Abstract
We investigated the effects of chronic mineralocorticoid receptor blockade with eplerenone on the development and progression of hypertension and end organ damage in Dahl salt-sensitive rats. Eplerenone significantly attenuated the progressive rise in systolic blood pressure (SBP) (204 ± 3 vs. 179±3 mmHg, p < 0.05), reduced proteinuria (605.5 ± 29.6 vs. 479.7 ± 26.1 mg/24h, p < 0.05), improved injury scores of glomeruli, tubules, renal interstitium, and vasculature in Dahl salt-sensitive rats fed a high-salt diet. These results demonstrate that mineralocorticoid receptor antagonism provides target organ protection and attenuates the development of elevated blood pressure (BP) in a model of salt-sensitive hypertension.
Introduction
Aldosterone plays a critical role in regulating body fluid, electrolytes, and blood pressure (BP) homeostasis, which is achieved by promoting the reabsorption of sodium and water and the secretion of potassium in the connecting tubules and collecting ducts in the kidneys. Accumulated evidence suggests that mineralocorticoid receptor (MR) activation mediated by increased aldosterone plays a crucial role in the development and progression of hypertension and end organ damage (Citation1–3). Extensive animal and clinical studies have demonstrated that MR antagonism lowers BP (Citation4–11) and provides cardiac, vascular, and renal protection via both hemodynamic and non-hemodynamic mechanisms (Citation5–8,11–16). Moreover, the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) demonstrated the addition of eplerenone, a selective MR antagonist, to standard medical therapy reduced morbidity and mortality among patients with acute myocardial infarction complicated by left ventricular dysfunction and heart failure (Citation17).
Hypertension affects approximately 50 million individuals in the United States (Citation18) and approximately 1 billion people worldwide (Citation19). A substantial proportion of individuals with hypertension in industrialized countries consume high levels of salt and Weinberger reported that approximately 50% of individuals with essential hypertension are salt-sensitive (Citation20). Abnormalities in aldosterone-mediated MR activation are associated with salt-sensitivity and hypertension (Citation21–25). The Dahl salt-sensitive (Dahl SS) rat, a rodent model of salt-sensitive hypertension, has been widely used to investigate the molecular mechanisms underlying the development of salt-induced hypertension and to evaluate the efficacy of pharmacologic interventions in salt-sensitive hypertension (Citation26–30). The selective MR antagonist, eplerenone, has been evaluated extensively in Dahl SS rats on a high-salt diet. However, reports on its efficacy for lowering BP were diverse: some studies reported a marked attenuation of the increase in BP (Citation4,7,8), whereas other studies reported no effects on BP in response to salt-loading in Dahl SS rats (Citation14,15).
Importantly, none of these studies have comprehensively investigated the effects of eplerenone on indices of end organ damage. In addition, few studies have assessed the BP lowering efficacy of eplerenone in Dahl SS rats on low-salt diet. To this end, the present study was designed to evaluate the effects of chronic MR antagonism with eplerenone on the development and progression of hypertension using radiotelemetry and to assess the effects of eplerenone on end organ structure and function and renal injury biomarkers in Dahl SS rats maintained on high- or low-salt diets.
Materials and Methods
Dahl SS rats (Harlan Labs. Inc: Indianapolis, IN, USA) were housed in a temperature- and humidity-controlled room with a 12:12-h dark-light cycle with food and water provided ad libitum. Rodent diets (#7034, low-salt diet, containing 0.3% NaCl; TD.92034, high-salt diet, containing 4% NaCl) were purchased from Harlan Teklad (Madison, WI, USA). Eplerenone, purified from Inspra tablets (Pfizer Inc., New York, NY, USA) was used to prepare eplerenone-medicated diets (based on Harlan Tekald #7034 and TD.92034) by Research Diets, Inc. (New Brunswick, NJ, USA). The eplerenone concentration in the diet was calculated to give a daily dose of 100 mg•kg−1d−1 eplerenone based on food intake relative to body weight.
All procedures utilizing experimental animals were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, and experimental protocols were approved in advance by the Institutional Animal Care and Use Committee at Merck Research Laboratories, Rahway, NJ.
Radiotelemetry Implant Surgery
Male Dahl SS rats (15 weeks old) were anesthetized with isoflurane and premedicated with buprenorphine (0.03 mg•kg−1, s.c.: Reckitt Benckiser healthcare Ltd., Hull, UK) prior to surgery. Telemetry devices (TA11PA-C40, Data Sciences International, DSI, St. Paul, MN, USA) were aseptically placed in a subcutaneous pocket on one side of the body with the catheter inserted into the descending aorta via the femoral artery. Penicillin G (150,000 U•kg−1, s.c. Bimeda Inc., Irwindale, CA, USA) was administered at the end of surgery. Rats were allowed to recover for 2 to 3 weeks prior to experimentation.
Experimental Design
Dahl SS rats with implanted radiotelemetry devices were housed individually in Nalgene metabolism cages (Braintree Scientific, Inc., Braintree, MA, USA) following a 1-week acclimation period. All animals were maintained on Harlan Teklad diet #7034 (0.3% NaCl) prior to study. The rats were randomly divided into four groups: Group 1, low-salt diet (0.3% NaCl), n = 8; Group 2, low-salt diet plus eplerenone 100 mg•kg−1d−1, n = 8; Group 3, high-salt diet (4% NaCl), n = 8; Group 4, high-salt diet plus eplerenone 100 mg•kg−1d−1, n = 8. Animals were maintained in these treatment groups for an 8-week period.
BP Measurement, Renal Function Assessment, and Kidney Injury Biomarkers Detection
Radiotelemetry signals were collected and analyzed using a DSI Dataquest System Version 4.1 (Data Sciences International, DSI, St. Paul, MN, USA). Mean, systolic, diastolic, and pulse arterial BPs and heart rate were determined on a beat-by-beat basis. Data was collected for 30 min every hour and was reported as average values for each animal over a 24-h period. Twenty-four hour food and water intake and urine output were monitored once a week during the study. Urinary electrolytes (including Na, K, and Cl), creatinine, and protein concentration were assessed by a Roche Modular Chemistry System (Roche Diagnostics, Indianapolis, IN, USA). Kidney injury biomarkers including lipocalin-2 (LPN), osteopontin (OPN), kidney injury molecule-1 (KIM-1), and renal papillary antigen 1 (RPA-1) were detected using a Kidney Injury Panel 1(rat) kit and Argutus AKI (rat) kit from Meso Scale Discovery, LLC (Gaithersburg, MA, USA). All these biomarkers have been validated in animal studies (Citation31).
Serum Aldosterone, Plasma Electrolytes, Creatinine, and Eplerenone Concentration Measurement
Upon study termination, blood was collected by jugular venepuncture from conscious animals and was used for measuring serum aldosterone by ELISA (IBL, Hamburg, Germany). Thereafter, animals were euthanized by CO2 inhalation and cardiac puncture was performed to collect blood for determination of plasma electrolytes and creatinine (Roche Diagnostics, Indianapolis, IN, USA) and eplerenone levels (determined by LC/MS/MS following protein precipitation with acetonitrile).
Figure 1. Effects of chronic eplerenone treatment on BP and heart rate in Dahl SS rats. Data are mean ± SEM (n = 8 for each group). Abbreviations: LS - low salt; HS - high salt; Epl - eplerenone. High salt statistically significantly increased sBP after 1 week of salt-loading (p < 0.05, HS alone vs. LS alone). sBP in LS + Epl 100 mg•kg−1d−1 and HS + Epl 100 mg•kg−1d−1 group was statistically significantly lower than the LS alone (since the first week of treatment) and HS alone group (since the second week of treatment), respectively (p < 0.05). Heart rate was statistically significantly higher in the HS alone group than the other three groups in the last 3 weeks of the study (p < 0.05) (color figure available online).
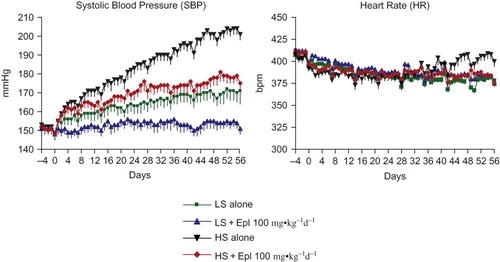
Figure 2. Effects of eplerenone on body weight, food intake, water intake, and urine output in Dahl SS rats. Data are mean ± SEM (n = 8 for each group). Abbreviations: LS - low salt; HS - high salt; Epl - eplerenone. Body weight and food intake were not statistically significantly different among all groups. Water intake and urine output were statistically significantly greater in the two high-salt diet groups than that in the two low-salt diet groups (*p < 0.05, HS groups vs. LS groups) (color figure available online).
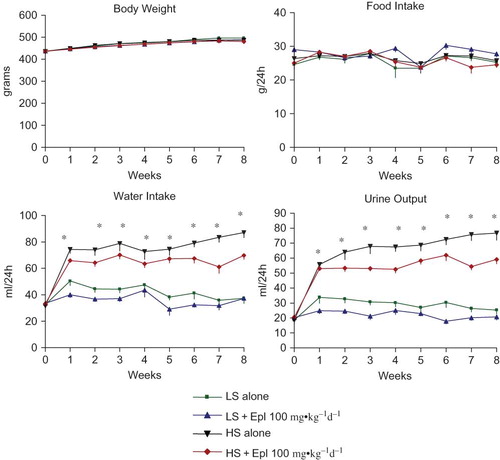
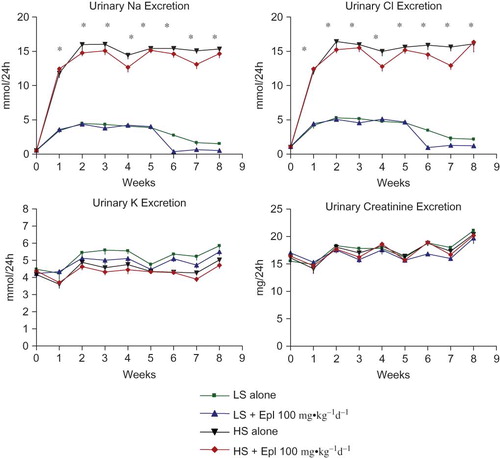
Figure 3. Effects of Eplerenone on urinary electrolytes and creatinine excretion in Dahl SS rats. Data are mean ± SEM (n = 8 for each group). Abbreviations: LS - low salt; HS - high salt; Epl - eplerenone. Urinary Na and Cl excretion were statistically significantly greater in the two high-salt diet groups than that in the two low-salt diet groups (*p < 0.05, HS groups vs. LS groups). Urinary K and creatinine excretion were not statistically significantly different among all groups (color figure available online).
Histopathology
Kidney and heart tissues were harvested from all animals, fixed in Prefer (Anatech LTD., Battle Creek, MI, USA) for at least 24 h, and were paraffin-embedded. Tissue sections were stained with hematoxylin and eosin (H&E) and the heart also with Masson’s trichrome strain (for collagen). The severity of histopathologic changes in renal tubules, interstitium, vasculature, and glomeruli were graded on a 1 to 5 scale corresponding to minimal, mild, moderate, marked, and severe (Citation32). Sections from both kidneys were examined and the final scores composite are a composite from all sections of each slice. Separate deparaffinized heart tissue sections were stained with Masson trichrome, which stains collagen-enriched areas as blue and cellular elements as red. Collagen deposition in the ventricle wall and perivascular area was graded on a 1 to 5 scale corresponding to minimal, mild, moderate, marked, and severe, based on the blue stained area size and intensity.
Statistical Analyses
All data are presented as means ± standard error of the mean (SE). A repeated measure analysis of variance (rmANOVA) was used for time course data analysis. One-way ANOVA followed by Newman-Keuls post-hoc test was used for comparisons of only one time point values in all groups. P value of <0.05 was considered to be of statistical significance.
Results
Effects of Eplerenone on Blood Pressure and Heart Rate
The baseline SBP of Dahl SS rats, while maintained on a low (0.3% NaCl) dietary salt and measured 2 to 3 weeks post-implantation of telemetry devices, was 151 ± 3 mmHg (). Systolic BP gradually increased by 18 ± 3 mmHg over the subsequent 8 weeks (from age 18 weeks to 25 weeks). This increase reflects the spontaneous development of hypertension characteristic of this rat strain. As expected, consumption of a high-salt (4% NaCl) diet accelerated the progression of hypertension. Thus, SBP increased by 52 ± 2 mmHg from 152 ± 3 mmHg to 204 ± 3 mmHg over an 8-week period of high-salt feeding. The extent of BP elevation in response to a high-salt diet was similar when measured during the day and night periods (data not shown). Chronic treatment with eplerenone (100 mg•kg−1d−1) completely blocked the SBP increase in animals fed a low-salt diet (170 ± 5 mmHg vs.154 ± 3 mmHg, p < 0.05) and significantly blunted the progression of hypertension in animals fed a high-salt diet (204 ± 3 mmHg vs.179 ± 3 mmHg, p < 0.05) (). Heart rates declined by less than 10% during the course of study and for the most part were not significantly different among groups receiving low or high salt diet with or without eplerenone (). There was a small increase in heart rate during the last 3 weeks for those animals on the high-salt diet that was not apparent in those animals receiving high salt plus eplerenone. Interestingly, we observed a consistent transient decrease in BP and heart rate contemporaneous with the weekly collection of urine samples from these animals. The magnitude of the decrease was similar for all groups of animals and we attribute these changes to the increased investigator presence during this procedure.
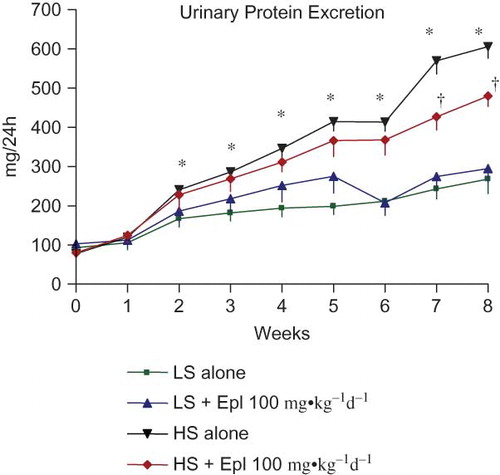
Figure 4. Time course of 24-h urinary protein excretion. Data are mean ± SEM (n = 8 for each group). Abbreviations: LS - low salt; HS - high salt; Epl - eplerenone. Urinary protein excretion was statistically significantly greater in the HS alone group than that in the LS alone group (*p < 0.05, HS alone vs. LS alone). Proteinuria was statistically significantly reduced in the HS + Epl 100 mg•kg−1d−1 group compared with the HS alone group in the 7th and 8th week of the study (†p < 0.05, HS + Epl 100 mg•kg−1d−1 vs. HS alone) (color figure available online).
Renal Excretory Effects of Eplerenone
The effects of eplerenone on body weight, food and water intake, urine output, and urinary excretion of Na, Cl, K, and creatinine are shown in and . Baseline data (reported as week 0) from all groups maintained with low-salt diet was collected 1 week prior to administration of eplerenone and to placing animals on a high- salt diet. Body weight or food intake did not vary among the groups. Water intake, urine output, and urinary excretion of Na and Cl were statistically significantly greater in the two high-salt diet groups than in the low-salt diet groups. Eplerenone did not exhibit natriuretic effects on Dahl SS rats on either a low-salt or high-salt diet at steady state. Urinary excretion of K and creatinine were not statistically significantly different between the groups. In addition, we tested the acute natriuretic effect of eplerenone in a separate cohort of age-matched Dahl SS rats and found that eplerenone elicited a significant natriuretic effect in the low-salt diet condition (24 h urinary Na excretion of 1.92 ± 0.37 mmol/24 h in animals that received low salt diet plus eplerenone 100 mg•kg−1d−1 vs. 1.26 ± 0.34 mmol/24 h in animals that received low salt diet alone, n = 5 per group, p < 0.05). However, under conditions of high-salt intake, sodium excretion was equivalent (P > 0.05) in control animals (9.24 ± 1.61 mmol/24 h, n = 5) and those that received eplerenone (9.35 ± 0.59 mmol/24 h, n = 5). Urinary protein excretion was markedly increased in Dahl SS rats on the high-salt diet, which was significantly attenuated (p < 0.05) with eplerenone after treatment for 7 to 8 weeks ().
Biomarkers of Kidney Injury
Urinary LPN and OPN are biomarkers of renal tubular epithelial injury. KIM-1, also known as TIM-1 (T cell immunoglobulin mucin domains–1), is a protein that is predominately expressed in proximal tubules and is a sensitive biomarker for proximal tubular injury. RPA-1 is considered as a collecting duct/renal papilla injury biomarker. All these biomarkers were increased as early as 2–4 weeks after salt-loading and were significantly diminished by eplerenone treatment towards the end of the study ().
Serum Aldosterone, Plasma Electrolytes, Creatinine, and Eplerenone Levels
Serum aldosterone levels and plasma Na, K, and creatinine concentrations were not different among groups ( and ). There was a trend towards an increase in plasma K in those animals that received high salt plus eplerenone but this did not reach statistical significance. The plasma eplerenone concentrations in the animals that received low salt and high salt were 77.3 ± 11.6 and 90.6 ± 19.4 nM, respectively ().
Organ Weights and Histopathologic Findings
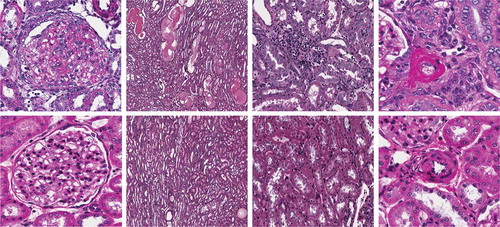
Animals on the high-salt diet had significantly increased heart and kidney weights, and eplerenone treatment tended to attenuate these changes (). Histologic evidence for renal injury was present in rats on either a low- or high-salt diet but was more severe for those animals on the high salt diet (). Thus, Dahl SS rats on the high-salt diet () exhibited severe focal-segmental or global glomerulosclerosis, perivascular fibrosis, inflammatory cell infiltration, interstitial fibrosis, tubular dilation, and tubular cast formation, and the quantitative injury scores of glomeruli, vasculature, interstitium, and renal tubules were significantly increased. Eplerenone treatment improved the renal histopathologic alterations induced by salt-loading (). With respect to heart collagen content, since collagen is a normal component of the interstitium, scores 1 and 2 reflect the normal range of histologic variations; salt-loading for 8 weeks did not significantly increase heart collagen content ().
Discussion
Our results demonstrate that Dahl SS rats exhibit a slow progression of hypertension when maintained on a low-salt diet, and consumption of a high-salt diet markedly accelerated the development of hypertension. Eplerenone completely blocked and significantly attenuated the progressive rise in SBP in Dahl SS rats under low-salt and high-salt conditions, respectively. This observation concurs with several recent reports that treatment with eplerenone substantially prevented the development of high salt-induced hypertension in Dahl SS rats (Citation4,7,8). However, in contrast to these findings, other investigators reported that eplerenone has no effects on BP in salt-loaded Dahl SS rats (Citation14,15). It is possible that differences in experimental protocol and technique may contribute to these different findings. For example, differences in the age of animals used and in different salt diets employed could influence the time course and magnitude of the hypertension and renal injury. As demonstrated here, the BP lowering efficacy of eplerenone is quite different in animals maintained on low- and high-salt diets. It is well documented that long-term exposure to a high-salt diet from a young age provokes severe hypertension and organ damage (Citation14,15), which may be refractory to mineralocorticoid antagonism. A second possible explanation for the different effects of eplerenone on BP in this and other studies could be related to the techniques used to measure BP. We employed radiotelemetry, generally viewed as the ‘‘gold standard’’ for BP measurement (Citation33), in conscious freely moving animals. However, studies that used the indirect tail cuff plethysmographpy (Citation14,15) carry limitations in accuracy and do not monitor BP during an entire 24-h period such that minor differences in BP could have been missed. Finally, the doses of eplerenone used by Ohtani et al. (Citation14) (12.5 or 40 mg•kg−1d−1) and Nagata et al. (Citation15) (30 or 100 mg•kg−1d−1) could be below those required for BP lowering and plasma exposure data for eplerenone were not provided in their studies. It is important to note that in our study, eplerenone was administered concomitantly with salt-loading at adult age, and the salt content is lower than that employed in most of the reports (Citation4,7,14,15).
With respect to mechanism of action, it is suggested that MR antagonists lower BP via enhanced natriuresis/diuresis or nonrenal mechanisms, including antagonizing MR in the central nervous system or cardiovascular tissues (Citation34–37). In the present study, no natriuretic/diuretic effect of eplerenone was detected under either low-salt or high-salt conditions by weekly monitoring of renal excretory function. We suspect that an early effect on natriuresis/diuresis could have been missed by assessing renal excretory function after eplerenone had been administered for 1 week. Accordingly, we performed an acute study with eplerenone (100 mg•kg−1d−1 for 3 days) in age-matched Dahl SS rats (without telemetry device implantation), and determined a significant natriuretic effect on the first day of treatment in the low-salt diet condition but not in the high-salt diet condition. The initial natriuretic/diuretic effect of eplerenone in animals on a low-salt diet could lead to a leftward shift of the steady state pressure-natruresis relationship curve, whereby sodium balance is maintained at a lower BP level. It is notable that the BP lowering effect of eplerenone in animals on a high-salt diet is quite different from the effect seen in animals on a low-salt diet, and the anti-hypertensive effect of eplerenone in high-salt fed animals cannot be explained by acute changes in natriuresis/diuresis. However, the contributions of nonrenal mechanisms such as antagonizing MR in the brain, heart, and vasculature to its BP efficacy remain to be determined.
The second purpose of the present study was to evaluate the effects of eplerenone on organ damage. It is interesting that Dahl SS rats, even on a low-salt diet, developed proteinuria. This effect may be attributed to the glomerular phenotype (loss of foot processes in podocytes) of this rat strain (Citation38). Although glomerular hypertension could accelerate proteinuria, the increase in glomerular pressure may not be a dominant contributor to the development of proteinuria in Dahl SS rats on low salt diet. Accordingly, a modest decrease in BP by eplerenone in Dahl SS rats on low salt diet, may not decrease urinary protein excretion. Animals on a high-salt diet developed more extensive renal injury as demonstrated by increased kidney injury biomarkers and histopathologic alterations in glomeruli, vasculature, and interstitium. The kidneys were enlarged, and proteinuria was dramatically accelerated. All the above-mentioned changes induced by a high-salt challenge were attenuated by eplerenone treatment. These findings further support the concept that eplerenone has renoprotective effects in salt-sensitive hypertensive renal injuries (Citation7,8). With respect to cardiac structure and function, it is apparent that a high-salt diet caused cardiac hypertrophy and increased heart rate; the latter could potentially reflect an early stage of cardiac dysfunction. Chronic treatment with eplerenone significantly reduced the cardiac changes observed in animals on a high-salt diet. Consistent with our findings, Ohtani et al. (Citation14) and Nagata et al. (Citation15) observed that salt-loading could induce heart failure when 8% salt was introduced to 7 weeks old Dahl SS rats for 5–6 weeks, subsequent treatment with eplerenone for 7–8 weeks results in attenuation in left ventricular hypertrophy and heart failure. Thus, MR antagonism is beneficial to the heart under high-salt conditions. It is not the scope of the present study to investigate the mechanism of organ protection afforded by eplerenone. However, it is reasonable to speculate that hemodynamic and/or nonhemodynamic, as well as other direct actions, might contribute to beneficial effects of eplerenone on tissues. An extensive body of evidence suggests salt-sensitive hypertension is associated with endothelial dysfunction, oxidative stress, inflammation, and fibrosis (Citation28–30), and eplerenone has been shown to suppress the induction of oxidative stress (Citation4,7,15), fibrosis/apoptosis, and inflammation (Citation7) in Dahl SS rats fed high-salt diet.
Figure 5. Time course of 24-h urinary kidney injury biomarkers. Data are mean ± SEM (n = 8 for each group). Abbreviations: LS - low salt; HS - high salt; Epl - eplerenone; LPN - lipocalin-2; OPN -osteopontin; KIM-1 -kidney biomarker molecule-1; RPA-1 -renal papillary antigen 1. All these biomarkers were increased as early as 2–4 weeks after salt-loading and were statistically significantly diminished by eplerenone treatment towards the end of the study. (*p < 0.05, HS alone vs. LS alone; †p < 0.05, HS + Epl 100 mg•kg−1d−1 vs. HS alone) (color figure available online).
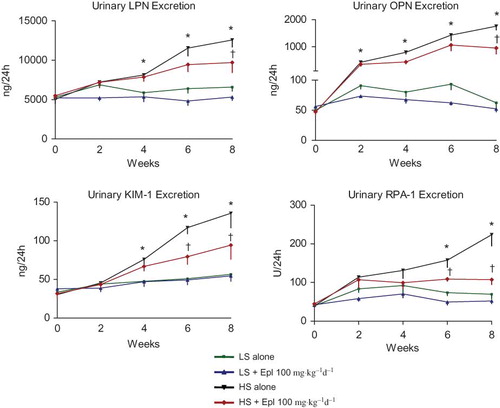
Figure 6. Serum aldosterone level at the end of an 8-week treatment. Data are mean ± SEM (n = 8 for each group). Abbreviations: LS - low salt; HS - high salt; Epl - eplerenone. There was no statistically significant difference in serum aldosterone level among all groups (color figure available online).
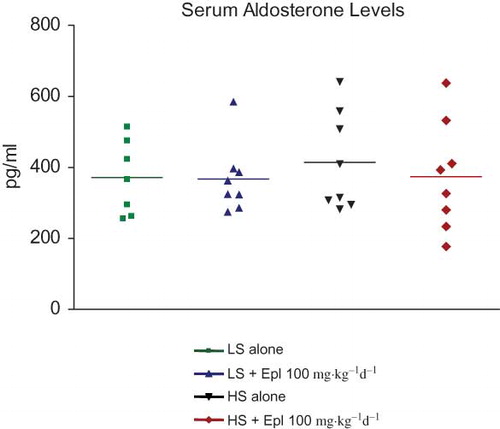
It is generally accepted that high-salt intake suppresses the renin-angiotensin system and aldosterone levels and low-salt intake has the opposite effect (Citation39). Previous reports showed that salt-loading in Dahl SS rats reduced plasma aldosterone levels (Citation8,14,15,40); however, tissue (heart and kidney) levels of aldosterone (Citation40) or MR mRNA or protein levels of MR (Citation8,14,15) were upregulated. These findings suggested that high dietary salt evokes inappropriate activation of the local aldosterone/MR pathway, which may play a causal role in the development of salt-sensitive hypertension. In the present study, high dietary salt did not change serum aldosterone levels, contrary to reports from other groups (Citation8,14,15,34). The underlying reason for this divergent finding is unclear at this stage. However, we speculate that the blood sampling method and time of blood collection may affect the measurement of serum aldosterone. In the present study, blood samples were collected around 10AM by jugular venepuncture in conscious animals. It is known that aldosterone secretion exhibits circadian rhythm in rats (Citation41,42) and humans (Citation43,44). Preliminary data (unpublished) from our laboratory indicates that aldosterone levels measured in conscious animals are at their lowest in the early morning. In addition, the stress caused by the procedure of jugular venepuncture in a conscious state might have an impact on aldosterone secretion. However, it is difficult to make an appropriate comparison with other published studies in which blood collection was conducted under anesthesia and the timing of the sample collection was not reported (Citation8,14,15,40). We did not determine tissue levels of aldosterone in the present study. Interestingly, Bayorh et al. (Citation40) reported that tissue levels of aldosterone are increased under conditions of high-salt administration to Dahl SS rats. Thus, it is conceivable that the beneficial effects of eplerenone (hemodynamic or organ protection) may be a consequence of inhibiting the effects of elevated tissue levels of aldosterone.
A concern associated with the clinical use of MR antagonists is hyperkalemia (Citation45). In the present study, urinary potassium excretion was slightly less in those animals that received eplerenone but the change did not reach statistical significance. Furthermore, plasma potassium levels were not statistically different among groups, although there was a trend for an increase in plasma potassium for those animals on a high-salt diet with eplerenone treatment. The somewhat modest effect on urinary potassium excretion and the absence of elevated plasma aldosterone in these studies may both be a consequence of submaximal MR blockade. Although we do not have a direct in-vivo measure of the extent of receptor blockade achieved, we do know that we achieved plasma levels of eplerenone of less than 100 nM (77.3 nM in animals on a low-salt diet and 90.6 nM in animals on a high-salt diet). Published data (Citation37) indicated that in rats, the IC50 of eplerenone for the aldosterone receptor was 360 nM. Thus, plasma levels of eplerenone of less than 100 nM would not be expected to produce complete receptor blockade, which may account for the minimal changes in potassium excretion and little change in the plasma concentration of aldosterone. Furthermore, it is also possible that higher doses of eplerenone, and hence higher plasma exposure, could produce a greater extent of BP lowering and organ protection.
Table 1. Plasma creatinine, Na, and K concentration at the end of 8 weeks treatment
Table 2. Plasma eplerenone level at the end of an 8-week treatment
Table 3. Body and organ weights
Table 4. Renal histopathologic scores
In conclusion, the findings of the present study demonstrate that MR antagonism attenuates the development and progression of hypertension and provides target organ protection in a model of salt-sensitive hypertension. Importantly, these data further support the promise of MR antagonists for the treatment of hypertension.
Figure 7. Representative light microscopic findings in glomeruli, renal tubules, interstitium, and vasculature. Upper panel: HS alone, glomerulus, renal tubules, interstitium, and renal artery (left→right). Lower panel: HS + Epl 100 mg·kg−1d−1, glomerulus, renal tubules, interstitium, and renal artery (left→right). High salt alone group shows excess mesangium and decreased capillary lumen in glomerulus, dilated tubules filled with proteinaceous materials, subendothelial fibrinoid material and endothelial proliferation in renal artery, and interstitium infiltration with mononuclear inflammatory cells. HS + Epl 100 mg·kg−1d−1 group exhibits normal appearance of glomerulus, decreased dilation and proteinaceous content in renal tubules, normal vascular appearance, and no infiltration with mononuclear inflammatory cells in interstitium (color figure available online).
Acknowledgments
We would like to acknowledge and thank Xiaolan Shen, Bo Wang, Christian N. Nunes, Leslie Ann Balogh, Carol Ann Keohane, Xiaoli Ping, Nina Jochnowitz, Loise Gichuru, Marianne Eybye, Huaibing He, and John Ronan for their excellent technical support to this work. We also acknowledge Dr. Shubing Wang for his assistance with statistical analyses and Dr. Edward D. Frohlich for his helpful suggestions on the manuscript.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 1991; 83:1849–1865.
- Tomaschitz A, Pilz S, Ritz E, Obermayer-Pietsch B, Pieber TR. Aldosterone and arterial hypertension. Nat Rev Endocrinol 2010;6:83–93.
- Marney AM, Brown NJ. Aldosterone and end-organ damage. Clin Sci (Lond) 2007;113:267–278.
- Bayorh MA, Mann G, Walton M, Eatman D. Effects of enalapril, tempol, and eplerenone on salt-induced hypertension in dahl salt-sensitive rats. Clin Exp Hypertens 2006;28:121–132.
- Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int 2003;63:1791–1800.
- Endemann DH, Touyz RM, Iglarz M, Savoia C, Schiffrin EL. Eplerenone prevents salt-induced vascular remodeling and cardiac fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension 2004; 43:1252–1257.
- Nagase M, Shibata S, Yoshida S, Nagase T, Gotoda T, Fujita T. Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension 2006;47:1084–1093.
- Du J, Fan YY, Hitomi H, Kiyomoto H, Kimura S, Kong CZ, Noma T, Kohno M, Nishiyama A, Nakano D. Mineralocorticoid receptor blockade and calcium channel blockade have different renoprotective effects on glomerular and interstitial injury in rats. Am J Physiol Renal Physiol 2009;297:F802–F808.
- Weinberger MH, Roniker B, Krause SL, Weiss RJ. Eplerenone, a selective aldosterone blocker, in mild-to-moderate hypertension. Am J Hypertens 2002;15: 709–716.
- Krum H, Nolly H, Workman D, He W, Roniker B, Krause S, Fakouhi K. Efficacy of eplerenone added to renin-angiotensin blockade in hypertensive patients. Hypertension 2002;40:117–123.
- White WB, Duprez D, St Hillaire R, Krause S, Roniker B, Kuse-Hamilton J, Weber MA. Effects of the selective aldosterone blocker eplerenone versus the calcium antagonist amlodipine in systolic hypertension. Hypertension 2003;41:1021–1026.
- Brandish PE, Forest TW, Chen H, Gatto NT, Molon-Noblot S, Zwierzynski I, Szczerba P, Adamson GE, Ma BK, Flores OA, Hershey JC. Eplerenone Decreases Inflammatory Foci in Spontaneously Hypertensive Rat Hearts With Minimal Effects on Blood Pressure. J Cardiovasc Pharmacol 2009;53:44–51.
- Zhou X, Ono H, Ono Y, Frohlich ED. Aldosterone antagonism ameliorates proteinuria and nephrosclerosis independent of glomerular dynamics in L-NAME/SHR model. Am J Nephrol 2004;24:242–249.
- Ohtani T, Ohta M, Yamamoto K, Mano T, Sakata Y, Nishio M, Takeda Y, Yoshida J, Miwa T, Okamoto M, Masuyama T, Nonaka Y, Hori M. Elevated cardiac tissue level of aldosterone and mineralocorticoid receptor in diastolic heart failure: Beneficial effects of mineralocorticoid receptor blocker. Am J Physiol Regul Integr Comp Physiol 2007;292:R946–R954.
- Nagata K, Obata K, Xu J, Ichihara S, Noda A, Kimata H, Kato T, Izawa H, Murohara T, Yokota M. Mineralocorticoid receptor antagonism attenuates cardiac hypertrophy and failure in low-aldosterone hypertensive rats. Hypertension 2006;47(4):656–664.
- Pitt B, Reichek N, Willenbrock R, Zannad F, Phillips RA, Roniker B, Kleiman J, Krause S, Burns D, Williams GH. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation 2003;108:1831–1838.
- Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309–1321.
- Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, MD: National Institutes of Health, 2003.
- World Health Organization (WHO). World Health Report 2002: reducing risks, promoting healthy life. Geneva: WHO, 2002.
- Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension 1996;27:481–490.
- Luft FC. Molecular genetics of salt-sensitivity and hypertension. Drug Metab Dispos 2001;29:500–504.
- Rodriguez-Iturbe B, Vaziri ND. Salt-sensitive hypertension–update on novel findings. Nep Physiol Rev 2005;85:679–715.
- Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev 2005;85:679–715.
- Cowley AW Jr. The genetic dissection of essential hypertension. Nat Rev Genet 2006;7:829–840.
- Fujita T. Aldosterone in salt-sensitive hypertension and metabolic syndrome. J Mol Med 2008;86:729–734.
- Roman RJ. Abnormal renal hemodynamics and pressure-natriuresis relationship in Dahl salt-sensitive rats. Am J Physiol 1986;251:F57–F65.
- Liang M, Yuan B, Rute E, Greene AS, Olivier M, Cowley AW Jr. Insights into Dahl salt-sensitive hypertension revealed by temporal patterns of renal medullary gene expression. Physiol Genomics 2003;12:229–237.
- Hayakawa H, Coffee K, Raij L. Endothelial dysfunction and cardiorenal injury in experimental salt-sensitive hypertension: effects of antihypertensive therapy. Circulation 1997;96:2407–2413.
- Meng S, Cason GW, Gannon AW, Racusen LC, Manning RD Jr. Oxidative stress in Dahl salt-sensitive hypertension. Hypertension 2003;41:1346–1352.
- Gu JW, Tian N, Shparago M, Tan W, Bailey AP, Manning RD Jr. Renal NF-kappaB activation and TNF-alpha upregulation correlate with salt-sensitive hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 2006;291:R1817–R1824.
- Ozer JS, Dieterle F, Troth S, Perentes E, Cordier A, Verdes P, Staedtler F, Mahl A, Grenet O, Roth DR, Wahl D, Legay F, Holder D, Erdos Z, Vlasakova K, Jin H, Yu Y, Muniappa N, Forest T, Clouse HK, Reynolds S, Bailey WJ, Thudium DT, Topper MJ, Skopek TR, Sina JF, Glaab WE, Vonderscher J, Maurer G, Chibout SD, Sistare FD, Gerhold DL. A panel of urinary biomarkers to monitor reversibility of renal injury and a serum marker with improved potential to assess renal function. Nat Biotechnol 2010;28:486–494.
- Crowley SD, Vasievich MP, Ruiz P, Gould SK, Parsons KK, Pazmino AK, Facemire C, Chen BJ, Kim HS, Tran TT, Pisetsky DS, Barisoni L, Prieto-Carrasquero MC, Jeansson M, Foster MH, Coffman TM. Glomerular type 1 angiotensin receptors augment kidney injury and inflammation in murine autoimmune nephritis. J Clin Invest 2009;119:943–953.
- Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE; Subcommittee of Professional and Public Education of the American Heart Association. Recommendations for blood pressure measurement in humans and experimental manimals. Part 2: Blood pressure measurement in experimental animals: a statement for professionals from the subcommittee of professional and public education of the American Heart Association council on high blood pressure research. Hypertension 2005;45:299–310.
- Hood SJ, Taylor KP, Ashby MJ, Brown MJ. The spironolactone, amiloride, losartan, and thiazide (SALT) double-blind crossover trial in patients with low-renin hypertension and elevated aldosterone-renin ratio. Circulation 2007;116:268–275.
- Huang BS, Van Vliet BN, Leenen FH. Increases in CSF [Na+] precede the increases in blood pressure in Dahl S rats and SHR on a high-salt diet. Am J Physiol Heart Circ Physiol 2004;287:H1160–H1166.
- Gomez-Sanchez EP, Fort C, Thwaites D. Central mineralocorticoid receptor antagonism blocks hypertension in Dahl S/JR rats. Am J Physiol Endocrinol Metab 1992;262:E96–E99.
- Brown NJ. Eplerenone: cardiovascular protection. Circulation 2003;107:2512–2518.
- Sterzel RB, Luft FC, Gao Y, Schnermann J, Briggs JP, Ganten D, Waldherr R, Schnabel E, Kriz W. Renal disease and the development of hypertension in salt-sensitive Dahl rats. Kidney Int 1988;33:1119–1129.
- Dluhy RG, Williams GH. Aldosterone–villain or bystander? N Engl J Med 2004;351:8–10.
- Bayorh MA, Ganafa AA, Emmett N, Socci RR, Eatman D, Fridie IL. Alterations in aldosterone and angiotensin II levels in salt-induced hypertension. Clin Exp Hypertens 2005;27:355–367.
- Hilfenhaus M. Circadian rhythm of the renin-angiotensin-aldosterone system in the rat. Arch Toxicol 1976;36:305–316.
- Gomez-Sanchez C, Holland OB, Higgins JR, Kem DC, Kaplan NM. Circadian rhythms of serum renin activity and serum corticosterone, prolactin, and aldosterone concentrations in the male rat on normal and low-sodium diets. Endocrinology 1976;99: 567–572.
- Ryoyu T, Isamu M, Ikeda Masatoshi I, Hideo K, Yoshiyu T, Shuichiro Y, Morise Toshio M, Hiroaki T. Circadian rhythm of plasma aldosterone and time dependent alterations of aldosterone regulators. J Steroid Biochemistry 1984;20:s321–323.
- Hurwitz S, Cohen RJ, Williams GH. Diurnal variation of aldosterone and plasma renin activity: timing relation to melatonin and cortisol and consistency after prolonged bed rest. J Appl Physiol 2004;96:1406–1414.
- Weir, M, Rolfe, M: Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol 2010;5:531–548.