Chen and colleagues [Citation1] in the current issue of Leukemia and Lymphoma have investigated a combination of homoharringtonine (HHT), aclarubicine, cytarabine and granulocyte colony-stimulating factor (G-CSF) (CHAG) in treating 36 patients (aged 16–61 years) with relapsed (19%) or refractory (67%) acute myeloid leukemia (AML) [Citation2]. HHT was given at 1 mg/m2 intravenously (IV) from day 1 to day 14. Complete remission (CR) was achieved in 81% and partial remission (PR) in 14% of the patients. The median duration to neutrophil recovery was 15 days. Cardiac toxicity was not noted in this study. One patient had liver dysfunction that spontaneously improved. The results are encouraging, with an excellent response rate that exceeds the expected 17–60% response rate for conventional therapies used in this setting [Citation3–9]. However, longer follow-up and ultimately randomized trials are warranted to assess the actual effect of this or other combinations.
In the early 1970s, HHT and other ester alkaloid cephalotaxins were isolated from the bark of the Cephalotaxus fortunei evergreen tree and found to have activity against murine leukemia by decreasing protein synthesis and inducing apoptosis [Citation10–12]. In one of the earliest Chinese studies, mixtures of HHT and harringtonine (HT) at different dosing schedules (IV or intramuscular [IM]) led to five CRs in 41 evaluable patients (12%). The study indicated that the maximum tolerated therapeutic effective dose was 4 mg daily IV for 14 days. Major dose-limiting toxicities were myelosuppression and cardiovascular abnormalities, including hypotension and arrhythmias [Citation13]. More Chinese phase I and II studies were summarized by Grem et al. [Citation14] in a landmark article that showed a CR rate of 24% (65 of 274 patients) in patients with AML.
As HHT is a G1/G2-phase specific drug that causes leukemic cell transformation into S-phase [Citation15], this provided the theoretical basis to combine it with other agents that are S-phase specific such as cytarabine [Citation16,Citation17]. In 1980, the HOAP (HHT, vincristine, cytarabine and steroids) regimen resulted in CRs in 82% (23/28) of newly diagnosed patients with AML [Citation18], but with more profound myelosuppression and cardiac toxicities. Similarly, HHT in combination with cytarabine and thioguanine was used in newly diagnosed patients with AML, resulting in a CR rate of 68% [Citation19].
The success of the Chinese studies motivated US investigators to use HHT in acute leukemias. In 1984, the first US phase I trial by Legha et al. [Citation20] investigated HHT daily bolus infusion over 10–360 min for 1–10 days, with dose escalation 0.2–8 mg/m2 daily in 43 patients with advanced malignancies, including five patients with acute leukemia; CR was achieved in three of the five patients. Hypotension was the limiting toxicity for doses > 4 mg/m2 daily. Other phase I bolus studies showed similar results and recommended a phase II dose of 3–4 mg/m2 daily for 5 days as a short infusion [Citation21]. Since hypotension seemed to be related to vasodilatation following the bolus infusion, HHT was administered through continuous infusion (CIVI). In a phase I–II study, Warrell et al. [Citation22] used CIVI HHT in 49 patients with relapsed or refractory AML and concluded that dosing at 5 mg/m2 daily for 9 days is a reasonable option, as any higher doses caused significant hypotension as in the bolus schedule. CR was achieved in 25% of the patients (consisting of both relapsed and refractory AML) and lasted for a median of 6 months. HHT CIVI was also effective in myelodysplastic syndrome (MDS) and MDS transformed to AML as shown by Feldman et al. [Citation23], where eight of 28 patients responded, but early death due to neutropenic complications was seen in 46%. Myelosuppression was an issue with CIVI, as full bone marrow recovery took up to 24 days. Subcutaneous (SQ) HHT was first used in a phase I trial in 2006 when Levy et al. [Citation24] treated 18 adults patients with relapsed/refractory AML, accelerated myeloproliferative neoplasms or refractory MDS with dose-escalated twice-daily SQ HHT 0.5–6 mg/m2 for 9 days. The study defined a maximum tolerated dose as 5 mg/m2 daily, resulting in two CRs in patients with AML (lasting 3 and 4 months) and hypoplastic bone marrow on day 10 in five of 14 patients, in addition to clearing peripheral blasts. Adverse events included hyperglycemia, fluid retention and infectious complications.
As the Chinese combined HHT with other chemotherapies based on theoretical synergism, the same was investigated in the USA in multiple phase II trials and in different patient populations (de novo, relapsed and refractory AML or MDS/transformed MDS). Feldman et al. [Citation25] used HHT 1.5–5 mg/m2 daily CIVI with 100 mg/m2 CIVI cytarabine for 7 days in the relapsed/refractory setting. CR was noted in five of 14 patients with relapsed AML, but none in refractory patients. Four of the responders were actually patients with acute promyelocytic leukemia (APL). The most significant adverse events were hypotension, fluid retention and hyperglycemia.
As some regimens (such as fludarabine, cytarabine, G-CSF and idarubicin; FLAG-IDA) [Citation26] incorporated G-CSF to increase blast percentage in S-phase [Citation27] to improve the cytotoxic effects of cell-cycle specific chemotherapy agents, so was done with HHT. Jin et al. [Citation28] studied G-CSF priming in the combination of HHT, cytarabine, aclarubicine and G-CSF in newly diagnosed patients with AML, resulting in an 83% CR rate after 1–2 cycles, a 3-year survival of 55–75% and treatment related death of 4% [Citation28]. Similar combinations were also used in the relapsed/refractory AML setting [Citation29], resulting in 52% CR and 64% overall response rate with a median OS of 12.5 months. A more recent combination included endostatin, G-CSF, cytarabine and HHT as salvage treatment for patients with relapsed/refractory AML, with a CR reported in five of six patients that provided a median survival of 16.2 months with preserved quality of life [Citation30].
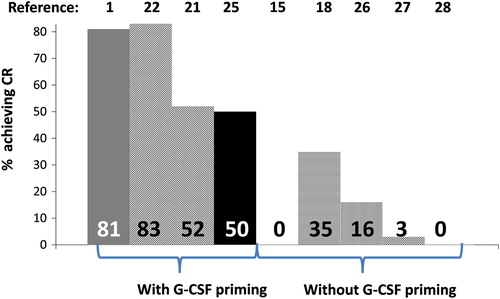
The difference in CR rates between the Chinese studies and the Western studies is perplexing. As the Chinese used G-CSF priming in relapsed/refractory patients, this may explain the difference in that scenario () [Citation31]. The reason for the higher CR rates in newly diagnosed patients between the Chinese studies and the Western studies remains unclear. There may be ethnic differences in the response to HHT between Chinese and Caucasian patients.
Figure 1. Achievement of complete remission (CR) following homoharringtonine-based regimens with and without granulocyte colony-stimulating factor (G-CSF) use.
The effects of different karyotypes were studied among 153 patients with de novo AML (non-APL) treated with HHT-based regimens who had available cytogenetics divided into favorable, intermediate or adverse risk groups [Citation32]; median OS was 4 months shorter if patients had an adverse karyotype. CR rates were also profoundly affected (88% in normal karyotype vs. 30% in trisomy 8, chromosome 3 abnormalities or complex karyotype) [Citation31]. These results are similar to those reported with conventional chemotherapy agents.
As the semi-synthetic analog of HHT, omacetaxine mepesuccinate (SYNRIBO®), was recently approved by the Food and Drug Administration for patients with chronic myeloid leukemia in chronic and accelerated phases who have failed two or more tyrosine kinase inhibitors, the interest in this drug may be increasing. Further, it would be interesting to investigate HHT's effect on the leukemic stem cell, as it was recently found to induce apoptosis in vitro in chronic myeloid leukemia (CML) and non-CML stem cells [Citation33], and in the murine model of CML and BCR–ABL positive B-acute lymphoblastic leukemia [Citation34], a feature not seen when traditional tyrosine kinase inhibitors are used for this disease. One question that remains is whether there will be sufficient Cephalotaxus fortunei trees to supply this medication for studies in these diseases.
Supplementary Material
Download Zip (978.1 KB)Potential conflict of interest:
Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- Chen L, Yin Q, Mi R, et al. CHAG priming regimen containing of cytarabine, aclacinomycin homoharringtonine and G-CSF for relapsed refractory acute myelogenous Leukemia: a modified combination chemotherapeutic combination. Leuk Lymphoma2013;54:2291–2293.
- Smith M, Barnett M, Bassan R, et al. Adult Acute Myeloid Leukaemia. Crit Rev Oncol Hematol 2004;50:197–222.
- Ohno R, Naoe T, Kanamaru A, et al. A double-blind controlled study of granulocyte colony-stimulating factor started two days before induction chemotherapy in refractory acute myeloid leukemia. Kohseisho Leukemia Study Group. Blood 1994;83:2086–2092.
- Vogler WR, McCarley DL, Stagg M, et al. A phase III trial of high-dose cytosine arabinoside with or without etoposide in relapsed and refractory acute myelogenous leukemia. A Southeastern Cancer Study Group trial. Leukemia 1994;8:1847–1853.
- Thomas X, Fenaux P, Dombret H, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) to increase efficacy of intensive sequential chemotherapy with etoposide, mitoxantrone and cytarabine (EMA) in previously treated acute myeloid leukemia: a multicenter randomized placebo-controlled trial (EMA91 Trial). Leukemia 1999; 13:1214–1220.
- Karanes C, Kopecky KJ, Head DR, et al. A phase III comparison of high dose ARA-C (HIDAC) versus HIDAC plus mitoxantrone in the treatment of first relapsed or refractory acute myeloid leukemia Southwest Oncology Group Study. Leuk Res 1999;23:787–794.
- Greenberg PL, Lee SJ, Advani R, et al. Mitoxantrone, etoposide, and cytarabine with or without valspodar in patients with relapsed or refractory acute myeloid leukemia and high-risk myelodysplastic syndrome: a phase III trial (E2995). J Clin Oncol 2004;22:1078–1086.
- Feldman EJ, Brandwein J, Stone R, et al. Phase III randomized multicenter study of a humanized anti-CD33 monoclonal antibody, lintuzumab, in combination with chemotherapy, versus chemotherapy alone in patients with refractory or first-relapsed acute myeloid leukemia. J Clin Oncol 2005;23:4110–4116.
- Milligan DW, Wheatley K, Littlewood T, et al. Fludarabine and cytosine are less effective than standard ADE chemotherapy in high-risk acute myeloid leukemia, and addition of G-CSF and ATRA are not beneficial: results of the MRC AML-HR randomized trial. Blood 2006;107:4614–4622.
- Powell RG, Weisleder D, Smith CR Jr. Antitumor alkaloids for Cephalataxus harringtonia: structure and activity. J Pharm Sci 1972; 61:1227–1230.
- O’Dwyer PJ, King SA, Hoth DF, et al. Homoharringtonine–perspectives on an active new natural product. J Clin Oncol 1986; 4:1563–1568.
- Wetzler M, Segal D. Omacetaxine as an anticancer therapeutic: what is old is new again. Curr Pharm Des 2011;17:59–64.
- Cephalotaxine esters in the treatment of acute leukemia. A preliminary clinical assessment. Chin Med J (Engl) 1976;2:263–272.
- Grem JL, Cheson BD, King SA, et al. Cephalotaxine esters: antileukemic advance or therapeutic failure?. J Natl Cancer Inst 1988;80:1095–1103.
- Mai WY, Lin MF. Induction of apoptosis by homoharringtonine in G1 phase human chronic myeloid leukemic cells. Chin Med J (Engl) 2005;118:487–492.
- te Boekhorst PA, Lowenberg B, Sonneveld P. Hematopoietic growth factor stimulation and cytarabine cytotoxicity in vitro: effects in untreated and relapsed or primary refractory acute myeloid leukemia cells. Leukemia 1994;8:1480–1486.
- Lowenberg B, van Putten W, Theobald M, et al. Effect of priming with granulocyte colony-stimulating factor on the outcome of chemotherapy for acute myeloid leukemia. N Engl J Med 2003;349:743–752.
- High remission induction (traditional sino-western HOAP) regimen for acute nonlymphocytic leukemia. Chin Med J (Engl) 1980; 93:565–568.
- Bian SG, Hao YS, Wang ZC. [Analysis of the therapeutic efficacy and prognostic factors of intensive chemotherapy in 91 patients with acute nonlymphoblastic leukemia]. Zhonghua Nei Ke Za Zhi 1990;29:22–25, 60.
- Legha SS, Keating M, Picket S, et al. Phase I clinical investigation of homoharringtonine. Cancer Treat Rep 1984;68:1085–1091.
- Stewart JA, Krakoff IH. Homoharringtonine: a phase I evaluation. Invest New Drugs 1985;3:279–286.
- Warrell RP Jr, Coonley CJ, Gee TS. Homoharringtonine: an effective new drug for remission induction in refractory nonlymphoblastic leukemia. J Clin Oncol 1985;3:617–621.
- Feldman EJ, Seiter KP, Ahmed T, et al. Homoharringtonine in patients with myelodysplastic syndrome (MDS) and MDS evolving to acute myeloid leukemia. Leukemia 1996;10:40–42.
- Levy V, Zohar S, Bardin C, et al. A phase I dose-finding and pharmacokinetic study of subcutaneous semisynthetic homoharringtonine (ssHHT) in patients with advanced acute myeloid leukaemia. Br J Cancer 2006;95:253–259.
- Feldman E, Arlin Z, Ahmed T, et al. Homoharringtonine in combination with cytarabine for patients with acute myelogenous leukemia. Leukemia 1992;6:1189–1191.
- Parker JE, Pagliuca A, Mijovic A, et al. Fludarabine, cytarabine, G-CSF and idarubicin (FLAG-IDA) for the treatment of poor-risk myelodysplastic syndromes and acute myeloid leukaemia. Br J Haematol 1997;99:939–944.
- te Boekhorst PA, Lowenberg B, Vlastuin M, Sonneveld P. Enhanced chemosensitivity of clonogenic blasts from patients with acute myeloid leukemia by G-CSF, IL-3 or GM-CSF stimulation. Leukemia 1993;7:1191–1198.
- Jin J, Jiang DZ, Mai WY, et al. Homoharringtonine in combination with cytarabine and aclarubicin resulted in high complete remission rate after the first induction therapy in patients with de novo acute myeloid leukemia. Leukemia 2006;20:1361–1367.
- Gu LF, Zhang WG, Wang FX, et al. Low dose of homoharringtonine and cytarabine combined with granulocyte colony-stimulating factor priming on the outcome of relapsed or refractory acute myeloid leukemia. J Cancer Res Clin Oncol 2011;137:997–1003.
- Fang B, Liu Y, Zhou J, et al. Salvage therapy with endostatin, low-dose homoharringtonine, and cytarabine in combination with granulocyte-colony stimulating factor for elderly patients with primary refractory acute myeloid leukemia. Am J Hematol 2012;87:126–127.
- Mi Y, Xue Y, Yu W, et al. Therapeutic experience of adult acute myeloid leukemia in a single institution of China and its relationship with chromosome karyotype. Leuk Lymphoma 2008;49:524–530.
- Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 2000;96:4075–4083.
- Allan EK, Holyoake TL, Craig AR, et al. Omacetaxine may have a role in chronic myeloid leukaemia eradication through downregulation of Mcl-1 and induction of apoptosis in stem/progenitor cells. Leukemia 2011;25:985–994.
- Chen Y, Hu Y, Michaels S, et al. Inhibitory effects of omacetaxine on leukemic stem cells and BCR-ABL-induced chronic myeloid leukemia and acute lymphoblastic leukemia in mice. Leukemia 2009;23:1446–1454.