ozdemir et al. Citation2014) is the only report of any neonicotinoid affecting cognition.
Acetamiprid was evaluated for potential cognitive effects using the complex Biel water maze testing paradigm, with sequential (Path A) and reverse (Path B) learning (EPA Citation2008). EPA noted an increased number of errors for trials 5–10 in Path B (NS) for the high-dose PND 22 F1 males, compared to controls, but no similar trend in F1 females or in Path A for males or females (). Treatment with acetamiprid had no effect on acquisition of the task in PND 22 males or females at any dose, and there were no effects on PND 62 in males or females at any dose. Based on the lack of consistency for the two paths and ages in males, and no effect on latency or any measure in females, the study scientists concluded that acetamiprid had no effect on learning or memory.
In the guideline study with thiacloprid, the high dose (40.8 mg/kg/d) increased latency for Trial 2 of passive avoidance for the F1 females at weaning, compared to controls (EPA Citation2002b). In this test, the rats are shocked if they move from the lighted chamber in which they are placed to an adjacent darkened chamber. The biological significance of this finding is unclear, as there was no effect on the number of trials-to-criterion for acquisition or retention at any dose and increased latency to cross for Trial 2 is consistent with more rapid, not impaired, task acquisition. There was also no effect on measures of learning or memory in the M-water maze, when the F1 offspring were tested as adults, and this finding occurred in only one sex, with no associated behavioral (e.g. motor activity) or neuropathology findings. Therefore, it is reasonable to accept the study scientists’ conclusion that this is a spurious finding, unrelated to treatment.
In summary, there were inconsistent findings on cognitive tests following gestational and/or lactational exposure to clothianidin (24 mg/kg/d), acetamiprid (45 mg/kg/d), and thiacloprid (25.6 and 40.8 mg/kg/d). Although the mixed results for clothianidin could be due to the different exposure duration and assessment paradigms, the only study that reported an effect (Ozdemir et al. Citation2014) had the greatest deficiencies, based on the criteria used to evaluate strength and human relevance (). For acetamiprid and thiacloprid, the study scientists did not consider the differences from control to represent an effect of treatment on cognitive behavior. In addition, these findings were only seen in weanling pups at dose levels that substantially increased neonatal pup death (acetamiprid), decreased body weight in dams during gestation and lactation (acetamiprid and thiacloprid) or decreased pup weight prior to weaning (thiacloprid) (). The guidelines for a DNT study require that the highest dose level induce “overt signs of maternal toxicity” (EPA Citation1998). This can cause a combination of stress and nutritional deficits to dams and/or their offspring, which can affect cognitive test results (Markham et al. Citation2010; Weinstock Citation2008).
Conclusions/weight of evidence
This review determined that there are relatively few studies published on the subject of DNT for the neonicotinoid insecticides and those studies generally have notable limitations relative to conformance criteria used to establish human relevance. A major challenge in translating the mode-of-action findings from published literature is associated with differences in metabolism and distribution (e.g. the lack of a blood–brain barrier in in vitro models); thus, these studies may be informative in certain respects but may not inform in vivo DNT responses. Other published studies are generally limited in critical aspects that pertain to human relevance, such as the selection of endpoints, sample size, and number of dose levels. By comparison, the sample size, route and duration of exposure, the selection of dose levels and the complement of tests used in the previously-unpublished DNT studies performed in accordance with EPA (Citation1998) guidelines are designed for hazard identification and use in risk assessments. Although these studies were not tailored to specifically investigate DNT associated with a nicotinic mode of action, the published literature with nicotine indicates the complement of tests in the study design is well suited for this purpose.
The collective information that is available shows no consistent pattern of effects among the six neonicotinoids or for any of these insecticides compared with nicotine. Dinotefuran produced no adverse effects in the guideline-compliant study at any dose level (EPA Citation2013b). The principal effects with the other neonicotinoids are associated with decreased body weight of F1 animals during development (e.g. delayed sexual maturation, decreased brain weight, and morphometric measurements) and acute toxicity (decreased motor activity associated with peak exposure via the diet and milk). Other findings among the individual studies, including differences in various brain measurements or performance in a neurobehavioral test, are inconsistent with a selective effect on the developing nervous system. These results may be useful for risk assessment purposes but the collective evidence indicates the neonicotinoid insecticides are not developmental neurotoxicants. This outcome is consistent with the relatively low activity of neonicotinoid insecticides toward nAChR subtypes that are expressed in mammals and other vertebrates and the absence of any other effect to suggest these compounds would be developmental neurotoxicants.
SUPPLEMENTAL_MATERIAL_for_Sheets_et_al_2015.pdf
Download PDF (988.6 KB)Acknowledgements
The authors want to acknowledge Alan Hoberman (Charles River Laboratories), Robert Garman (Consultants in Veterinary Pathology, Inc.), Mark Nemec and Melissa Beck (formerly with WIL Laboratories), Eddie Sloter (WIL Labs), Edmund Lau and Steave Su (Exponent, Inc.), Sandra Allen and Gill Milburn (Regulatory Science Associates, formerly at Syngenta Central Toxicology Laboratory), and Barry Stuart (formerly with Bayer CropScience) for their involvement in the guideline DNT studies cited in this review or further analysis of the findings. We also acknowledge the assistance of Edward Scollon (Syngenta Crop Protection) and Gabriele Schmuck (Bayer HealthCare, Animal Health) for reviewing studies reported in the literature.
Declaration of interest
The authors are employed or funded by companies that manufacture and sell the neonicotinoid insecticides that were evaluated in this review. The authors are experts on the toxicology of these insecticides, with responsibility for addressing regulatory toxicology issues and reviews. RHC is involved in litigation for dinotefuran; however, the subject of litigation does not pertain to DNT and EPA concluded dinotefuran had no effect in the DNT study, as reflected in this review. None of the other authors is involved in litigation for the neonicotinoid insecticides reviewed in this paper. This review is premised upon the publically-available literature, the publically-available EPA reviews of the DNT studies and the DNT study reports and data analysis. All authors contributed to the conception, design, data analysis and interpretation, drafting and critically revising this review paper, including sections unrelated to the insecticide produced by the company for which they worked. At the end of the review and drafting process and immediately prior to submission of the manuscript, a copy of the final draft was sent to company representatives for comment. Revisions made in response to comments and suggested edits were minor and did not alter or contribute to the opinions and conclusions. Thus, the analysis, interpretation and conclusions presented herein are solely those of the authors.
References
- Abbott L, Winzer-Serhan H. Smoking during pregnancy: lessons learned from epidemiological studies and experimental studies using animal models. Crit Rev Toxicol 2012;42:279–303.
- Abou-Donia MB, Goldstein LB, Bullman S, Tu T, Wasi AK, Ankelika MD, Abdel-Rahman AA. Imidacloprid induces neurobehavioral deficits and increases expression of glial fibrillary acidic protein in the motor cortex and hippocampus in offspring rats following in utero exposure. J Toxicol Environ Health Part A 2008;71:119–130.
- Abou-Donia MB, Khan WA, Dechkovskaia AM, Goldstein LB, Bullman SL, Rahman AA. In utero exposure to nicotine and chlorpyrifos alone, and in combination produces persistent sensorimotor deficits and Purkinje neuron loss in the cerebellum of adult offspring rats. Arch Toxicol 2006;80:620–631.
- Adams J. Editor’s note. Neurotoxicol Teratol 2010;32:406–417.
- Akayama A, Minamida I. Discovery of a new systemic insecticide, nitenpyramandits insecticidal properties. In: Yamamoto I, Casida JE, editors. Nicotinoid insecticides and the nicotinic acetylcholine receptor. Tokyo: Springer-Verlag; 1999. pp 127–148.
- Bal-Price A, Crofton KM, Leist M, Allen S, Arand M, Buetler T, Fritsche E, et al. International Stakeholder NETwork (ISTNET): creating a developmental neurotoxicity (DNT) testing road map for regulatory purposes. Arch Toxicol 2015;89:269–287.
- Bal-Price AK, Hogberg HT, Buzanska L, Lenas P, van Vliet E, Hartung T. In vitro developmental neurotoxicity (DNT) testing: relevant models and endpoints. Neurotoxicology 2010;31:545–554.
- Bolon B, Garman R, Jensen K, Krinke G, Stuart B. Ad Hoc Working Group of the STP Scientific and Regulatory Policy Committee: a 'best practices' approach to neuropathologic assessment in developmental neurotoxicity testing – for today. Toxicol Pathol 2006;34:296–313. Erratum: Toxicol Pathol 34(3):296–313.
- Bolon B, Garman RH, Gundersen HJG, Johnson GA, Kaufmann W, Krinke G, Little PB, et al. Continuing Education Course #3: current practices and future trends in neuropathology assessment for developmental neurotoxicity testing. Toxicol Pathol 2011;39:289–293.
- Bruin J, Gerstein H, Holloway A. Long-term consequences of fetal and neonatal nicotine exposure: a critical review. Toxicol Sci 2010;116:364–374.
- Buckingham SD, Lapied B, Corronc HL, Grolleau F, Sattelle DB. Imidacloprid actions on insect neuronal acetylcholine receptors. J Exp Biol 1997;200:2685–2692.
- Chao SL, Casida JE. Interaction of imidacloprid metabolites and analogs with nicotinic acetylcholine receptor of mouse brain in relation to toxicity. Pestic Biochem Physiol 1997;58:77–88.
- Chapin RE, Adams J, Boekelheide K, Gray LE, Hayward SW, Lees PS, Mcintyre BS, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of Bisphenol A. Birth Defects Res B Dev Reprod Toxicol 2008;83:157–395.
- Clark S, Nakad R. Pharmacotherapeutic management of nicotine dependence in pregnancy. Obstet Gynecol Clin North Am 2011;38:297–311.
- Clifford A, Lang L, Chen R. Effects of maternal cigarette smoking during pregnancy on cognitive parameters of children and young adults: a literature review. Neurotoxicol Teratol 2012;34:560–570.
- Court JA, Martin-Ruis C, Graham A, Perry E. Nicotinic receptors in human brain: topography and pathology. J Chem Neuroanat 2000;20:281–298.
- Crosby EB, Bailey JM, Oliveri AN, Levin ED. Neurobehavioral impairments caused by developmental imidacloprid exposure in zebrafish. Neurotox Teratol 2015;49:81–90.
- Cutler AR, Wilkerson AE, Gingras JL, Leven ED. Prenatal cocaine and/or nicotine exposure in rats: preliminary findings on long-term cognitive outcome and genital development at birth. Neurotoxicol Teratol 1996;18:635–643.
- Davis M. The mammalian startle response. In: Eaton RC, editor. Neural mechanisms of startle behavior. New York: Plenum; 1984. pp 287–351.
- Desesso JM. Comparative gestational milestones invertebrate development. In: Hood RD, editor. Developmental and reproductive toxicology: a practical approach. 3rd ed. New York: Informa Healthcare; 2012. pp 93–138 (Chapter 6).
- Desesso JM, Watson RE, Keen CL, Hazelden KP, Haws LC, Li AA. Analysis and integration of developmental neurotoxicity and ancillary data into risk assessment: a case study of dimethoate. J Toxicol Environ Health Part A 2009;72:94–109.
- Dutch National Institute for Public Health and the Environment (RIVM). 2014. http://www.rivm.nl/en/Documents_and_publications/Scientific/Models/PROAST.
- Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res C Embryo Today 2008;84:30–44.
- Dwyer JB, Mcquown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol Ther 2009;122:125–139.
- Ellis-Hutchings RG, Rasoulpour RJ, Terry C, Carney EW, Billington R. Human relevance framework evaluation of a novel rat developmental toxicity mode of action induced by sulfoxaflor. Crit Rev Toxicol 2014;44:45–62.
- Environmental Protection Agency. (1998). Health effects test guidelines; OPPTS 870.6300; Developmental neurotoxicity study. EPA 712-C-98-239, Washington, DC. Available: http://www.epa.gov/opptsfrs/home/guidelin.htm.
- Environmental Protection Agency (2002a). Data evaluation record for imidacloprid: developmental neurotoxicity study – rat; DP Barcode D286291; TXR#00501055.
- Environmental Protection Agency (2002b). Data evaluation record for YRC2894 (Thiacloprid): developmental neurotoxicity study – rat; DP Barcode D281298;TXR#0050517.
- Environmental Protection Agency (2007). Thiamethoxam. Review of developmental neurotoxicity study including brain morphometry data in low- and mid-dose groups. US Environmental Protection Agency. TXR#0054533. 9 March 2007. Available from: http://www.epa.gov/pesticides/chemical/foia/cleared-reviews/reviews/060109/060109-2007-03-09a.pdf
- Environmental Protection Agency (2008). Data evaluation record for acetamiprid developmental neurotoxicity study and EPA response to rebuttals submitted by Nisso America. PC Code 099050. TXR# 0052563
- Environmental Protection Agency Office of Pesticide Programs (2012). Guidance for considering and using open literature toxicity studies to support human health risk assessment. Procedures for reviewing relevant effects data published in the open literature for use in OPP’s human health risk assessments. Available from: http://www.epa.gov/pesticides/science/lit-studies.pdf (Accessed 3 March 2014).
- Environmental Protection Agency (2013a). Acetamiprid: pesticide tolerances. EPA-HQ-OPP-2012-0626; FRL-9391-2, Federal Register/vol. 78, No. 118, pp. 36671–36677.
- Environmental Protection Agency (2013b). Dinotefuran: review of developmental neurotoxicity study. DP Barcode 384323. TXR No. 0055570.
- Environmental Protection Agency (2014). IRIS toxicological review of benzo[a]pyrene (External Review Draft 2014; Preface p. XX). Available from: http://cfpub.epa.gov/ncea/iris_drafts/recordisplay.cfm?deid=280022
- Eppolito AK, Smith RF. Long-term behavioral and developmental consequences of pre- and perinatal nicotine. Pharmacol Biochem Behav 2006;85:835–841.
- Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry 2001;40:630–641.
- FQPA Food Quality Protection Act, Public Law, August 3, 1996. U.S. Congress, Washington, DC, Public Law 104-170. Federal Registry 1996;51:34014–34025.
- Garman RH, Fix AS, Jortner BS, Jensen KF, Hardisty JF, Claudio L, Ferenc S. Methods to identify and characterize developmental neurotoxicity for human health risk assessment. II: neuropathology. Environ Health Perspect 2001;109:93–100.
- Garman RH, Li AA, Kaufmann W, Auer RN, Bolon B. Recommended methods for brain processing and quantitative analysis in rodent developmental neurotoxicity studies. Toxicol Pathol 2015; [Epub ahead of print]. doi: 10.1177/0192623315596858.
- Health Canada, PMRA – Clothianidin. (2002). A human health risk assessment for clothianidin (TI-435) – proposal for tolerance of residues in/on canola and corn; EPA OPPTS.
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol 1992;14:221–228.
- Holson RR, Freshwater L, Maurissen JPJ, Moser VC, Phang W. Statistical issues and techniques appropriate for developmental neurotoxicity testing: a report from the ILSI Research Foundation/Risk Science Institute expert working group on neurodevelopmental endpoints. Neurotox Teratol 2008;30:326–348.
- Huang LZ, Liu X, Griffith WH, Winzer-Serhan UH. Chronic neonatal nicotine increases anxiety but does not impair cognition in adult rats. Behav Neurosci 2007;121:1342–1352.
- Insecticide Resistance Action Committee (IRAC) Guidelines for Management of Resistance to Group 4 Insecticides; March 2015; Version 2.0. Available at: http://www.iraconline.org/documents/group-4-irm-guidelines/text=pdf.
- Jeschke P, Nauen R, Schindler M, Elbert A. Overview of the status and global strategy for neonicotinoids. J Agric Food Chem 2011;59:2897–2908.
- Jo YH, Talmage DA, Role LW. Nicotinic receptor-mediated effects on appetite and food intake. J Neurobiol 2002;53:618–632.
- Kaufmann W, Groters S. Developmental neuropathology in DNT-studies – a sensitive tool for the detection and characterization of developmental neurotoxicants. Reprod Toxicol 2006;22:196–213.
- Keil AP, Daniels JL, Hertz-Picciotto I. Autism spectrum disorder, flea and tick medication, and adjustments for exposure misclassification: the CHARGE (Childhood Autism Risks from Genetics and Environment) case–control study. Environ Health 2014;13:3–10.
- Kimura-Kuroda J, Komuta Y, Kuroda Y, Hayashi M, Kawano H. Nicotine-like effects of the neonicotinoid insecticides acetamiprid and imidacloprid on cerebellar neurons from neonatal rats. PLoS One 2012;7:e32432.
- Koch M. The neurobiology of startle. Prog Neurobiol 1999;59:107–128.
- Lacy RT, Mactutus CF, Harrod SB. Prenatal IV nicotine exposure produces a sex difference in sensorimotor gating of the auditory startle reflex in adult rats. Int J Dev Neurosci 2011;29:153–161.
- LeSage MG, Gustaf E, Dufek MB, Pentel PR. Effects of maternal intravenous nicotine administration on locomotor behavior in pre-weanling rats. Pharmacol Biochem Behav 2006;85:575–583.
- Levin ED, Briggs SJ, Christopher NC, Rose JE. Prenatal nicotine exposure and cognitive performance in rats. Neurotoxicol Teratol 1993;15:251–260.
- Levin ED, Slotkin TA. Developmental neurotoxicity of nicotine. In: Slikker W, Chang LW, editors. Handbook of developmental neurotoxicology. San Diego: Academic Press; 1998. pp 587–615.
- Li AA, Lowe KA, McIntosh LJ, Mink PJ. Evaluation of epidemiology and animal data for risk assessment: chlorpyrifos developmental neurobehavioral outcomes. J Toxicol Environ Health Part B: Crit Rev 2012;15:109–184.
- Lunchick C. Occupational and residential exposure and risk assessment for PNR 1427 dog and cat collars formulated with imidacloprid and flumethrin. Bayer HealthCare Report No. 33861; 2010.
- Makris SL, Raffaele K, Allen S, Bowers WJ, Hass U, Alleva E, Calamandrei G, et al. A retrospective performance assessment of the developmental neurotoxicity study in support of OECD Test Guideline 426. Environ Health Perspect 2009;117:17–25.
- Markham JA, Taylor AR, Taylor SB, Bell DB, Koenig JI. Characterization of the cognitive impairments induced by prenatal exposure to stress in the rat. Front Behav Neurosci 2010;4:1–15.
- Matsuda K, Buckingham SD, Kleier D, Rauh JJ, Grauso M, Sattelle DB. Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol Sci 2001;22:573–580.
- Maurissen M. Practical considerations on the design, execution and analysis of developmental neurotoxicity studies. Neurotox Teratol 2010;32:121–123. [Also referenced in the journal’s guide to authors: http://www.elsevier.com/journals/neurotoxicology-and-teratology/0892-0362/guide-for-authors (accessed 20 April 2015).
- Muhammad A, Mychasiuk R, Nakahashi A, Hossain SR, Gibb R, Kolb B. Prenatal nicotine exposure alters neuroanatomical organization of the developing brain. Synapse 2012;66:950–954.
- Nagata K, Song JH, Shono T, Narahashi T. Modulation of the neuronal nicotinic acetylcholine receptor-channel by the nitromethylene heterocycle imidacloprid. J Pharmacol Exp Ther 1998;285:731–738.
- Neurotoxicology and Teratology Guide for Authors. 2015. Available from: http://www.elsevier.com/journals/neurotoxicology-and-teratology/0892-0362/guide-forauthors#4001 (Accessed 22 September 2015).
- Norton S. Hyperactive behavior of rats after lesions of the globus pallidus. Brain Res Bull 1976;1:93–202.
- OECD. (2007). Organization for Economic Cooperation and Development, Test Guideline 426. OECD Guideline for testing of chemicals. Developmental neurotoxicity study. http://www.oecd.org/document/40/0,3343,en_2649_34377_37051368_1_1_1_1,00.html.
- Ozdemir HH, Kara M, Yumrutas O, Uckardes F, Eraslan E, Demir CF, Bal R. Determination of the effects on learning and memory performance and related gene expressions of clothianidin in rat models. Cogn Neurodyn 2014;8:411–416.
- Papke RL, Ono F, Stokes C, Urban J, Boyd RT. The nicotinic acetylcholine receptors of zebrafish and an evaluation of pharmacological tools used for their study. Biochem Pharmacol 2012;84:352–365.
- Pauly J, Slotkin T. Maternal tobacco smoking, nicotine replacement and neurobehavioural development. Acta Paediatr 2008;97:1331–1337.
- Raffaele KC, Fisher JE, Hancock S, Hazelden K, Sobrian SK. Determining normal variability in a developmental neurotoxicity test: a report from the ILSI Research Foundation/Risk Science Institute expert working group on neurodevelopmental endpoints. Neurotoxicol Teratol 2008;30:288–325.
- Raffaele KC, Rowland J, May B, Makris SL, Schumacher K, Scarano LJ. The use of developmental neurotoxicity data in pesticide risk assessments. Neurotoxicol Teratol 2010;32:563–572.
- Rasoulpour RJ, Ellis-Hutchings RG, Terry C, Millar NS, Zablotny CL, Gibb A, Marshall V, et al. A novel mode-of-action mediated by the fetal muscle nicotinic acetylcholine receptor resulting in developmental toxicity in rats. Toxicol Sci 2012;127:522–534.
- Reiter LW. Chemical exposures and animal activity: utility of the figure-eightmaze. In: Hayes AW, Schnell RC, Miyaeds TS, editors. Developments in the science and practice of toxicology. London, UK: Elsevier Science Publishers; 1983. pp 73–84.
- Roy TS, Seidler FJ, Slotkin TA. Prenatal nicotine exposure evokes alterations of cell structure in hippocampus and somatosensory cortex. J Pharmacol Exp Ther 2002;300:124–133.
- Schep LJ, Slaughter RJ, Beasley DM. Nicotinic plant poisoning. Clin Toxicol (Philadelphia, PA) 2009;47:771–781.
- Sheets LP. The neonicotinoid insecticides. In: Massaro EJ, editor. Neurotoxicology handbook. vol. 1. Oxford, UK: Humana Press Inc.; 2002. pp 79–87.
- Schneider T, Ilott N, Brolese G, Bizarro L, Asherson PJ, Stolerman IP. Prenatal exposure to nicotine impairs performance of the 5-choice serial reaction time task in adult rats. Neuropsychopharmacology 2011;36:1114–1125.
- Schneider T, Bizarro L, Asherson PJE, Stolerman IP. Hyperactivity, increased nicotine consumption and impaired performance in the five-choice serial reaction time task in adolescent rats prenatally exposed to nicotine. Psychopharmacol 2012;223:401–415.
- Sheets LP. Imidacloprid. In: Wexler P, editor. Encyclopedia of toxicology, 3rd ed. vol. 2. London, UK: Elsevier Inc., Academic Press; 2014. pp 1000–1003.
- Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C. Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Pollut Res Int 2015;22:5–34.
- Slikker W, Xu ZA, Levin ED, Slotkin TA. Mode of action: disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction-developmental neurotoxicity of nicotine. Crit Rev Toxicol 2005;35:703–711.
- Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther 1998;285:931–945.
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol 2004;198:132–151.
- Tanaka T. Effects of maternal clothianidin exposure on behavioral development in F1 generation mice. Toxicol Ind Health 2012a;28:697–707.
- Tanaka T. Reproductive and neurobehavioral effects of clothianidin administered to mice in the diet. Birth Defects Res B Dev Reprod Toxicol 2012b;95:151–159.
- Thomas JD, Garrison ME, Slawecki CJ, Ehlers C, Riley EP. Nicotine exposure during the neonatal brain growth spurt produces hyperactivity in preweanling rats. Neurotoxicol Teratol 2000;22:695–701.
- Tiesler C, Heinrich J. Prenatal nicotine exposure and child behavioural problems. Eur Child Adolesc Psychiatry 2014;23:913–929.
- Tizabi Y, Russell LT, Nespor SM, Perry DC, Grunberg NE. Prenatal nicotine exposure: effects on locomotor activity and central [125I]alpha-BT binding in rats. Pharmacol Biochem Behav 2000;66:495–500.
- Tomizawa M, Casida JE. Minor structural changes in nicotinoid insecticides confer differential subtype selectivity for mammalian nicotinic acetylcholine receptors. Br J Pharmacol 1999;127:115–122.
- Tomizawa M, Casida J. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Ann Rev Entomol 2003;48:339–364.
- Tomizawa M, Casida J. Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu Rev Pharmacol Toxicol 2005;45:247–268.
- Tomizawa M, Lee D, Casida J. Neonicotinoid insecticides: molecular features conferring selectivity for insect versus mammalian nicotinic receptors. J Agric Food Chem 2000;48:6106–6024.
- Tsuji R, Crofton KM. Developmental neurotoxicity guideline study: issues with methodology, evaluation and regulation. Congenit Anom (Kyoto) 2012;52:122–128.
- Tyl RW, Crofton KM, Moretto A, Moser V, Sheets LP, Sobotka TJ. Identification and interpretation of developmental neurotoxicity effects: a report from the ILSI Research Foundation/Risk Science Institute expert working group on neurodevelopmental endpoints. Neurotox Teratol 2008;30:349–381.
- Vaglenova J, Parameshwaran K, Suppiramanian V, Breese CR, Pandiella N, Birru S. Longlasting teratogenic effects of nicotine on cognition: gender specificity and role of AMPA receptor function. Neurobiol Learn Memory 2008;90:527–536.
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev 2008;32:1073–1086.
- West CD, Kemper TL. The effect of a low protein diet on the anatomical development of the rat brain. Brain Res 1976;107:221–237.
- Winzer-Serhan UH. Long-term consequences of maternal smoking and developmental chronic nicotine exposure. Front Biosci 2008;13:636–649.
- Wisborg K, Henriksen T, Jespersen L, Secher N. Nicotine patches for pregnant smokers: a randomized controlled study. Obstet Gynecol 2000;96:967–971.
- Yamamoto I, Yabuta G, Tomizawa M, Saito T, Miyamoto T, Kagabu S. Molecular mechanism for selective toxicity of nicotinoids and neonicotinoids. J Pesticide Sci 1995;20:33–40.
- Yamamoto I, Tamizawa M, Saito T, Miyamoto T, Walcott E, Sumikawa K. Structural factors contributing to insecticidal and selective actions of neonicotinoids. Arch Insect Biochem Physiol 1998;37:24–32.
- Yamamoto I. Nicotine to nicotinoids: 1962 to 1997. In: Yamamoto I, Casida J, editors. Nicotinoid insecticides and the nicotinic acetylcholine receptor. Tokyo: Springer-Verlag; 1999. pp 3–27.
- Yamamoto I, Casida JE. Nicotinoid insecticides and the nicotinic acetylcholine receptor. Tokyo: Springer-Verlag; 1999.
- Yang W, Carmichael SL, Roberts EM, Kegley SE, Padula AM, English PB, Shaw GM. Residential agricultural pesticide exposures and risk of neural tube defects and orofacial clefts among offspring in the San Joaquin Valley of California. Am J Epidemiol 2014;179:740–748.
- Zhang A, Kayser H, Maienfisch P, Casida J. Insect nicotinic acetylcholine receptor: conserved neonicotinoid specificity of [3H]-imidacloprid binding site. J Neurochem 2000;75:1294–1303.
