Abstract
There are limited effective therapies for most patients with relapsed diffuse large B-cell lymphoma (DLBCL). We conducted a phase II trial of the multi-targeted vascular endothelial growth factor receptor (VEGFR) kinase inhibitor, sunitinib, 37.5 mg given orally once daily in adult patients with relapsed or refractory DLBCL. Of 19 enrolled patients, 17 eligible patients were evaluable for toxicity and 15 for response. No objective responses were seen and nine patients achieved stable disease (median duration 3.4 months). As a result, the study was closed at the end of the first stage. Grades 3–4 neutropenia and thrombocytopenia were observed in 29% and 35%, respectively. There was no relationship between change in circulating endothelial cell numbers (CECs) and bidimensional tumor burden over time. Despite some activity in solid tumors, sunitinib showed no evidence of response in relapsed/refractory DLBCL and had greater than expected hematologic toxicity.
Introduction
Despite the improved cure rates achieved by adding rituximab to anthracycline-based chemotherapy in aggressive-histology B-cell lymphomas [Citation1–4 ], relapsed or primary refractory diffuse large cell lymphomas continue to pose major clinical challenges, with disappointing results. Selected patients may be eligible for second-line therapy using high dose chemotherapy (HDCT) and autologous stem cell transplant (ASCT), but only 25–30% are cured [Citation5]. In the remaining patients, palliative chemotherapy combined with corticosteroids offers temporary relief in some cases, but survival is typically short [Citation6]. Therefore, there is an urgent need to identify alternative clinical approaches that either replace or enhance chemotherapy, offering better disease control with less toxicity.
Sunitinib maleate (SUTENT; Pfizer Inc., New York, NY), is an oral multi-targeted tyrosine kinase inhibitor of vascular endothelial growth factor (VEGF) receptors (VEGFR-1, -2, and -3) and platelet derived-growth factor receptors (PDGFR-α and -β) in addition to KIT, FLT3, RET, and CSF-1 [Citation7,Citation8]. This broad range of receptor inhibition may confer both antiangiogenic effects and direct anti-tumor effects, depending on the tumor subtype. Sunitinib 50 mg given on the schedule of 4 weeks on/2 weeks off provides progression-free and overall survival benefit in renal cell carcinoma (RCC) and progression-free survival benefit in imatinib-resistant gastrointestinal stromal tumors (GISTs) [Citation8–10 ]. Given the previously identified angiogenic phenotype of large cell lymphomas [Citation11–18 ], and the evidence that VEGF and PDGF may promote lymphoma cell growth in both a paracrine and an autocrine fashion [Citation19–21 ], the NCIC (NCIC) Clinical Trials Group undertook a phase II study to evaluate the efficacy of sunitinib in patients with relapsed or refractory diffuse or mediastinal (thymic) large B-cell lymphoma (DLBCL and PMBCL) or transformed B-cell lymphomas. We chose a dose of 37.5 mg p.o. daily with no planned breaks, since this had demonstrated comparable benefit in GIST without an increase in toxicity [Citation22], and the evidence from laboratory studies suggested that antiangiogenic agents have greater efficacy when given continuously without interruption [Citation23,Citation24].
Materials and methods
Patients
Adults aged 18 or older with relapsed or refractory DLBCL, PMBCL, or transformed lymphomas were eligible. Additional key inclusion criteria included at least one and no more than two prior cytotoxic chemotherapy regimens (one must have been anthracycline-containing). Salvage chemotherapy with HDCT/ASCT and up to one other chemotherapy and non-chemotherapy regimen (e.g. radiation) were permitted. Eligible patients must have been able to stop selected CYP3A4 inhibitors/inducers prior to starting sunitinib, have adequate cardiac function, have measurable bidimensional disease, and have an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1. Key exclusion criteria were concurrent use of other antilymphoma therapy, prior use of sunitinib, other antiangiogenic agents, or multi-targeted receptor tyrosine kinase (RTK) inhibitors, uncontrolled hypertension, symptomatic cardio- or cerebrovascular disease, therapeutic anticoagulation, human immunodeficiency virus (HIV), and brain metastases. In addition, patients were excluded if they had a history of cerebrovascular accident, pulmonary embolism, or myocardial infarction within 12 months prior to study enrollment.
The study was approved by the institutional review boards of the participating NCIC Clinical Trials Group institutions and was registered with clinicaltrials.gov. Written informed consent was obtained from all patients before study participation.
Study design
This was a non-randomized, non-blinded multicenter phase II trial of sunitinib in patients with relapsed or refractory DLBCL or PMBCL conducted by the NCIC Clinical Trials Group. Sunitinib was supplied by the Cancer Therapy Evaluation Program (CTEP) of the US National Cancer Institute.
The primary endpoint of this study was objective response. Response was defined as per the report of the international workshop to standardize response criteria for non-Hodgkin lymphoma (NHL) [Citation25]. The secondary endpoints included progression-free survival, toxicity, and the evaluation of antiangiogenic activity as determined by serial assessment of the number of circulating endothelial cells (CECs), apoptotic CECs (aCECs), and their precursors (CEPs).
Treatment
Patients self-administered sunitinib 37.5 mg orally once daily in 4-week cycles. Dose modifications were made for toxicities graded according to the Cancer Therapy Evaluation Program, National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Up to two dose reductions (25 mg then 12.5 mg) were permitted for pre-specified toxicities. Grades 3–4 hematologic and grade 3 non-hematologic adverse events (AEs) generally required one dose reduction after resolution to ≤grade 2. Grade 4 non-hematologic AEs generally led to study discontinuation. Grade 2 hypertension was treated with antihypertensive medications until the blood pressure (BP) was controlled to a mild hypertension range. The drug was held for grade 3 hypertension until BP was controlled, then resumed with one dose reduction. Grade 4 hypertension led to study discontinuation. Patients requiring more than two dose reductions were removed from the study. No dose re-escalations were permitted. Patients who did not recover from toxic effects as required within 2 weeks were removed from protocol therapy.
Assessments
Patients were clinically assessed every 4 weeks. Tumor imaging with computed tomography (CT) scans and assessment of cardiac function by electrocardiography (ECG) and multi-gated acquisition (MUGA) scan were performed at baseline and every 8 weeks while the patient remained on study. Anatomic response assessments were performed locally at each site based on the largest bidimensional marker lesions identified at baseline. In the absence of serious or unmanageable toxicity, patients with complete response (CR), partial response (PR), or stable disease (SD) continued on therapy until disease progression or for a maximum of 12 cycles (1 year). Earlier discontinuation of therapy was permissible if continued treatment was no longer considered in the patient's best interest. In addition, patients who progressed (treatment failure) went off study at the time progression was documented clinically and/or radiographically. At the conclusion of the trial, a central review of X-rays and/or scans was to be carried out for any investigator-claimed responses.
CECs and CEPs were measured in three Ontario centers at baseline, day 1 of cycles 2 and 3, every 3 months thereafter, and at study discontinuation. Flow cytometric analysis was performed in one central location using previously published methods [Citation26].
Statistical methods
A Simon two-stage design was used [Citation27]. A response rate of 5% was not considered promising, while a 20% response rate was worthy of further study. If no responses were seen in the first cohort of 15 evaluable patients, no further accrual would take place. If one or more responses were seen in group 1, then an additional 10 patients would be accrued. The study would be considered positive and sunitinib of interest in DLBCL and its variants if at least three responses were seen in the group of 25 patients (alpha: 0.12; beta: 0.89). All time-to-event data were described using the Kaplan–Meier method. Blood levels of CECs, aCECs, and CEPs (cells/μL) were plotted as percent change from baseline in comparison with the sum of bidimensional measurements of marker lymph nodes over time.
Results
Patient characteristics
A total of 19 patients were enrolled between February 2007 and September 2008 at seven Canadian sites. Two patients were deemed ineligible (one no histological diagnosis, one pulmonary embolism <12 months prior to entry). Seventeen patients were evaluable for toxicity and 15 were evaluable for response. Baseline patient characteristics are outlined in . The median age was 65 and median time from lymphoma diagnosis was 20.3 months (range 5.8–132 months). Fourteen patients had a diagnosis of DLBCL, 10 had immediately preceding chemosensitive disease (complete or partial response to last treatment), and five had relapsed post-HDCT and -ASCT. The majority (11 patients) had an elevated serum lactate dehydrogenase (LDH) at the time of study enrollment.
Table I. Baseline characteristics.
Treatment delivery
The median number of cycles of sunitinib received was 2 (1–5), with only five patients remaining on drug for three or more cycles. Only six of 17 patients received ≥90% of the planned dose intensity, with 14 patients missing doses and five undergoing dose reductions necessitated by toxicities ( and ).
Table II. Most common adverse events according to grade.
Table III. Hematological adverse events.
Safety
The most commonly reported non-hematologic treatment-related AEs thought to be at least possibly related to sunitinib were: fatigue (59%), anorexia (47%), nausea (47%), diarrhea (35%), vomiting (29%), mucositis, clinical exam and functional/symptomatic (24% and 18%, respectively), heartburn (24%), and hypertension (24%), with most of these events of mild or moderate intensity (grades 1 or 2) (). One patient had a grade 2 asymptomatic reduction in left ventricular (LV) systolic function, two developed grade 1 pleural effusion, and four developed elevated thyroid stimulating hormone (TSH) on treatment, although only two required thyroid replacement. One patient had a grade 4 pericardial effusion develop on study, but this was deemed to be related to progressive lymphoma, not to sunitinib. Neutropenia and thrombocytopenia were grade 3 or more in five and six patients, respectively (), and were the most common reason for dose omission or reduction. Six patients (35%) discontinued treatment due to AEs, four of which were hematologic, and eight patients discontinued therapy due to disease progression. There were no treatment-related deaths.
Efficacy
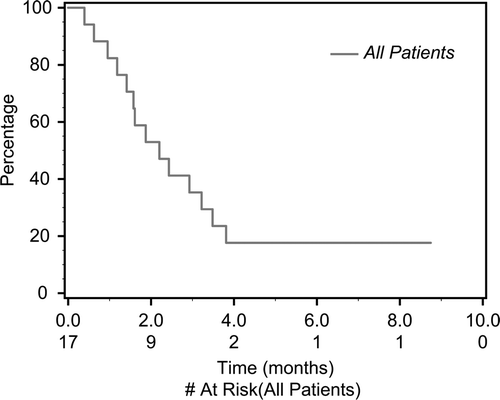
Of 17 eligible patients, 15 were evaluable for response. One patient received only two doses of drug, and one patient did not have restaging scans. Of those evaluable, no patient experienced a clinical response to sunitinib after central radiology review. As a result, the study was closed to accrual according to the protocol. Nine patients (53%) achieved stable disease as best response (median duration 3.4 months; range: 1.4–8.7 months), and six (35%) had primary progressive disease. Overall progression-free survival (PFS) () was 2.2 months (95% confidence interval [CI] 1.41–3.48). All patients are currently off study, eight due to disease progression, one due to symptomatic progression, and six due to toxicity, and two withdrew consent.
Analysis of biomarkers
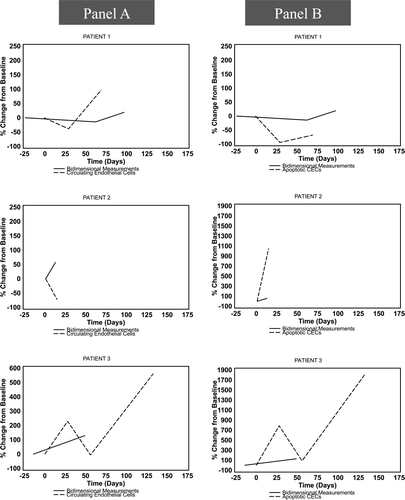
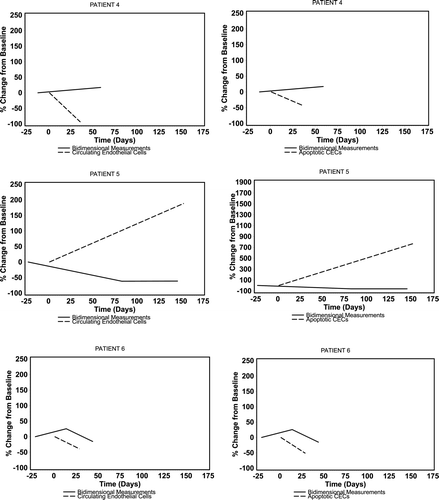
CECs, aCECs, and CEPs were assessed at baseline in 10 patients and in two or more serial measurements in seven patients (six of whom had restaging CT scans for comparison). The median baseline CEC count was 2.9 cells/μL (range 1.13–7.03 cells/μL), of which 86% (range 30–99%) were viable. CEP levels were too low to be serially followed. There was no discernible relationship between the change in absolute or apoptotic CECs over time and clinical response or change in bidimensional measurements (). Sixty-seven percent of the patients with stable disease had a normal LDH at baseline compared with 0% in patients with primary progressive disease.
Discussion
As in solid tumors, neo-angiogenesis may contribute to the pathogenesis and poor prognosis in many aggressive-histology lymphomas. The detection of VEGF A, B, and C isoforms and their receptors on many large cell lymphoma samples suggests that the VEGF pathway is critically important, and may contribute to disease progression in both an autocrine and a paracrine fashion [Citation12,Citation17,Citation18,Citation28,Citation29].
The anti-VEGF monoclonal antibody bevacizumab has been evaluated in relapsed DLBCL, resulting in one partial response and eight patients with stable disease as best response, out of 30 evaluable patients; 6-month PFS, the primary study endpoint, was 15% (95% CI 5–26%) [Citation30]. Unfortunately, a multicenter phase III trial comparing CHOP-R (cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab) with CHOP-R + bevacizumab was recently discontinued due to excess cardiac morbidity, and the clinical benefits of adding an antiangiogenic agent to standard treatment are still unknown.
The evaluation of agents targeting VEGF signaling in NHL, notably DLBCL, is of interest. Sunitinib (SU11248) was a logical agent to study, since it is an orally bioavailable inhibitor affecting RTKs involved in tumor proliferation and angiogenesis, including VEGFR-1, -2, and -3, and PDGFR-α and -β.
In this multicenter phase II study, sunitinib 37.5 mg p.o. daily did not produce objective radiologic responses in patients with relapsed or refractory DLBCL or transformed lymphoma, with short-lived stable disease as the best achievable response in 53% of patients. This contrasts with objective response rates of 25.5–36.5% in metastatic RCC [Citation10], 23% in bevacizumab-refractory metastatic RCC [Citation31], 16% in advanced pancreatic neuroendocrine tumors [Citation32], and 11% in advanced non-small cell lung cancer [Citation33]. The lack of response in lymphoma is congruent with the sunitininb experience in heavily pretreated chronic lymphocytic leukemia (CLL) [Citation34].
These negative results may be explained by a number of factors. First, diffuse large cell lymphomas are rapidly proliferating tumors that may not be suited to treatment with cytostatic agents used as monotherapy. The median time on drug (2 months) may have been too short to demonstrate any efficacy in many patients whose baseline elevated LDH suggested highly mitotic tumors. Second, the dose of sunitinib chosen for testing in this patient population, 37.5 mg daily, may have been too low, despite the continuous schedule used. Most clinical trials of sunitinib reporting significant objective response rates have used 50 mg daily for 4 out of 6 weeks. We selected the lower dose to permit continuous administration and to avoid the rebound increase in markers of angiogenesis observed after angiogenesis inhibitors are stopped [Citation35–37 ]. However, despite a lower daily dose, we encountered unexpected excessive myelosuppression induced by sunitinib, which compromised the ability to administer even the reduced dosage intended in this study on schedule, and accounted for many of the AEs that led to study treatment discontinuation. This may be because 37.5 mg daily is a comparable if not slightly higher total dose over a 6-week period than the total dose of the 50 mg syncopated 6-week schedule. Or perhaps, in this patient population who have been previously treated with multiagent chemotherapy (including alkylators and anthracyclines) and, frequently, ASCT, an interrupted schedule (as has been evaluated in patients with solid tumors) may have allowed greater drug delivery. Our hematologic adverse event experience is not dissimilar to that reported in heavily pretreated patients with CLL given sunitinib 37.5 mg p.o. daily [Citation34]. In that trial, 16 of 18 (89%) patients experienced grade 3 or higher adverse events to sunitinib, with 56% ≥grade 3 thrombocytopenia and 27% ≥grade 3 neutropenia. These data, together with those from our trial, suggest that patients with lymphoid cancers may experience greater myelosuppression because of the presence of extensive marrow involvement, as might be expected in patients with CLL, or due to the nature of their prior therapy, such as exposure to purine analogs or following ASCT.
Clinical trials for traditional cytotoxic drugs are often designed to show an improvement in the objective response rate. Many of the newer anti-cancer agents, including those targeting angiogenesis, may have a primarily cytostatic rather than a cytotoxic effect, and may delay progression and/or death while having little effect on tumor size. In the absence of randomized trials wherein time-to-event endpoints such as time to tumor progression can be reliably compared with a control group [Citation8,Citation10], surrogate biomarkers are needed to help validate the mechanistic hypotheses of action, identify responsive patients and optimal biologic doses, and predict the outcomes of regimens that include anti-VEGF agents.
CECs and their progenitors (CEPs) are rarely found in the blood of healthy subjects, but may be elevated in patients with neoplastic disease and correlate with angiogenesis [Citation38]. Preclinically, CECs appear to correlate with tumor volumes in SCID (severe combined immunodeficiency) mice bearing human lymphoma [Citation39]. Increased CD133+CD34+VEGFR-2+ endothelial precursor cells (EPCs) are detectable in the peripheral blood of patients with aggressive lymphomas and decrease in number following complete response to chemotherapy [Citation40]. Additionally, bevacizumab reduced the frequency of viable CECs and CEPs in patients with rectal cancer [Citation41], and in a previous study in patients with relapsed aggressive lymphomas, CECs and CEPs declined during metronomic low-dose cyclophosphamide and high dose celecoxib [Citation42].
The lack of correlation between tumor response as measured by bidimensional measurements and changes in CECs contrasts with the observations in patients with RCC treated with sunitinib on a 50 mg daily for 4 weeks, with 2 weeks off, schedule [Citation43]. While on sunitinib, opposite kinetics of two circulating CD34bright cell populations, hematopoietic progenitor cells (HPCs) and small CECs, were observed, with the HPCs decreasing and the CECs increasing but normalizing to pretreatment values during the 2-week drug-free period. This suggested that sunitinib was directly targeting the immature tumor vessels. In another study, sunitinib was reported to cause a greater increase in CECs in patients with GIST, and this increase was associated with clinical benefits compared with patients with progressive disease [Citation44].
The problematic reproducibility and validity of measuring low frequency CECs by flow cytometry is known, but our negative findings may simply reflect the limited power of this analysis due to serial monitoring of a small number of patients (n = 8) for a median of two cycles.
Finally, cytostatic agents probably work best when used in combination with chemotherapy. Despite this, it would be unrealistic to pursue more drug development with sunitinib in combination with chemotherapy in the absence of single-agent activity (or tolerability). The absence of any objective responses negatively predicts for eventual regulatory approval of a given therapeutic agent in solid tumors. There is no reason to suppose that in lymphomas, which are often more sensitive to a specific chemotherapeutic agent than are solid tumors, this observation with respect to targeted agents would not also hold true. Indeed, all recently approved new agents in lymphoma demonstrated objective responses when given as single agents [Citation45].
Conclusion
Sunitinib administered 37.5 mg p.o. daily was inactive in patients with relapsed or refractory DLBCLs and resulted in greater hematological and other toxicities compared to the experience in populations with solid tumors. No convincing pharmacodynamic evidence of antiangiogenic activity was demonstrable by CEC and CEP biomarker analysis, with the qualification that limited serial sampling was possible.
In our opinion, this study illustrates the challenge of studying novel targeted therapies including antiangiogenesis agents in rapidly proliferating lymphomas.
Supplementary Material
Download Zip (4.7 MB)Acknowledgements
We acknowledge the Canadian Cancer Society as having funded this trial through a core grant to the NCIC Clinical Trials Group and CTEP for supplying the investigational agent of this trial.
We acknowledge the principal investigators Dr. Judith Sutherland from BCCA Kelowna, BC and Dr. Muhammad Salim from Allen Blair Cancer Center, Regina, SK and the following physicians who contributed patients to this trial: Drs. Andrew Belch and Anthony Reiman from the Cross Cancer Center, Edmonton, AB; Dr. Stephen Couban from QEII Health Sciences Center, Halifax, NS; Drs. Matthew Cheung and Kevin Imrie from the Odette Cancer Center, Toronto, ON; Dr. Vishal Kukreti from Princess Margaret Hospital, Toronto, ON; and Dr. Kevin Murphy from the BCCA Fraser Valley Center, Surrey, BC.
Potential conflict of interest:
Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol 2005;23:4117–4126.
- Coiffier B. Rituximab in combination with CHOP improves survival in elderly patients with aggressive non-Hodgkin's lymphoma. Semin Oncol 2002;29:18–22.
- Pfreundschuh M, Truemper L, Gill D, et al. First analysis of the completed Mabthera International (MInT) trial in young patients with low-risk diffuse large B-cell lymphoma (DLBCL): addition of rituximab to a CHOP-like regimen significantly improves outcome of all patients with the identification of a very favorable subgroup with IPI = O and no bulky disease. Blood 2004;104(Suppl. 1): Abstract 157.
- Habermann T, Weller E, Moprrison VA, et al. Rituximab-CHOP versus CHOP with or without maintenance rituximab in patients 60 years of age or older with diffuse large B-cell lymphoma (DLBCL): an update. Blood 2004;104(Suppl. 1): Abstract 127.
- Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med 1995;333:1540–1545.
- Vose JM, Bierman PJ, Anderson JR, et al. Progressive disease after high-dose therapy and autologous transplantation for lymphoid malignancy: clinical course and patient follow-up. Blood 1992;80:2142–2148.
- Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol 2007;25:884–896.
- Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006;368:1329–1338.
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:3584–3590.
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115–124.
- Jorgensen JM, Sorensen FB, Bendix K, et al. Expression level, tissue distribution pattern, and prognostic impact of vascular endothelial growth factors VEGF and VEGF-C and their receptors Flt-1, KDR, and Flt-4 in different subtypes of non-Hodgkin lymphomas. Leuk Lymphoma 2009;50:1647–1660.
- Jorgensen JM, Sorensen FB, Bendix K, et al. Angiogenesis in non-Hodgkin's lymphoma: clinico-pathological correlations and prognostic significance in specific subtypes. Leuk Lymphoma 2007;48:584–595.
- Bertolini F, Paolucci M, Peccatori F, et al. Angiogenic growth factors and endostatin in non-Hodgkin's lymphoma. Br J Haematol 1999;106:504–509.
- Bertolini F, Fusetti L, Mancuso P, et al. Endostatin, an antiangiogenic drug, induces tumor stabilization after chemotherapy or anti-CD20 therapy in a NOD/SCID mouse model of human high-grade non-Hodgkin lymphoma. Blood 2000;96:282–287.
- Bertolini F, Fusetti L, Rabascio C, Cinieri S, Martinelli G, Pruneri G. Inhibition of angiogenesis and induction of endothelial and tumor cell apoptosis by green tea in animal models of human high-grade non-Hodgkin's lymphoma. Leukemia 2000;14:1477–1482.
- Bertolini F, Mancuso P, Gobbi A, Pruneri G. The thin red line: angiogenesis in normal and malignant hematopoiesis. Exp Hematol 2000;28:993–1000.
- Gratzinger D, Zhao S, Marinelli RJ, et al. Microvessel density and expression of vascular endothelial growth factor and its receptors in diffuse large B-cell lymphoma subtypes. Am J Pathol 2007;170:1362–1369.
- Gratzinger D, Zhao S, Tibshirani RJ, et al. Prognostic significance of VEGF, VEGF receptors, and microvessel density in diffuse large B cell lymphoma treated with anthracycline-based chemotherapy. Lab Invest 2008;88:38–47.
- Wang ES, Teruya-Feldstein J, Wu Y, Zhu Z, Hicklin DJ, Moore MA. Targeting autocrine and paracrine VEGF receptor pathways inhibits human lymphoma xenografts in vivo. Blood 2004;104:2893–2902.
- Dias S, Hattori K, Zhu Z, et al. Autocrine stimulation of VEGFR-2 activates human leukemic cell growth and migration. J Clin Invest 2000;106:511–521.
- Dias S, Hattori K, Heissig B, et al. Inhibition of both paracrine and autocrine VEGF/VEGFR-2 signaling pathways is essential to induce long-term remission of xenotransplanted human leukemias. Proc Natl Acad Sci USA 2001;98:10857–10862.
- George S, Blay JY, Casali PG, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer 2009;45:1959–1968.
- Shaked Y, Emmenegger U, Man S, et al. The optimal biological dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity. Blood 2005;106:3058–3061.
- Bertolini F, Paul S, Mancuso P, et al. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res 2003;63:4342–4346.
- Cheson BC, Horning SJ, Coiffer B, et al. Report of international workshop to standardize response criteria for non-Hodgkin's lymphomas. J Clin Oncol 1999;17:1244–1253.
- Mancuso P, Colleoni M, Calleri A, et al. Circulating endothelial cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood 2006;108:452–459.
- Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989;10:1–10.
- Ganjoo K. Antiangiogenesis: a new approach to the treatment of lymphoma. Leuk Lymphoma 2007;48:454–455.
- Ribatti D, Vacca A, Nico B, Fanelli M, Roncali L, Dammacco F. Angiogenesis spectrum in the stroma of B-cell non-Hodgkin's lymphomas. An immunohistochemical and ultrastructural study. Eur J Haematol 1996;56:45–53.
- Stopeck AT, Unger JM, Rimsza LM, et al. A phase II trial of single agent bevacizumab in patients with relapsed, aggressive non-Hodgkin lymphoma: Southwest Oncology Group study S0108. Leuk Lymphoma 2009;50:728–735.
- Rini BI, Michaelson MD, Rosenberg JE, et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol 2008;26:3743–3748.
- Kulke MH, Lenz HJ, Meropol NJ, et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol 2008;26:3403–3410.
- Socinski MA, Novello S, Brahmer JR, et al. Multicenter, phase II trial of sunitinib in previously treated, advanced non-small-cell lung cancer. J Clin Oncol 2008;26:650–656.
- Shanafelt T, Zent C, Byrd J, et al. Phase II trials of single-agent anti-VEGF therapy for patients with chronic lymphocytic leukemia. Leuk Lymphoma 2010;51:2222–2229.
- Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci USA 2007;104:17069–17074.
- Mancuso MR, Davis R, Norberg SM, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest 2006;116:2610–2621.
- Shaked Y, Ciarrocchi A, Franco M, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science 2006;313:1785–1787.
- Blann AD, Woywodt A, Bertolini F, et al. Circulating endothelial cells. Biomarker of vascular disease. Thromb Haemost 2005;93:228–235.
- Monestiroli S, Mancuso P, Burlini A, et al. Kinetics and viability of circulating endothelial cells as surrogate angiogenesis marker in an animal model of human lymphoma. Cancer Res 2001;61:4341–4344.
- Igreja C, Courinha M, Cachaco AS, et al. Characterization and clinical relevance of circulating and biopsy-derived endothelial progenitor cells in lymphoma patients. Haematologica 2007;92:469–477.
- Willett CG, Boucher Y, Duda DG, et al. Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. J Clin Oncol 2005;23:8136–8139.
- Buckstein RJ, Crump M, Shaked Y, et al. High dose celecoxib and metronomic ‘low-dose’ cyclophosphamide is effective and safe therapy in patients with relapsed and refractory aggressive histology NHL. Ann Oncol 2005;16(Suppl. 5): Abstract 331.
- Vroling L, van der Veldt AA, de Haas RR, et al. Increased numbers of small circulating endothelial cells in renal cell cancer patients treated with sunitinib. Angiogenesis 2009;12: 69–79.
- Norden-Zfoni A, Desai J, Manola J, et al. Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clin Cancer Res 2007;13:2643–2650.
- El-Maraghi RH, Eisenhauer EA. Review of phase II trial designs used in studies of molecular targeted agents: outcomes and predictors of success in phase III. J Clin Oncol 2008;26:1346–1354.